
The incidence and risk factors of acute pain after preoperative needle localization of pulmonary nodules: a cross-sectional study
Introduction
As increasing numbers of people undergo lung cancer screening by low-dose computed tomography (CT), millions of small pulmonary nodules (<1 cm in size) or ground-glass opacities (GGOs) are detected early (1-3). Among those small pulmonary nodules or GGOs, a substantial percentage of nodules may require video-assisted thoracic surgery (VATS) to intervene (1). However, these small pulmonary nodules are difficult to be identified under VATS and thoracic surgeons often need to perform preoperative CT-guided localization to accurately localize the nodules intraoperatively (4-6).
Until recently, none of the available preoperative CT-guided localization methods are optimal and may predispose patients to potential harm (7,8). Among the preoperative CT-guided localization methods, hookwire localization remains the mainstay (9-11), but it may also lead to complications or significant risks (e.g., pneumothorax, pulmonary hemorrhage, substantial pain, and wire dislodgement) (12). The majority of previous studies mainly focused on the efficacy and safety of hookwire needle localization (7,8), but the related pain is not fully examined. A retrospective study involving 57 patients reported that the incidence of pain after hookwire localization was 7%, but they did not clearly state the method used to assess pain nor at which state (13). Other studies assessed pain as the exploratory component of complications after hookwire localization (14,15), but the severity and related risk factors are still limited. Exploring potential predictors may be helpful to prevent acute pain in high-risk population.
Based on our institutional experience, patients often experience moderate to severe pain after CT-guided localization before VATS. If not controlled well, acute pain after needle localization may cause severe discomfort or anxiety in patients. Herein, we conducted a cross-sectional study in a tertiary center, aiming to accurately determine the incidence and risk factors of acute pain after hookwire CT-guided localization. Specifically, we investigated the incidence of pain at rest, during deep breathing and movement after CT-guided localization and explored potential risk factors related to substantial acute pain. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-557/rc).
Methods
Ethics and registration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shanghai Chest Hospital (IRB #KS (P) 2142) and informed consent was taken from all the patients. This trial was registered at the Chinese Clinical Trial Registry prior to subject enrolment (No. ChiCTR2100051447; principal investigator, Yuwei Qiu; date of registration, September 23, 2021).
Patients and study design
This cross-sectional study was conducted at Shanghai Chest Hospital. Eligible patients were between 18 and 75 years old, had an American Society of Anesthesiologists (ASA) physical status of I–III, were scheduled for elective video-assisted thoracoscopic surgery, and had small lung nodules requiring preoperative CT-guided localization.
Patients were excluded if they had clinically significant cardiovascular diseases, were unable to perform the visual analogue scale (VAS) (16), or had undergone previous thoracic surgeries, received chronic pain medication, were diagnosed with herpes zoster accompanied by debilitating pain, or other factors that precluded an accurate pain assessment.
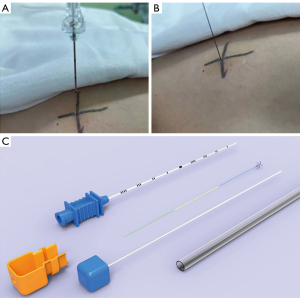
Preoperatively, the patients were admitted to radiology procedure suite for localization. Local anesthesia using 2% lidocaine was administered for all patients by the radiologists performing localization. First, the position of the small pulmonary nodules was determined by high-resolution CT scanning (SIEMENS, SOMATOM Force, Bayern, Germany). Next, a localization needle (20# gauge size and length of 9 mm, GHIATAS Beaded Breast Localization Wire, Bard Peripheral Vascular Inc., Arizona, United States) or a disposable pulmonary nodule locating needle (model: SS510-10 Senscure, Ningbo, China) was used for localization (Figure 1). When the hook was released, an anchor needle would firmly grasp the adjacent lung tissue. CT reexamination was then used to confirm the positional relationship between the locating needle and the pulmonary nodules. VATS surgery was performed within 1–2 hours.
Outcome assessment
Primary outcome
The primary outcome of this study was the incidence of moderate to severe pain during deep breathing after CT-guided needle localization.
