
Adjuvant treatment for patients with incidentally resected limited disease small cell lung cancer—a retrospective study
Introduction
Historically, small cell lung cancer (SCLC) has been considered a non-operable disease. Two completed prospective randomized control trials (1,2) suggested that surgery had no benefits compared to radiation in the treatment of limited-stage SCLC. Over the past 20 years, several retrospective single-institution (3-7) or database-based studies (8-15) have reported favorable results for surgical resection in patients with early-stage SCLC. Under the current National Comprehensive Cancer Network (NCCN) guidelines (16), American College of Chest Physician (ACCP) guidelines (17), and European Society for Medical Oncology (ESMO) guidelines (18), surgery with adjuvant therapy is now recommended for the treatment of patients with clinical T1–2N0M0 or stage I SCLC.
It is currently recommended that only T1–2N0M0 or stage I SCLC cases receive surgical resection (16-18); however, a considerable number of patients with N1 or N2 lymph node metastatic SCLC ultimately undergo surgical resection in clinical practice. This may partially attributed to some patients receiving an incidental diagnosis after resection for what was initially presumed to be non-small cell lung cancer (NSCLC), pulmonary metastatic disease, or other diseases when the decision for surgical resection is made (6,11). This problem is not new; already three decades ago, incidental SCLC findings occurred in 4–12% of surgeries for solitary lung nodules (19).
Surgery without chemotherapy has been shown to provide no benefit to patients with SCLC (1). Thus, adjuvant therapy, as a salvage treatment, might improve the survival of patients with incidentally resected SCLC. However, the proper adjuvant therapy for SCLC patients who undergo resections (both purposely and incidentally) is still unclear.
According to ESMO guidelines, adjuvant chemotherapy (ad-chemo) is recommended for pT1–2N1 patients who receive complete surgical resection (R0), while adjuvant chemo-radiotherapy (ad-CRT) is recommended for N2 patients (18). According to NCCN guidelines, ad-CRT is recommended for both N1 and N2 patients, though data to support this recommendation are sparse (16). In sum, the use of proper adjuvant therapy for N1–2 cases remains controversial and needs further study.
NCCN (16) and ACCP guidelines (17) would suggest that ad-chemo is recommended for patients with T1–2N0 (stage I) resected SCLC. Due to the relative infrequency of such surgical candidates, this recommendation is only supported by 4 quite dated phase-II single-arm studies (20-23) and a database-based retrospective study (24). It is clear that further research needs to be conducted on the use of adjuvant therapy for patients with incidentally resected N1-2 SCLC. In addition, further research also needs to be conducted on the use of adjuvant therapy for patients with incidentally resected N0 cases because the evidence is limited.
This study aimed to evaluate outcomes of patients with incidentally resected SCLC to explore the use of salvage adjuvant therapy, stratified by absence (pN0) or presence (pN1-2) of pathologic lymph node metastasis. We hypothesized that ad-chemo and/or ad-CRT could improve survival of patients after incidentally resected. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-616/rc).
Methods
Patient selection
Consecutive patients who underwent surgical resection and were diagnosed with SCLC after resection from January 2005 to December 2014 at the Shanghai Pulmonary Hospital were retrospectively included in this study. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had been treated with palliative intent; (II) had positive surgical margin; (III) had been diagnosed with other malignant tumors; (IV) died within 30 days after surgery; and/or (V) were lost to follow-up during the designated period. The number of cases in the area during the study period determined the sample size. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Shanghai Pulmonary Hospital (No. K20-196Y), and individual consent for this retrospective analysis was waived.
Diagnosis and treatment
The preoperative workup for lung resection patients at this institution routinely included chest computed tomography (CT), brain magnetic resonance imaging, a whole-body bone scan, an ultrasound or CT scan of the abdomen, and fiberoptic or electronic bronchoscopy. Positron emission tomography/CT was not mandatory for all patients. All patients received an exfoliative cell examination of sputum and a bronchoscopic brush biopsy. Endobronchial ultrasound-guided transbronchial needle aspiration was performed in patients with enlarged mediastinal lymph nodes. Patients with peripheral nodules also underwent transthoracic needle biopsy.
