
Comparison of operation time, efficacy and safety between through-the-scope stent and over-the-while stent in malignant central airway obstruction: a multi-center randomized control trial
Introduction
Malignant central airway obstruction (CAO) is common in advanced-stage lung cancers and pulmonary metastatic carcinomas (1,2). Patients suffering from these diseases are often complicated by post-obstructive pneumonia, severe dyspnea, and poor prognosis. Interventional bronchoscopic procedures might be suitable for CAO patients owing to their immediate alleviation of airway stenosis (3,4).
Airway stent implantation is an effective interventional bronchoscopic procedure for the treatment of CAO or airway stenosis (5-7). At present, self-expandable metallic (SEM) airway stents, such as over-the-while (OTW) stents of Chinese Nanjing micro-tech Company and Ultraflex stents from USA Boston Scientific Company, are implanted under the guidance of a guide-wire. These stents should be implanted under flouroscopy, which is time-consuming and requires unnecessary exposure to radiation (8-12) or direct bronchoscope visualization.
In a previous pilot study, we established the feasibility of a novel SEM through-the-scope (TTS) stent delivery system in CAO patients (13), which can pass through the working channel of a flexible bronchoscope. Furthermore, it can also be directly implanted into airway stenosis via flexible bronchoscopy, which does not require a guide-wire and avoids X-ray exposure. These changes might reduce the stent implantation operation time, increase the accuracy of implantation, and reduce the risk of suffocation. However, there is paucity of data regarding TTS stent placement. As a kind of novel SEM airway stent, there were only 3 studies reported the application of TTS stent in malignant CAO patients. All these studies were case series reports and the total numbers were limited (no more than 40 patients). More data about TTS stent efficacy and safety might provide further evidence for clinical application. Meanwhile, there was no comparison of data between TTS stent and common OTW stent. This prospective multi-center randomized controlled trial, we evaluated the efficacy and safety of the novel TTS stent system. We present the following article in accordance with the CONSORT reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-565/rc).
Methods
Patients
This prospective six-center study was conducted in the Respiratory Diseases Departments of the following institutions: the First Affiliated Hospital of Soochow University (Suzhou, 01), the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, 02), the Affiliated Hospital of Qingdao University (Qingdao, 03), Jiangsu Province Hospital (Nanjing, 04), Xiamen Second People’s Hospital (Xiamen, 05), Nantong First People’s Hospital (Nantong, 06). This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Soochow University (No. 2017063). The Research Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University, the Research Ethics Committee of the Affiliated Hospital of Qingdao University, the Research Ethics Committee of Jiangsu Province Hospital, the Research Ethics Committee of Xiamen Second People’s Hospital and the Research Ethics Committee of Nantong First People’s Hospital were informed and agreed with the study. Informed consent was obtained from each patient. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Male and non-pregnant female patients aged 18 to 75 years old who were diagnosed with unresectable malignant tumors and CAO were eligible for inclusion. CAO was defined as airway occlusion >50% at the trachea, right or left main bronchus, or right intermediate bronchus, as determined by computed tomography (CT) scan or bronchoscopy examination. The exclusion criteria were as follows: (I) patients that did not require stent implantation; (II) those with two or more sites of airway occlusion requiring stent implantation; (III) patients with lesions near the tracheal carina (less than 2 cm); (IV) those with a normal airway diameter >20 mm; (V) patients from which informed consent could not be obtained.
Trial design
This open-label, individually randomized controlled trial was conducted from May 15, 2017, to December 30, 2018 (the date of enrollment of the last patient) at six sub-centers in China. Eligible patients were randomly assigned (2:1 ratio) to receive implantation of the TTS stent or OTW stent, in addition to standard treatment. The permuted block randomisation sequence, including stratification, was prepared by a statistician not involved in the trial using SAS software, version 9.4. The sample size (144 subjects) was assessed and calculated by a statistician before the trial. The standard treatment was determined by the attending physician based on the patients’ disease conditions, including supplemental oxygen, chemotherapy, radiotherapy, or molecular targeted therapy. The subjects underwent blood gas analysis, chest CT scan, and bronchoscopy before and after the stent implantation, and were routinely followed by bronchoscopy 1 week after stent implantation.