10–15 minutes after needle localization, an investigating researcher assessed pain intensity using a 10 cm VAS (0 cm = no pain and 10 cm = worst imaginable pain) in the preanesthesia room (16). We assessed pain at rest, during deep breathing and physical movement (defined as moving the ipsilateral arm). Furthermore, we graded the pain severity of the VAS using the specific adjectives: 0 cm = no pain, 1–3 cm = mild pain, 4–6 cm = moderate pain, and 7–10 cm = severe pain) (16). A VAS score ≥4 cm was considered to indicate moderate to severe pain (16).
Exposure variables
The predefined exposure variables were based on the existing literature or clinical experience. The potential predictors of acute pain included patient demographics [age, gender, and body mass index (BMI), education levels (middle school, high school, or above)], preoperative psychological stress and CT-guided localization factors. The Chinese version of the State-Trait Anxiety Inventory (STAI) was used to reflect patients’ psychological stress before CT-guided needle localization (17). STAI is a reliable instrument used to test both the levels of state and trait anxiety, in which the state anxiety reflects an immediate state of patients’ anxiety at a particular moment (18,19). CT-guided localization factors included the numbers of localization needles, surgical site (left thorax, right thorax, and bilateral thorax), type of localization needles (GHIATAS Beaded Breast Localization Wire or Senscure localization needle), distance between the skin and final needle position around the lung lesion, distance between the parietal pleura and final needle position around the lung lesion, and the specific location of the needle puncture on the chest wall. Here, we classified the location of needle punctures on the chest wall into the following categories: anterior chest wall (defined from the parasternal line to the anterior axillary line), lateral chest wall (defined from the anterior axillary line to the posterior axillary line), and posterior chest wall (defined from the posterior axillary line to the spine).
Other outcomes
Patients’ vital signs including non-invasive systolic blood pressure, diastolic blood pressure, and pulse rate after needle localization were monitored and collected.
Statistical analysis
Statistical analyses were performed using SPSS software, Version 25 of the SPSS System (IBM, USA). Descriptive data were presented as mean ± standard deviations (SD), median (interquartile ranges), or percentage. One-way ANOVA was applied for between-group testing of continuous variables. The Chi-square or Fisher’s Exact tests were used for categorical variables.
Multivariable logistic regression models were developed to examine the predictors of moderate to severe pain during deep breathing after hookwire CT-guided needle localization. Acute pain intensity during deep breathing was differentiated as “moderate to severe pain” vs. “mild or no pain” according to the VAS. Factors with P<0.1 in the univariate analysis were evaluated as potential covariates in stepwise multivariable logistic regression analyses with forward selection. Multivariate stepwise logistic regression was performed on all variables retained from the univariate analysis. A post-hoc analysis included a comparison between the identified risk factors in terms of acute pain intensity. A two-sided significance level of 5% (P<0.05) and confidence intervals (CIs) of 95% were used.
Our sample size was determined as follows. The primary outcome of this study was the incidence and risk factors of moderate to severe pain during deep breathing after CT-guided needle localization. However, research was limited regarding the true incidence of pain, thus we conducted a pilot study and found that the incidence of moderate to severe pain at deep breath was 48% (24/50). We planned to include 300 patients and estimated to have about 150 events, which should be more than sufficient for testing 9 predictors under the rule of thumb of 10 events per predictor (20).
Results
A total of 335 patients were screened for inclusion between September 24, 2021 and December 31, 2021. After excluding 35 patients (18 patients had rescheduled their surgery, 9 patients refused to participate, 2 patients had preoperative fever, and 6 patients had canceled preoperative CT-guided localization), 300 patients were included in the final analysis (Figure 2). All patients were followed up according to the study protocol.
The mean age of the patients was 51 (SD =12) years, and 63% were female. GHIATAS beaded breast localization wire was used in 88.7% of the patients and Senscure localization needle was used in 11.3%. Single needle localization was achieved in 76.3% of the patients, with the remaining 23.7% having two or more needle localizations for multiple pulmonary nodules. Needle localization on the specific chest wall occurred in 268 (89%) patients and 32 (11%) patients received needle localization on mixed locations of the chest wall. The mean procedure time for CT-guided hookwire localization was 8 min (range, 5–10 min). The patients’ demographics and localization-related variables are shown in Table 1 and Table 2.