Patients without a definite SCLC diagnosis, but in whom lung cancer was highly suspected, underwent surgical resection based on treatment principles for NSCLC. All patients underwent surgery without induction chemotherapy. After resection, it was recommended that all patients receive 4–6 courses of adjuvant platinum-based chemotherapy. For patients with malignant lymph nodes, ad-CRT was an alternative option. Some patients also received prophylactic cranial irradiation (PCI). All adjuvant therapy was performed before tumor recurrence. Tumor, node, metastasis (TNM) staging was determined according to the 7th edition of the TNM classification system for lung cancer (25). The pathologic diagnoses were confirmed by 2 senior pathologists.
Patients were stratified into pN0 or pN1–2 groups according to their pathological lymph nodes status and further grouped according to different adjuvant therapies (surgery alone, ad-chemo, ad-CRT, ad-chemo + PCI and ad-CRT + PCI). For pN0 cases, survival were compared between surgery alone and ad-chemo groups and for pN1/2 cases, survival were compared among surgery alone, ad-chemo and ad-CRT groups, because the number of patients in some adjuvant therapies were too small.
Outcome
The primary outcome was overall survival (OS). OS was defined as the time from receiving surgery to death or the last follow-up time-point. Survival was updated by telephone contact annually. Patients who were not deceased were censored at the date they were last known to be alive. Patients’ outcomes were recorded up to December 31, 2020.
Statistical analysis
Continuous variables were compared using the Student’s t-test. Unordered categorical variables were analyzed using Pearson’s chi square test or the Fisher exact test, and ordered categorical variables were analyzed using the Mann-Whitney test. Survival curves were constructed using the Kaplan-Meier method and compared using the log-rank test. Multivariable cox regression models using a stepwise backwards (Wald) method were constructed to identify the relevant variables affecting survival. Independent variables included age, gender, symptoms, comorbidities, ward characteristics, laterality, surgical approaches, surgical methods, surgical margins, postoperative complications, histologic types, pT category, pN category, and types of adjuvant therapy. Only factors that were significantly associated with a specific outcome in univariate analysis (P<0.05) were included in the multivariate analysis. A 2-sided P value <0.05 was considered statistically significant. The statistical analysis was performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA), and the survival curves were drawn using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA).
Results
Therapy information and baseline characteristics
Between 2005 and 2014, 15,368 patients who underwent surgical resection were diagnosed with primary lung cancer at the Shanghai Pulmonary Hospital in China. A total of 290 (1.9%) of these patients were diagnosed with SCLC, and 193 (1.3%) patients were diagnosed incidentally after resection. One hundred and sixty one patients met the eligibility criteria and were included in this study (see Figure 1). Among these 161 incidental cases, 103 (64.0%) patients received ad-chemo, 13 (8.1%) received ad-CRT, 9 (5.6%) received ad-chemo and PCI, and 1 (0.6%) received ad-CRT and PCI, while the remaining 35 (21.7%) received surgery alone. The baseline characteristics of the patient cohort are listed in Table 1. There were no missing values for all the relevant variables. Of the patients who received adjuvant therapy, a higher proportion had symptoms before diagnosis, compared to patients who received no adjuvant therapy (63.3% vs. 81.8%, P=0.041).