Data collection
Before and after the stent implantation, the baseline characteristic data of all patients were collected, including dyspnea score, anaesthesia methods, and airway stenosis grade. The airway stenosis rate was calculated by the ratio of stenosis airway diameter and normal airway diameter according to a CT scan. Dyspnea was measured using the modified British medical research council (mMRC) score. Karnofsky (KPS) score and arterial blood gas exchange analysis, including partial pressure of oxygen (PaO2) and partial pressure of carbon dioxide (PaCO2), were all collected. The entire process of all surgical procedures was recorded by video. Complications relating to stent implantation were also collected until 7 days after stent implantation, including bleeding, stent displacement, fracture, and airway restenosis.
Outcome measures
The primary outcome measure was the operative time for the implantation procedure of an airway stent into the airway stenosis. The secondary outcome measures were the efficacy of the stent implantation and the successful rate of the stent implantation. Successful stent implantation was defined as a stent that fully covered the airway stenosis, with the upper and lower ends exceeding the lesion by more than 0.5 cm. Moreover, successful stent implantation was represented by an improvement of the airway stenosis to less than 50% of the homologous normal airway as well as alleviation of the dyspnea symptoms. The efficacy of stent implantation was defined as the improvement of both dyspnea index score and airway stenosis ratio (less than 50% after stent implantation).
The stent implantation procedure time (operative time) was calculated as the time from the insertion of the bronchoscope into the airway to removal of the bronchoscope from the airway and was measured by at least three different physicians using the video recording. The time-point of the insertion of the bronchoscope into the airway was defined as insertion of the bronchoscope into the glottis (in patients with local anesthesia) or artificial airway (in patients with local anesthesia using a laryngeal mask or endotracheal intubation). The time-point of removal of the bronchoscope from the airway was defined as removal of the bronchoscope from the glottis (in patients with local anesthesia) or artificial airway (in patients with local anesthesia using a laryngeal mask or endotracheal intubation) following successful stent implantation.
Statistical analysis
SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA) was utilized for statistical analysis. Primary efficacy analysis was conducted on an intention-to-treat (ITT) basis and included all patients who had undergone randomization. The time to clinical improvement was assessed after all patients had reached day 7. Multiple imputation will be used for missing data. The primary analysis will use the ITT principle and a per protocol analysis will be undertaken to assess the robustness of the findings.
The t-test/calibration or t-test/Wilcoxon rank-sum test was used to compare quantitative data such as age at baseline. The chi-square test/exact probability method was used to compare qualitative data. The Wilcoxon rank-sum test or Cochran-Mantel-Haenszel (CMH) chi-square test was used for ordered classification data. The main efficacy indicator was the operative time of airway stent implantation. The operative times of different research groups were compared, and the 95% confidence intervals (CIs) were calculated using the group t-test or t-test for comparison between groups. The Chi-square test was used to compare the clinical effectiveness of the airway stent and the success rate of the release stent. P<0.05 was considered as significant.
Results
Patients
The trial was conducted from May 15, 2017 to December 30, 2018 at six clinical sites. A total of 148 subjects were enrolled in this study. Of these, 14 subjects were excluded and 134 subjects were included in the ITT analysis, which accounted for 90.54% of the total number of subjects (Figure 1).
There were no significant between-group differences in the demographic characteristics at enrollment, including age, underlying diseases, malignant diseases, driver gene condition, and CAO sites (Table 1). The most common tumor was non-small cell lung cancer (47.31% in the TTS group and 63.04% in the OTW group). Esophageal cancer accounted for about 20% of malignant diseases resulting in CAO. Most patients had no cancer driver genes. The most common CAO sites were the trachea (46.24% in the TTS group and 51.11% in the OTW group) and the right main bronchus (29.67% in the TTS group and 35.56% in the OTW group).