Table 1
| Variables | Mean ± SD or n (%) (N=300) |
|---|---|
| Age (years) | 51±12 |
| Sex | |
| Female | 190 (63.3) |
| Male | 110 (36.7) |
| ASA physical status | |
| I | 186 (62.0) |
| II | 96 (32.0) |
| III | 18 (6.0) |
| Body mass index (kg/m2) | 23.0±3.0 |
| Educational level | |
| Middle school | 100 (33.3) |
| High school or above | 200 (66.7) |
| Smoking status | |
| Current | 16 (5.3) |
| Former | 45 (15.0) |
| Never | 239 (79.7) |
| Alcohol use | |
| Current | 31 (10.3) |
| Former | 17 (5.7) |
| Never | 252 (84.0) |
| Comorbidities | |
| Hypertension | 67 (22.3) |
| Diabetic mellitus | 24 (8.0) |
| Coronary heart disease | 5 (2.0) |
| Arrhythmia | 8 (2.7) |
ASA, American Society of Anesthesiologists.
Table 2
| Variables | N=300 |
|---|---|
| Localization characteristics | |
| Numbers of location needles, n (%) | |
| 1 | 229 (76.3) |
| 2 | 62 (20.7) |
| 3 | 9 (3.0) |
| Types of localization device, n (%) | |
| GHIATAS Beaded Breast Localization Wire | 266 (88.7) |
| Senscure localization needle | 34 (11.3) |
| Direction of thorax, n (%) | |
| Left thorax | 114 (38.0) |
| Right thorax | 181 (60.3) |
| Bilateral thorax | 5 (1.7) |
| Specific location of puncture on chest wall, n (%) | |
| Anterior chest wall | 52 (17.3) |
| Lateral chest wall | 92 (30.7) |
| Posterior chest wall | 124 (41.3) |
| Mixed | 32 (10.7) |
| Distance from skin to needle tip, mm | 63±16 |
| Distance from parietal pleura to needle tip, mm | 23±13 |
| Mean procedure time, min | 8 [5, 10] |
| Pain characteristics | |
| Median VAS of pain at rest, cm | 2 [2, 3] |
| Proportions of pain at rest, n (%) | |
| VAS 0–3 | 240 (79.9) |
| VAS 4–6 | 53 (17.8) |
| VAS 7–10 | 7 (2.3) |
| Median VAS of pain during deep breathing, cm | 4 [2, 5] |
| Proportions of pain during deep breathing, n (%) | |
| VAS 0–3 | 148 (49.2) |
| VAS 4–6 | 123 (41.1) |
| VAS 7–10 | 29 (9.7) |
| Median VAS of pain during movement, cm | 3 [2, 5] |
| Proportions of pain during movement, n (%) | |
| VAS 0–3 | 163 (54.3) |
| VAS 4–6 | 106 (35.3) |
| VAS 7–10 | 31 (10.3) |
| Median VAS among different specific chest wall locations | |
| Anterior chest wall | 3 [2, 4] |
| Lateral chest wall | 4 [3, 5] |
| Posterior chest wall | 3 [2, 5] |
| Rescue medication, n (%) | 16 (5.3) |
| Median of pre-procedural state anxiety | 47 [45, 49] |
Data are presented as mean (standard deviation) or median [inter-quartile range], or n (%). VAS, visual analogue scale.
Prevalence of acute moderate to severe pain after CT-guided needle localization
After CT-guided pulmonary nodule localization, 17.8% of the patients had a VAS between 4 and 6 cm and 2.3% had a VAS between 7 and 10 cm at rest. However, during deep breathing, acute pain was more prevalent, with 41.1% of patients experiencing a VAS between 4 and 6 cm and 9.7% with a VAS between 7 and 10 cm. In total, moderate to severe pain during deep breathing or movement occurred in 50.8% and 45.7% of patients respectively, as shown in Table 2.
Predictors of moderate to severe pain during deep breathing
Univariate analysis showed that age (P=0.068), number of localization needles (P=0.029), and the specific location of the puncture on the chest wall (P=0.024) were potential risk factors related to moderate to severe pain during deep breathing (Table 3).