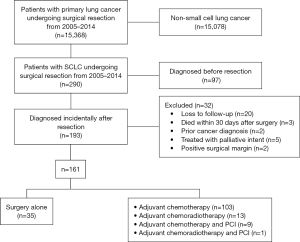
Table 1
| Characteristic | Patient cohort (N=161) | Adjuvant therapy (n=126) | No adjuvant therapy (n=35) | P |
|---|---|---|---|---|
| Age (years)a | 0.332 | |||
| Mean ± SD | 61.1±9.6 | 60.6±9.2 | 63.0±10.7 | |
| Median (Q1, Q3) | 61.0 (55.0, 68.0) | 61.0 (55.0, 67.0) | 64.0 (56.0, 72.0) | |
| Gender, n (%) | 0.970 | |||
| Male | 140 (87.0) | 109 (88.6) | 31 (88.6) | |
| Female | 21 (13.0) | 17 (13.4) | 4 (11.4) | |
| Laterality, n (%) | 0.347 | |||
| Left | 85 (52.8) | 64 (50.8) | 21 (60.0) | |
| Right | 76 (47.2) | 62 (49.2) | 14 (40.0) | |
| Symptoms, n (%) | 0.041 | |||
| Without | 53 (32.9) | 47 (36.7) | 6 (18.2) | |
| With | 108 (67.1) | 81 (63.3) | 27 (81.8) | |
| Comorbidities, n (%) | 0.222 | |||
| Without | 108 (67.1) | 81 (64.3) | 27 (77.1) | |
| With | 53 (32.9) | 45 (35.7) | 8 (22.9) | |
| Ward character, n (%) | 1.000 | |||
| General ward | 137 (85.1) | 107 (84.9) | 30 (85.7) | |
| Priority ward | 24 (14.9) | 19 (15.1) | 5 (14.3) | |
| FEV1%, mean ± SD | 85.8±16.5 | 81.2±14.8 | 87.0±16.8 | 0.169 |
| Year of diagnosis, n (%) | 0.546 | |||
| 2005–2009 | 55 (34.2) | 45 (35.7) | 10 (28.6) | |
| 2010–2014 | 106 (65.8) | 81 (64.3) | 25 (71.4) | |
| Surgical approach, n (%) | 0.864a | |||
| Open | 120 (74.5) | 94 (74.6) | 26 (74.3) | |
| VATS | 40 (24.8) | 31 (24.6) | 9 (25.7) | |
| VATS converted to open | 1 (0.4) | 1 (0.8) | 0 (0) | |
| Surgical method, n (%) | 0.513 | |||
| Sub-lobectomy | 10 (6.2) | 7 (5.6) | 3 (8.6) | |
| Lobectomy | 118 (73.3) | 91 (72.2) | 27 (77.1) | |
| Pneumonectomy | 33 (20.5) | 28 (22.2) | 5 (14.3) | |
| Surgical margin, n (%) | 0.786 | |||
| >2 cm | 138 (85.7) | 107 (88.6) | 31 (88.6) | |
| ≤2 cm | 23 (14.3) | 19 (15.1) | 4 (11.4) | |
| Complications, n (%) | 0.059 | |||
| Without | 137 (85.1) | 111 (88.1) | 26 (74.3) | |
| With | 24 (14.9) | 15 (11.9) | 9 (25.7) | |
| Pathologic T category, n (%) | 0.060 | |||
| T1 | 60 (37.3) | 43 (34.1) | 17 (48.6) | |
| T2 | 67 (41.6) | 57 (45.2) | 10 (28.6) | |
| T3 | 25 (15.5) | 17 (13.5) | 8 (22.9) | |
| T4 | 9 (5.6) | 9 (7.1) | 0 (0.0) | |
| Pathologic N category, n (%) | 0.343 | |||
| N0 | 70 (43.5) | 51 (40.5) | 19 (54.3) | |
| N1 | 24 (14.9) | 20 (15.9) | 4 (11.4) | |
| N2 | 67 (41.6) | 55 (43.7) | 12 (34.3) | |
| Histologic types, n (%) | 0.127 | |||
| SCLC | 77 (47.8) | 56 (44.4) | 21 (60.0) | |
| Combined SCLC | 84 (52.2) | 70 (55.6) | 14 (40.0) | |
| Length of postoperative stay, n (%) | 0.664 | |||
| <14 days | 120 (74.5) | 95 (75.4) | 25 (71.4) | |
| ≥14 days | 41 (25.5) | 31 (24.6) | 10 (28.6) |
a, 1 patient who underwent VATS but converted to open surgery was not included in the model when testing. SCLC, small cell lung cancer; FEV1, forced expiratory volume in 1 s; VATS, video-assistant thoracoscopic surgery.