Table 1
| Characteristics | TTS group (n=91) | OTW group (n=43) | t/χ2/Z | P |
|---|---|---|---|---|
| Male/female | 68/23 | 32/11 | χ2=0.0013 | 0.972 |
| Age (years), mean ± SD | 62.76±9.82 | 65.07±9.60 | t=−1.3105 | 0.192 |
| Underlying diseases, n (%) | 0.660 | |||
| Cardiovascular diseases | 24 (26.37) | 14 (32.56) | ||
| Cerebrovascular diseases | 0 (0.00) | 2 (4.56) | ||
| Diabetes | 9 (9.89) | 4 (9.30) | ||
| Chronic lung diseases | 5 (5.49) | 4 (9.30) | ||
| Malignant diseases, n (%) | 0.427 | |||
| NSCLC | 43 (47.25) | 27 (62.79) | ||
| SCLC | 7 (7.69) | 1 (2.33) | ||
| Thyroid cancer | 4 (4.40) | 4 (9.30) | ||
| Esophageal cancer | 20 (21.98) | 8 (18.60) | ||
| Others cancers | 17 (18.68) | 3 (6.98) | ||
| EGFR/ALK (lung cancer), n | Z=−1.097 | 0.280 | ||
| Yes | 4 | 1 | ||
| No | 50 | 31 | ||
| Unknown | 37 | 13 | ||
| CAO sites, n (%) | Z=0.5630 | 0.573 | ||
| Trachea | 43 (46.24) | 23 (50.00) | ||
| Left main bronchus | 23 (24.73) | 7 (15.22) | ||
| Right main bronchus | 27 (26.01) | 16 (34.78) | ||
| KPS, mean ± SD | 65.45±19.48 | 65.71±16.60 | t=−0.0540 | 0.957 |
| Airway obstruction rate (%), mean ± SD | 81.77±14.54 | 81.14±16.72 | t=0.2224 | 0.824 |
| Stenosis patterns, n (%) | ||||
| Endoluminal | 19 (20.88) | 10 (23.26) | χ2=0.097 | 0.755 |
| Extrinsic | 18 (19.78) | 7 (16.28) | χ2=0.236 | 0.627 |
| Mixed | 54 (59.34) | 26 (60.47) | χ2=0.015 | 0.901 |
| Dyspnea index score, mean ± SD | 2.98±1.26 | 2.84±1.04 | Z=−0.928 | 0.355 |
| Arterial blood gas, mean ± SD | ||||
| pH | 7.42±0.04 | 7.43±0.04 | t=−0.4730 | 0.637 |
| PaO2 | 84.48±39.64 | 95.75±57.38 | t=−0.9712 | 0.337 |
| PaCO2 | 40.53±7.56 | 39.17±5.33 | t=0.9966 | 0.322 |
| Anesthesia, n (%) | 0.245 | |||
| Local anesthesia | 45(49.45) | 25(58.14) | ||
| Tracheal cannula | 2 (2.20) | 0 (0.00) | ||
| Laryngeal mask | 23 (25.27) | 14 (32.56) | ||
| Hard lens | 21 (23.08) | 4 (9.30) |
CAO, central airway obstruction; TTS, through-the-scope; OTW, over-the-while; SD, standard deviation; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; KPS, Karnofsky; PaO2, partial pressure of oxygen; PaCO2, partial pressure of carbon dioxide.
The airway obstruction rate in the TTS group was similar to that in the OTW group (81.77%±14.54% vs. 81.14%±16.72%, P=0.824). No difference was observed in the dyspnea index scores (2.98±1.26 vs. 2.84±1.04, P=0.532), KPS (65.45±19.48 vs. 65.71±16.60, P=0.957), and arterial blood gas results (including pH, PaO2, and PaCO2, Table 2) between the two groups. There were no important between-group differences in the anesthesia methods during the stent implantation procedures. Local anesthesia (50.55% in the TTS group and 55.56% in the OTW group) and laryngeal mask (25.27% in the TTS group and 31.11% in the OTW group) were used in most patients.