Table 3
| Variable | Moderate to severe pain during deep breathing after needle localization | ||
|---|---|---|---|
| Absent (n=148) | Present (n=152) | P value | |
| Age, years | 52±12 | 49±12 | 0.068$ |
| Female, n (%) | 88 (59.5) | 102 (67.1) | 0.169 |
| Educational level, n (%) | 0.235 | ||
| High school or above | 104 (70.3) | 97 (63.8) | |
| Middle school | 44 (29.7) | 55 (36.2) | |
| Body mass index (kg/m2) | 23.12±3.01 | 22.82±2.82 | 0.383 |
| Pre-procedural State Anxiety | 47±3 | 47±3 | 0.363 |
| Direction of thorax! | 0.370 | ||
| Left thorax | 60 (41.4) | 55 (36.7) | |
| Right thorax | 85 (58.6) | 95 (63.3) | |
| Numbers of needles | 0.029* | ||
| Single needle location | 121 (81.8) | 108 (71.1) | |
| Multiple needle location | 27 (18.2) | 44 (28.9) | |
| Specific location on chest wall& | 0.024* | ||
| Anterior chest | 35 (24.0) | 28 (19.6) | |
| Lateral chest | 42 (28.8) | 60 (41.9) | |
| Posterior chest | 69 (47.3) | 55 (38.5) | |
| Types of location device, n (%) | 0.265 | ||
| GHIATAS Beaded Breast Localization Wire | 129 (87.2) | 137 (90.1) | |
| Senscure location needle | 19 (12.8) | 15 (9.9) | |
| Systolic blood pressure after localization | 135±19 | 134±24 | 0.483 |
| Diastolic blood pressure after localization | 82±11 | 81±13 | 0.335 |
| Pulse rate after localization | 75±8 | 75±9 | 0.627 |
Data are presented as mean ± standard deviation, median (inter-quartile range), or n (%). Variables when $P<0.1 were included in final multivariate model. *, significant difference between groups (P<0.05). !, patients localized at bilateral thorax are not included in the analysis here. The actual total number of patients are 145 in Absent column and 150 in Present column. &, patients with mixed locations on the chest wall are not included in the analysis. The actual total number of patients are 146 in Absent column and 143 in Present column.
Multivariate logistic regression analysis showed that multiple needle locations (compared to single needle location: OR: 2.363, 95% CI: 1.157–4.825, P=0.018) and the specific location of the needle punctures on the chest wall (lateral chest wall versus anterior chest wall OR: 2.235, 95% CI: 1.106–4.518, P=0.025; posterior chest wall versus anterior chest wall OR: 1.198, 95% CI: 0.611–2.349, P=0.599) were identified as significant predictors of moderate to severe pain after CT-guided needle localization (Table 3). Other factors included in this study (i.e., age, sex, BMI, educational level, preoperative level of anxiety, type of needles, direction of needle puncture, and distance from the skin to the tips of the needles) were not related to moderate to severe pain (P>0.05, Table 4).
Table 4
| Variables | Z | P value | OR | 95% CI |
|---|---|---|---|---|
| Age per year increase | 1.201 | 0.273 | 0.989 | 0.969–1.009 |
| Multiple location needles vs. single location needle | 5.570 | 0.018* | 2.363 | 1.157–4.825 |
| Specific location on the chest wall | ||||
| Lateral vs. anterior | 5.020 | 0.025* | 2.235 | 1.106–4.518 |
| Posterior vs. anterior | 0.277 | 0.599 | 1.198 | 0.611–2.349 |
*, significant difference (P<0.05). OR, odds ratio; CI, confidence interval.
Post-hoc analysis
Non-parametric tests were used to compare the effect of the specific location of needle puncture on acute pain intensity. The results showed that there were significant differences between the three locations of needle puncture on the chest wall (P=0.036). The median VAS (interquartile ranges) was 3 [2–4] cm, 4 [3–5] cm, and 3 [2–5] cm in the anterior chest wall, lateral chest wall, and posterior chest wall, respectively (Table 2).
Association between acute pain intensity during deep breathing and patients’ other outcomes
We did not find that there were any differences in systolic blood pressure, diastolic blood pressure, and pulse rate between patients regardless of whether or not they experienced moderate to severe pain. Furthermore, 5.3% (16/300) of patients were treated with cyclooxygenase-2 (COX-2) inhibitors without perceptible relief.
Discussion
This cross-sectional study was the first to thoroughly investigate acute pain following preoperative localization of pulmonary nodules. About a half of the patients included in this study experienced moderate to severe pain after preoperative needle localization, including 50.8% of patients during deep breathing and 45.7% during movement. We found that multiple punctures and the specific locations of punctures on the chest wall were significantly associated with moderate to severe pain after preoperative needle localization, irrespective of age, gender, BMI, levels of preoperative anxiety, type of needles, direction of needle punctures, or penetration depth.