OS for the entire cohort
The median follow-up time was 33.6 months (interquartile range, 15.7–67.4 months). The median OS of the entire cohort was 36.6 months (95% CI: 28.5–44.8 months), and the 5-year OS rate was 36.5%. Median survival times (MST) and 5-year OS rates for the pathologic stage I, II, and III patients were “not reached” and 53.6%, 39.1 months and 40.8%, 20.7 months and 22.2%, respectively (log-rank P<0.001).
Comparison of different types of adjuvant therapy in pN0 cases
Of the 70 patients with pN0 SCLC who were diagnosed incidentally, 45 (64.3%) received ad-chemo, 19 (27.1%) received surgery alone, and the remaining 6 (8.6%) patients received ad-chemo + PCI. The Kaplan-Meier analysis demonstrated that ad-chemo was associated with better OS than surgery alone [median OS: not reached vs. 30.9 (95% CI: 11.0–56.7) months; 5-year OS rates: 61.7% vs. 35.1%; log-rank P=0.037; see Figure 2]. After adjusting for age, postoperative complications, comorbidities, surgical methods and surgical margin, multivariable Cox regression showed that ad-chemo was associated with a lower risk of death than surgery alone (HR: 0.373, 95% CI: 0.141 to 0.985; see Table 2).
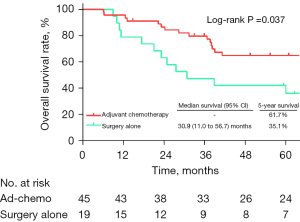
Table 2
| Variable | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Age ≥60 years | 3.456 | 1.069–11.172 | 0.038 |
| Postoperative complications | 3.074 | 0.952–9.928 | 0.060 |
| Comorbidities | 2.874 | 1.029–8.025 | 0.044 |
| Surgical methods | |||
| Pneumonectomy vs. lobectomy | 15.569 | 3.763–64.415 | <0.001 |
| Sub-lobectomy vs. lobectomy | 4.604 | 1.568–13.521 | 0.005 |
| Surgical margin ≤2 cm | 4.911 | 1.348–17.883 | 0.016 |
| Ad-chemo vs. surgery alone | 0.373 | 0.141–0.985 | 0.047 |
OS, overall survival; SCLC, small cell lung cancer; CI, confidence interval.
Comparison of different types of adjuvant therapy in pN1–2 cases
Of the 91 patients with pathologic positive lymph nodes (pN1–2) who were diagnosed incidentally, 58 (63.7%) received ad-chemo, 13 (14.3%) received ad-CRT, 16 (17.6%) patients received surgery alone, and the other 4 (4.4%) patients received some other types of adjuvant therapy (3 received ad-chemo + PCI and 1 received ad-CRT + PCI). When comparing survival among the patients who received ad-chemo, ad-CRT, and surgery alone, the Kaplan-Meier analysis showed that ad-CRT was associated with the longest survival [median OS: 69.1 (95% CI: 5.5–132.8) months; 5-year OS: 59.4%; see Figure 3]. Ad-chemo was not associated with better survival than surgery alone [median OS: 18.7 (95% CI: 14.6–22.9) vs. 25.4 (95% CI: 5.8–44.9) months; 5-year OS: 15.3% vs. 6.0%; log-rank P=0.848]. After adjusting for surgical margin, symptoms, ward character and laterality, multivariable Cox regression analysis showed that ad-CRT was associated with a lower risk of death (HR: 0.279, 95% CI: 0.102–0.761), while ad-chemo was not (HR: 0.869, 95% CI: 0.459–1.645) compared to surgery alone (see Table 3).