Table 2
| Outcome measures | TTS group (n=91) | OTW group (n=43) | t/χ2/Z | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | ||||
| Airway stenosis rate (%), mean ± SD | 81.77±14.54 | 32.56±5.38 | 0.000 | 81.14±16.72 | 34.25±5.65 | 0.000 | |||
| Dyspnea index score, mean ± SD | 2.98±1.26 | 1.17±0.76 | 0.000 | 2.84±1.04 | 1.21±0.71 | 0.000 | |||
| Efficacy of stent implantation (%) | 89 (97.80) | 39 (90.70) | 0.093 | ||||||
| Success rate (%) | 91 (100.00) | 42 (97.67) | 0.323 | ||||||
| Rate of first-time success (%) | 71 (78.02) | 32 (74.42) | χ2=0.1837 | 0.668 | |||||
| Stent implantation times (min) | 1.45 (0.87–2.20) | 4.05 (3.33–5.00) | t=−8.2744 | 0.000 | |||||
CAO, central airway obstruction; TTS, through-the-scope; OTW, over-the-while; SD, standard deviation.
Primary outcome and secondary outcome
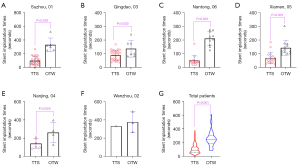
Table 2 and Figures 2,3 display the primary outcome and secondary outcomes. The time of stent implantation in the TTS group was 87 (52–132) seconds, which was significantly shorter than that in the OTW group [243 (200–300) seconds, P<0.001]. The shortest stent implantation times in the TTS and OTW groups were 28 seconds and 78 seconds, while the longest times were 375 and 610 seconds in these two groups, respectively.

As shown in Table 2 and Figure 2, the TTS stents significantly improved the dyspnea index scores (1.17±0.76 vs. 2.98±1.26) and relieved the airway obstruction rates (32.56%±5.38% vs. 81.77%±14.54%) of CAO patients. These results were similar to those in the OTW stents group. In the TTS group, two patients failed to show an airway obstruction improvement to less than 50%, while four patients failed this in the OTW group. Although the efficacy of stent implantation for patients in the TTS group was marginally higher than that in the OTW group (97.80% vs. 90.70%, P=0.093), the difference was not statistically significant.
In the TTS group, 70 stents were implanted successfully the first time, whereas 20 stents required adjustment 1 to 2 times after the first implantation. In the OTW group, 32 stents were implanted successfully the first time, 10 stents required adjustment 1 to 2 times after the first implantation, and one stent failed to implant. However, no differences were observed in the rates of success (100.00% vs. 97.67%, P=0.323) or first-time success (78.02% vs. 74.42%, P=0.668) between the TTS and OTW groups.
Safety
All complications are shown in Table 3. The most common complications after stent implantation were secretion retention, granulation, and hemoptysis. Secretion retention occurred in 98.8% and 97.67% of patients in the TTS and OTW groups, respectively, with no significant differences. Although hemoptysis occurred in 26.37% and 30.23% of patients in the TTS and OTW groups, it was mild (less than 50 mL/24 h) and occurred during or after the implantation procedure. Only one patient in the TTS group and one patient in the OTW experienced moderate hemoptysis (50–100 mL/24 h), which improved after treatment with drugs (sodium kalosulfonate).
Table 3
| Complications | TTS group (n=91), n (%) | OTW group (n=43), n (%) | Z | P |
|---|---|---|---|---|
| Secretion retention | 90 (98.80) | 42 (97.67) | −0.545 | 0.586 |
| Granulation | 26 (28.57) | 18 (41.86) | −1.523 | 0.128 |
| Haemorrhage | 24(26.37) | 13 (30.23) | −0.465 | 0.642 |
| Local infection | 3 (3.30) | 4 (9.30) | −1.453 | 0.210 |
| Displacement | 1 (1.10) | 0 (0.00) | −0.687 | 1.000 |
| Restenosis | 14 (15.38) | 13 (30.23) | −1.993 | 0.064 |
TTS, through-the-scope; OTW, over-the-while.