Initially developed for the localization of breast nodules, hookwire has been widely applied for the localization of pulmonary nodules in China since 2009 (21), and involves passing a puncture needle directly through the lung tissue to anchor the lesion. Previous research has mainly focused on the safety and efficacy of hookwire localization during VATS resection (1,12,19,21). Hookwire localization has a high localization accuracy, short operation time, and few postoperative complications, and is therefore well suited to detect small pulmonary nodules in thoracic centers (22,23). However, this method still has some limitations. Patients may feel substantial pain or discomfort due to the rigid wire traversing the chest wall and remaining in place until surgical resection (24,25), and some patients appear unable to lie still due to severe pain.
There are no studies that thoroughly investigate acute pain after needle localization prior to VATS. Yoshida et al. reported that the incidence of pain after hookwire localization was 7%, but they did not clearly state which method was used (13). Hu et al. compared the efficacy and safety of localization of small pulmonary nodules with microcoil and hookwire (26). Their data found that 24.2% (8/33) of patients in the hookwire group experienced moderate to severe pain at rest (26). We found that 20.1% of patients experienced moderate to severe pain at rest, which was consistent with Hu et al.’s findings. In our study, pain was assessed at different statuses including at rest, during deep breathing, or movement. Patients reported the pain as localized, sharp, and constant at rest, which was greatly aggravated by deep breathing or movements such as turning in bed, sitting or changing directions in our study. Possible sources of nociceptive input of preoperative needle localization remained unclear and might be multifactorial. We assumed that this mechanism is related to needle puncture of the skin or muscles, intercostal nerve injury, or irritation and inflammation of the lung parenchyma or pleura. As mentioned above, hookwire localization anchored the small pulmonary nodules by inducing the rigid wire from the skin to the lung parenchyma, and then keeping the rigid wire in place until surgical resection. Although the needle puncture was performed under local infiltration with 2% lidocaine, the rigid wire may also damage muscles, cause lesion to the intercostal nerve, or provoke visceral pain of the lung parenchyma, ultimately leading to moderate to severe pain.
We found that two specific factors were associated with the increased incidence of moderate to severe pain during deep breathing: additional numbers of penetration needles and the penetration location on the chest wall. Tian et al. reported that multiple punctures increased the incidence of pneumothorax and intrapulmonary hemorrhage compared with single puncture during CT-guided microcoil localization of pulmonary nodules (27), but they did not analyze differences in pain. Our data indicates that compared to a single puncture, multiple punctures with more than one needle may increase to increase the risk of moderate to severe pain by two-fold. Multiple punctures with more than one needle may increase the risk of damage to the muscles, intercostal nerves, bony structure, and pulmonary parenchyma or pleura. We speculate that this was similar to the fact that a single chest tube was associated with less pain without increasing the risk of recurrent effusion compared to the routine two chest drainage tubes (28). Interestingly, the specific location of needle punctures on the chest wall contributed to a substantial proportion of acute pain. Previous reviews have reported that the intercostal nerves aroused from the ventral rami of the thoracic spinal nerves and were mixed with both sensory and motor fibers. The collateral branch of the intercostal nerves provides sensory innervations to the pleura, peritoneum, partial anterior, and lateral chest walls (29). Damage or irritation to the intercostal nerves may affect thoracic pain. In our results, the lateral chest seemed to be the most vulnerable region of pain, and was statistically significant compared to the anterior-lateral area. This might result from increased likelihood of intercostal nerve injury. Similarly, some evidence has demonstrated that the incidence of long-term pain after sternotomy or anterior thoracotomy was less than that after lateral thoracotomy (30). In terms of specific neurophysiological studies on the role of nerve damage during persistent pain in thoracotomy, Benedetti et al. found evidence of nerve damage in both acute and chronic pain following posterolateral thoracotomy (31). Anterior thoracotomy was less likely to cause nerve dysfunction compared with the posterolateral approach (32).