Table 3
| Variable | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Surgical margin ≤2 cm | 2.707 | 1.148–6.385 | 0.023 |
| Symptoms | 1.715 | 1.008–3.042 | 0.047 |
| Adjuvant therapy (ref. = surgery alone) | |||
| Ad-chemo | 0.869 | 0.459–1.645 | 0.666 |
| Ad-CRT | 0.279 | 0.102–0.761 | 0.013 |
| Priority ward vs. common ward | 0.293 | 0.105–0.822 | 0.020 |
| Laterality (left vs. right) | 0.539 | 0.318–0.913 | 0.022 |
OS, overall survival; SCLC, small cell lung cancer; CRT, chemo-radiotherapy; CI, confidence interval.
Discussion
In this single-institution retrospective study, we evaluated the role of adjuvant therapy in patients with SCLC who incidentally received complete surgical resection. For pN0 patients, ad-chemo was associated with significantly better OS than surgery alone. For pN1-2 patients, ad-CRT was associated with longer OS than surgery alone or ad-chemo, while ad-chemo was not associated with better OS than surgery alone.
Only about 5% of SCLC cases are considered surgically resectable (26), and due to the rarity of resectable SCLC cases, no prospective studies have been conducted comparing surgery alone, ad-chemo, and ad-CRT in patients with SCLC. Recently, 2 studies (24,27) evaluated the optimal adjuvant therapy for pT1–2N0 and N1-2 cases, respectively.
Based on the National Cancer Data Base (NCDB), Yang et al. (24) performed the only study to evaluate the role of adjuvant therapy in patients with pT1–2N0M0 SCLC who underwent resection, and found that ad-chemo was associated with better survival than surgery alone (MST 59.8 vs. 42.1; 5-year OS: 50.0% vs. 40.4%). Their results provided support for the current NCCN guidelines that also recommend ad-chemo after resection for T1–2N0M0 SCLC patients (16). However, Yang’s study (24) was limited in terms of information regarding surgical complications and recurrence, which made identifying adjuvant treatment indistinguishable from palliative treatment and severely impacted any interpretation of results (28). The current study had the advantage of including such information beyond what was presented by in Yang et al. In our study, patients with resected pN0 patients were found to benefit from ad-chemo after resection, and had a 63.2% 5-year survival rate. The multivariate Cox model showed that ad-chemo was an independent prognostic factor for pN0 patients who received resection. Our single-institution result, with surgical complications, pulmonary functions and recurrence information, verified and corroborates the conclusions illustrated by Yang et al. (24).
Urushiyama et al. (27) compared ad-chemo and ad-CRT in patients with resected N1–2 SCLC in a retrospective study using the Diagnosis Procedure Combination (DPC) database in Japan. Median recurrence-free survival (RFS) was 1,146 days in the ad-chemo group and 873 days in the ad-CRT group. RFS was significantly longer in ad-chemo patients than ad-CRT patients in the univariable analysis. However, in the multivariable analysis, RFS did not differ significantly between the ad-CRT and ad-chemo groups (HR: 1.29; 95% CI: 0.91–1.84). In Urushiyama’s study (27), recurrence could not be recorded if patients were discharged to another hospital or home before recurrence, limiting the validity of results. As deaths in hospitals other than those participating in the DPC database could not be recorded, findings on OS were not available in Urushiyama’s study.
An additional study utilizing the NCDB by Wong et al. (29) reported that postoperative radiotherapy (PORT) significantly improved the 5-year OS rates of patients with pathologic N2 SCLC from 18.6% to 29.0% but did not improve the survival of patients with pathologic N1 SCLC. In this study (29), 44.9% of patients did not receive chemotherapy in the no-PORT group, while only 3.8% of patients did not receive chemotherapy in the PORT group; the poor survival of the no-PORT group might be explained by the higher rate of patients who did not receive chemotherapy. The advantage of PORT in N2 disease retained its significance in the multivariate analysis; however, a formal comparison of surgery alone, ad-chemo, and ad-CRT might still be necessary.