The incidence of restenosis in the TTS group (15.38%) was lower than that in the OTW group (30.23%), with a statistical trend (P=0.064). Although there were no significant differences, this might indicate some clinical changes. As for the complication of granulation, similar conditions were observed. The rate of granulation in the TTS group was lower than that in the OTW group (28.57% vs. 41.86%, P=0.128); although this difference was not statistically significant, there were some clinical variations. One patient in the TTS group suffered pneumothorax and recovered after closed thoracic drainage.
Discussion
In this randomized controlled trial, we confirmed that the novel TTS stent effectively relieved airway stenosis in CAO patients. The TTS stent implantation operation time was significantly lower than that of the OTW stent. Moreover, no additional complications were observed with the TTS stent as compared with the OTW stents. Our findings demonstrated the efficacy and safety of TTS stents in the treatment of malignant CAO patients. Although TTS stent implantation only reduced the operative time by 2.6 min, this represents an important clinical implication in CAO patients; for patients with more than 50% airway obstruction, the saved time of stent implantation can significantly reduce the risk of hypoxia and asphyxia.
There are multiple approaches to improve airway stenosis in malignant CAO patients, including mechanical debulking, cryotherapy, thermal therapy, and airway stent implantation (14-16). The metallic airway stent is a crucial treatment method for malignant airway stenosis, which is part of multimodal approach in malignant CAO patients (17-19). However, there are some limitations in the implantation procedures of previous stents, which should be implanted under X-ray or rigid/flexible bronchoscope visualization. This is time-consuming and requires unnecessary radiation exposure and higher technical conditions (16-19). Our previous pilot study demonstrated the preliminary application of a novel metallic airway stent in CAO patients (13), which could be inserted into the scope of a flexible bronchoscope and be implanted under direct visualization. This study confirmed that the TTS stent provided a similar efficacy and required less operative time as compared with the OTW stents. These results confirmed our previous reports and reduced the procedure risk in CAO patients, especially in patients with severe stenosis or worse KPS scores. Moreover, TTS stent implantation can be performed directly using a flexible bronchoscope and does not require a guide-wire, rigid bronchoscope, or X-ray. It provides a simple and easy method for stent implantation in malignant CAO patients.
Stent-related complications were the most important limitations of TTS implantation in CAO patients (20-25). The complications of this TTS stent have been reported in a previous pilot study by our team (13). In this RCT, we re-evaluated the stent complications in a larger cohort. Secretion retention and granulation remain the most common complications after stent implantation, haemorrhage and restenosis were also common. The incidences of all complications were similar between the TTS and OTW groups. Although the incidences of granulation and restenosis in the TTS group were lower than those in the OTW group (28.39% vs. 41.86%, 15.79% vs. 29.79%), the differences were not statistically significant. Our results demonstrated that there were no additional complications after TTS stent implantation as compared to the OTW stent; however, it is unclear whether the incidences of long-term complications are similar.
Compared with our previous pilot study, the incidence of stent-related complications was significantly higher, which might be attributable to several factors. Firstly, the sites and severities of the airway stenoses differed between the two studies. The present study enrolled more patients with stenosis in the trachea or left main bronchus. Furthermore, the stenosis grade in the present study was also significantly higher than that in the previous report (81.77% vs. 68.7%). Secondly, the characteristics of the enrolled patients, including their underlying and malignant diseases, varied between the two studies. Finally, patients included in the present study were enrolled randomly from six hospitals, as compared to the previous retrospective single center study.