Some studies have explored new devices that could impact soft tissue injury and therefore mitigate the pain response. A semi-rigid hookwire was reported to have an improved ease of use compared to a double-thorn hookwire or microcoil, representing a promising therapeutic direction (32). A multicenter, prospective study of a novel technique for pulmonary nodule localization found that Senscure needles had a high success rate, feasibility, and good tolerance in all patients, without significant pain (14). Contrary to Fan et al.’s findings (14), we did not observe that Senscure needles were associated with less pain. A possible explanation for this discrepancy may be that only 11.3% of patients underwent localization using Senscure location needles, as compared to 88.7% of patients who received GHIATAS. The higher cost of Senscure’s needle may restrict its application in clinical practice.
Our cross-sectional study has some strengths to explore risk factors of acute pain after CT-guided localization of pulmonary nodules. However, limitations still exist. Firstly, this was a single-center observational study and undoubtedly had inherent limitations. Secondly, we did not include the effect of procedure proficiency on acute pain. In our center, CT-guided localization was primarily conducted by the radiologist team in which each radiologist performed 1,000 consecutive cases every year. This substantial procedural experience allowed radiologists to achieve very favorable localization outcomes. Thirdly, we assessed acute pain at 10–15 minutes after needle localization when the initial pain was stable. However, the pain intensity may change as time passed. Finally, whether acute pain was associated with complications due to CT-guided localization (pneumothorax or hemorrhage) was uncovered in our study and should be addressed in the future.
In summary, moderate to severe acute pain during deep breathing or movement after preoperative localization of pulmonary nodules is very common. The number of localization needles and the specific location of punctures on the chest wall were identified to be significant predictors of moderate to severe pain. As with all observational analyses, causality cannot be assumed, and more trials are needed to further verify our results. Awareness of the potential risk factors for moderate to severe acute pain might reduce the incidence after CT-guided localization and allow for the implementation of preventative strategies accordingly.
Acknowledgments
The authors would like to thank Lingming Yu from the Department of Radiology at Shanghai Chest Hospital for his assistance. The authors also appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: This work was supported by the Shanghai Municipal Commission of Health (No. 202040200, to Yuwei Qiu) and the Shanghai Shen Kang Hospital Development Center Project (No. SHDC2020CR4063).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-557/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-557/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-557/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shanghai Chest Hospital (IRB #KS (P) 2142) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang Y, Fu F, Chen H. Management of Ground-Glass Opacities in the Lung Cancer Spectrum. Ann Thorac Surg 2020;110:1796-804. [Crossref] [PubMed]
- Migliore M, Fornito M, Palazzolo M, et al. Ground glass opacities management in the lung cancer screening era. Ann Transl Med 2018;6:90. [Crossref] [PubMed]
- Kobayashi Y, Ambrogio C, Mitsudomi T. Ground-glass nodules of the lung in never-smokers and smokers: clinical and genetic insights. Transl Lung Cancer Res 2018;7:487-97. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Li C, Liu B, Jia H, et al. Computed tomography-guided hook wire localization facilitates video-assisted thoracoscopic surgery of pulmonary ground-glass nodules. Thorac Cancer 2018;9:1145-50. [Crossref] [PubMed]
- Imperatori A, Nardecchia E, Cattoni M, et al. Perioperative identifications of non-palpable pulmonary nodules: a narrative review. J Thorac Dis 2021;13:2524-31. [Crossref] [PubMed]
- Xu Y, Ma L, Sun H, et al. CT-guided microcoil localization for pulmonary nodules before VATS: a retrospective evaluation of risk factors for pleural marking failure. Eur Radiol 2020;30:5674-83. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Klinkenberg TJ, Dinjens L, Wolf RFE, et al. CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol 2017;115:898-904. [Crossref] [PubMed]
- Kleedehn M, Kim DH, Lee FT, et al. Preoperative Pulmonary Nodule Localization: A Comparison of Methylene Blue and Hookwire Techniques. AJR Am J Roentgenol 2016;207:1334-9. [Crossref] [PubMed]
- Yao F, Wang J, Yao J, et al. Reevaluation of the efficacy of preoperative computed tomography-guided hook wire localization: A retrospective analysis. Int J Surg 2018;51:24-30. [Crossref] [PubMed]
- Hanauer M, Perentes JY, Krueger T, et al. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: report of 181 patients. J Cardiothorac Surg 2016;11:5. [Crossref] [PubMed]
- Yoshida Y, Inoh S, Murakawa T, et al. Preoperative localization of small peripheral pulmonary nodules by percutaneous marking under computed tomography guidance. Interact Cardiovasc Thorac Surg 2011;13:25-8. [Crossref] [PubMed]
- Fan L, Yang H, Yu L, et al. Multicenter, prospective, observational study of a novel technique for preoperative pulmonary nodule localization. J Thorac Cardiovasc Surg 2020;160:532-539.e2. [Crossref] [PubMed]
- Yang F, Zhao H, Sui X, et al. Comparative study on preoperative localization techniques using microcoil and hookwire by propensity score matching. Thorac Cancer 2020;11:1386-95. [Crossref] [PubMed]
- Qiu Y, Wu J, Huang Q, et al. Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: A noninferiority randomised trial. Eur J Anaesthesiol 2021;38:S97-S105. [Crossref] [PubMed]
- Zhou Z, Wang Y, Niu Y, et al. How we assess the perioperative anxiety of surgical patients with pulmonary nodules: the revision of state-trait anxiety inventory. J Cardiothorac Surg 2020;15:324. [Crossref] [PubMed]
- Shek DT. The Chinese version of the State-Trait Anxiety Inventory: its relationship to different measures of psychological well-being. J Clin Psychol 1993;49:349-58. [Crossref] [PubMed]
- Hah JM, Cramer E, Hilmoe H, et al. Factors Associated With Acute Pain Estimation, Postoperative Pain Resolution, Opioid Cessation, and Recovery: Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open 2019;2:e190168. [Crossref] [PubMed]
- Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373-9. [Crossref] [PubMed]
- Zhou JH, Li WT, Chen HQ, et al. CT-guided hookwire localization of small solitary pulmonary nodules in video-assisted thoracoscopic surgery. Zhonghua Zhong Liu Za Zhi 2009;31:546-9. [PubMed]
- Pittet O, Christodoulou M, Pezzetta E, et al. Video-assisted thoracoscopic resection of a small pulmonary nodule after computed tomography-guided localization with a hook-wire system. Experience in 45 consecutive patients. World J Surg 2007;31:575-8. [Crossref] [PubMed]
- Zhang B, Zhang Y, Le H, et al. Intraoperative localization in minimally invasive surgery for small pulmonary nodules: a retrospective study. Transl Cancer Res 2021;10:3470-8. [Crossref] [PubMed]
- Shah RM, Spirn PW, Salazar AM, et al. Localization of peripheral pulmonary nodules for thoracoscopic excision: value of CT-guided wire placement. AJR Am J Roentgenol 1993;161:279-83. [Crossref] [PubMed]
- Wang J, Yao J, Xu L, et al. Comparison of cyanoacrylate and hookwire for localizing small pulmonary nodules: A propensity-matched cohort study. Int J Surg 2019;71:49-55. [Crossref] [PubMed]
- Hu L, Gao J, Chen C, et al. Comparison between the application of microcoil and hookwire for localizing pulmonary nodules. Eur Radiol 2019;29:4036-43. [Crossref] [PubMed]
- Tian Y, An J, Zou Z, et al. Computed Tomography-Guided Microcoil Localization of Pulmonary Nodules: Effects of Multiple Punctures. Thorac Cardiovasc Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Batchelor TJP, Ljungqvist O. A surgical perspective of ERAS guidelines in thoracic surgery. Curr Opin Anaesthesiol 2019;32:17-22. [Crossref] [PubMed]
- Alzahrani T. Pain relief following thoracic surgical procedures: A literature review of the uncommon techniques. Saudi J Anaesth 2017;11:327-31. [Crossref] [PubMed]
- Kalso E, Mennander S, Tasmuth T, et al. Chronic post-sternotomy pain. Acta Anaesthesiol Scand 2001;45:935-9. [Crossref] [PubMed]
- Benedetti F, Amanzio M, Casadio C, et al. Postoperative pain and superficial abdominal reflexes after posterolateral thoracotomy. Ann Thorac Surg 1997;64:207-10. [Crossref] [PubMed]
- Benedetti F, Vighetti S, Ricco C, et al. Neurophysiologic assessment of nerve impairment in posterolateral and muscle-sparing thoracotomy. J Thorac Cardiovasc Surg 1998;115:841-7. [Crossref] [PubMed]