The rigid bronchoscope takes more convenience for the interventional bronchoscopy treatment. However, rigid bronchoscope is not common in PR China, because of its cost and lack of anesthesiologist. Electronic bronchoscope and fiberoptic bronchoscope are more common in China. There is part of patients (25 cases) received rigid bronchoscope in a sub-center (site 05). Silicone stents are suitable for malignant and benign CAO. However, the advert effect of migration significantly limited the application of silicone stents. Moreover, implantation of silicone stents requires rigid bronchoscopy. The cost of silicone stents is 3 to 4 times of metallic stents in PR China. These causes lead to the common of metallic stents in MCAO patients in our country. Although metallic airway stents seem take more advert effects than silicone stents especially in benign airway stenosis, the implantation of metallic stents could rapidly improve the dyspnea of MCAO patients. Meanwhile, the airway obstruction rate of MCAO patients might be serious in our country. In this study, the medium airway obstruction rate is more than 81%, which indicated more serious disease. In these conditions, metallic airway stents might be a better choice, as metallic stents were more available than silicone stents in our country.
There are some limitations in this study that should be noted. First, there were no long-term follow-up results. This drawback is attributable to the design of the trial. The primary endpoint was the stent implantation operative time and did not include a long-term investigation after stent implantation. In future research, we will evaluate the long-term efficacy and complications of the TTS stent in malignant CAO patients. Second, this study only enrolled patients with one site of airway stenosis. Some patients with two or more sites of airway stenosis were excluded. These subjects might require Y-shaped stents or more than one stent, which significantly increases the risks and complexity involved in implantation. Therefore, we have designed another real-world study to observe the efficacy and safety of the TTS stent in CAO patients with one or more sites of airway stenosis with long-term follow-up.
In conclusion, this study confirmed the efficacy of the TTS stent in malignant CAO patients. The operative time of TTS stent implantation is significantly shorter than that of the OTW airway stent, which might reduce the risk and flexibility of stent implantation. Also, there were no additional short-term stent-related complications. This novel TTS airway stent might be a substitute for malignant CAO patients. However, the long-term safety still needs to be investigated in a large-scale real-world study.
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: This study was funded by the Jiangsu Province Special Program of Medical Science (No. BE2016672) and the Suzhou Science and Technology Planning Project (Nos. SLT201917, SKY2021026).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-565/rc
Trial Protocol: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-565/tp
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-565/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-565/coif). HD received Royalties on the Dutau Novatech rigid bronchoscope and is the consultant for Novatech. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Research Ethics Committee of the First Affiliated Hospital of Soochow University (No. 2017063). The Research Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University, the Research Ethics Committee of the Affiliated Hospital of Qingdao University, the Research Ethics Committee of Jiangsu Province Hospital, the Research Ethics Committee of Xiamen Second People’s Hospital and the Research Ethics Committee of Nantong First People’s Hospital were informed and agreed with the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chaddha U, Hogarth DK, Murgu S. Bronchoscopic Ablative Therapies for Malignant Central Airway Obstruction and Peripheral Lung Tumors. Ann Am Thorac Soc 2019;16:1220-9. [Crossref] [PubMed]
- Murgu SD, Egressy K, Laxmanan B, et al. Central Airway Obstruction: Benign Strictures, Tracheobronchomalacia, and Malignancy-related Obstruction. Chest 2016;150:426-41. [Crossref] [PubMed]
- Jiang M, Xu H, Yu D, et al. Risk-score model to predict prognosis of malignant airway obstruction after interventional bronchoscopy. Transl Lung Cancer Res 2021;10:3173-90. [Crossref] [PubMed]
- Ratwani AP, Davis A, Maldonado F. Current practices in the management of central airway obstruction. Curr Opin Pulm Med 2022;28:45-51. [Crossref] [PubMed]
- Chen Y, Zhou ZQ, Feng JX, et al. Hybrid stenting with silicone Y stents and metallic stents in the management of severe malignant airway stenosis and fistulas. Transl Lung Cancer Res 2021;10:2218-28. [Crossref] [PubMed]
- Rosell A, Stratakos G. Therapeutic bronchoscopy for central airway diseases. Eur Respir Rev 2020;29:190178. [Crossref] [PubMed]
- Marchioni A, Andrisani D, Tonelli R, et al. Integrated intErventional bronchoscopy in the treatment of locally adVanced non-small lung cancER with central Malignant airway Obstructions: a multicentric REtrospective study (EVERMORE). Lung Cancer 2020;148:40-7. [Crossref] [PubMed]
- Guibert N, Saka H, Dutau H. Airway stenting: Technological advancements and its role in interventional pulmonology. Respirology 2020;25:953-62. [Crossref] [PubMed]
- Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71. [Crossref] [PubMed]
- Dutau H, Di Palma F, Thibout Y, et al. Impact of Silicone Stent Placement in Symptomatic Airway Obstruction due to Non-Small Cell Lung Cancer - A French Multicenter Randomized Controlled Study: The SPOC Trial. Respiration 2020;99:344-52. [Crossref] [PubMed]
- Ortiz-Comino RM, Morales A, López-Lisbona R, et al. Silicone Stent Versus Fully Covered Metallic Stent in Malignant Central Airway Stenosis. Ann Thorac Surg 2021;111:283-9. [Crossref] [PubMed]
- Herth FJ, Eberhardt R. Airway stent: what is new and what should be discarded. Curr Opin Pulm Med 2016;22:252-6. [Crossref] [PubMed]
- Jiang JH, Zeng DX, Wang CG, et al. A Pilot Study of a Novel through-the-Scope Self-Expandable Metallic Airway Stents Delivery System in Malignant Central Airway Obstruction. Can Respir J 2019;2019:7828526. [Crossref] [PubMed]
- Wilson GE, Walshaw MJ, Hind CR. Treatment of large airway obstruction in lung cancer using expandable metal stents inserted under direct vision via the fibreoptic bronchoscope. Thorax 1996;51:248-52. [Crossref] [PubMed]
- Mahmood K, Wahidi MM, Thomas S, et al. Therapeutic bronchoscopy improves spirometry, quality of life, and survival in central airway obstruction. Respiration 2015;89:404-13. [Crossref] [PubMed]
- Razi SS, Lebovics RS, Schwartz G, et al. Timely airway stenting improves survival in patients with malignant central airway obstruction. Ann Thorac Surg 2010;90:1088-93. [Crossref] [PubMed]
- Usuda K, Iwai S, Yamagata A, et al. Clinical outcomes and survival following placement of self-expandable metallic stents for central airway stenosis and fistula. Thorac Cancer 2021;12:48-56. [Crossref] [PubMed]
- Chin CS, Litle V, Yun J, et al. Airway stents. Ann Thorac Surg 2008;85:S792-6. [Crossref] [PubMed]
- Husain SA, Finch D, Ahmed M, et al. Long-term follow-up of ultraflex metallic stents in benign and malignant central airway obstruction. Ann Thorac Surg 2007;83:1251-6. [Crossref] [PubMed]
- Emam W, Mostafa Y, Madkour A, et al. Bronchoscopic management as an alternative treatment in non-operable benign tracheal stenosis. Int J Clin Pract 2021;75:e14058. [Crossref] [PubMed]
- Ma KC, Li M, Haas AR, et al. Efficacy and safety of airway stenting to treat anastomotic complications after lung transplant: a cohort study. J Thorac Dis 2020;12:3539-48. [Crossref] [PubMed]
- Xiong XF, Xu L, Fan LL, et al. Long-term follow-up of self-expandable metallic stents in benign tracheobronchial stenosis: a retrospective study. BMC Pulm Med 2019;19:33. [Crossref] [PubMed]
- Fortin M, Lacasse Y, Elharrar X, et al. Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis. Respiration 2017;93:430-5. [Crossref] [PubMed]
- Casal RF. Update in airway stents. Curr Opin Pulm Med 2010;16:321-8. [Crossref] [PubMed]
- Murgu SD, Colt HG. Complications of silicone stent insertion in patients with expiratory central airway collapse. Ann Thorac Surg 2007;84:1870-7. [Crossref] [PubMed]