To our knowledge, this is the first study to compare surgery alone, ad-chemo, and ad-CRT for patients with incidentally resected pN1–2 SCLC. We found that ad-CRT significantly improved OS compared to surgery alone or ad-chemo. Our findings support the recommendation in the NCCN guidelines to use adjuvant treatment in patients with pathologic positive lymph nodes. In addition, we found that ad-chemo did not improve OS compared to surgery alone for incidentally resected pN1–2 SCLC cases, which is the first time such a finding has been reported.
This study had several limitations. First, this study carries the inherent bias of a retrospective randomized study, and our conclusions would ideally be further verified in a prospective manner. It was recommended that all patients received ad-chemo, but some patients refused to receive adjuvant therapy, which may have impacted those patients’ outcome. The baseline characteristics between the patients who received adjuvant therapy and those who did not were compared, and only a proportion of patients with symptoms differed significantly between the two groups (see Table 1). Patients with symptoms or who are less fit might be less likely to receive aggressive therapy. For pN0 cases, we compare ad-chemo to surgery alone, but no patients received ad-CRT, so we cannot provide new data about tri-modality therapy among these patients. For pN1/2 cases, ad-CRT was an alternative option to ad-chemo, and we do not know the exact reasons why patients chose to receive ad-CRT and not ad-chemo. The possible reason might be physicians recommended one over the other, but we had no data to prove it. Second, our analysis was limited by the sample size. Patients with pN1 or pN2 SCLC were analyzed together, not separately, because of the limitation of the small sample size. Even the multivariate analysis for patients with pN1/2 SCLC did not reveal that pN2 was associated with a higher risk of death than pN1; thus, a separate analysis of pN1 or pN2 cases needs to be conducted in the future. Third, only 10 patients (6 with pN0 SCLC and 4 with pN1–2 SCLC) received PCI after resection. These 10 patients were not included in the survival analysis because of the limitation of the small sample size. PCI may provide a benefit to patients with stage IIB or III SCLC who have received complete resection (30,31), and optimal adjuvant therapy for pN1–2 incidentally resected patients might include PCI. Despite these limitations, which we acknowledge, this study closes a knowledge gap regarding the benefits of salvage adjuvant therapy following incidental resection of SCLC, with important clarifying results as stratified by nodal status. We have set the foundation for future prospective evaluations.
Conclusions
Patients who incidentally receive surgical resection and are diagnosed with limited disease SCLC after resection should be offered adjuvant therapy as a salvage treatment. For patients with incidentally resected pN0 SCLC, ad-chemo should be considered after resection. For patients with incidentally resected pN1/2 SCLC, ad-chemo does not improve OS compared to surgery alone in our cohort, and these patients should receive ad-CRT as an adjuvant therapy.
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: This work was supported by the Natural Science foundation of Shanghai (No. 19ZR1442700) and Innovation Research Team Foundation of Shanghai Pulmonary Hospital (No. FKCX1905).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-616/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-616/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-616/coif). MBA received consulting fees from Astra and Zeneca. AF received consultations fees from Amgen, AstraZeneca, Roche, Astellas, Takeda, BMS, MSD, Pfizer, Merck, Novartis and Janssen for work unrelated to the subject of this manuscript. AA reports advisory board: MSD Oncology, Roche, Takeda, Pfizer, Bristol-Myers Squibb, AstraZeneca, Eli-Lilly; speaker bureau from Eli-Lilly, AstraZeneca, Amgen; research funding from Boehringer Ingelheim, AstraZeneca, Bristol Myers Squibb; expert testimony from Roche, AstraZeneca, Bristol Myers Squibb; travels, accommodations, expenses from Bristol Myers Squibb, AstraZeneca, Amgen; outside the submitted work. FG received consulting fees for advisory boards or consultations from: Eli Lilly, Roche, Boehringer Ingelheim, AstraZeneca, Pierre Fabre, BMS, MSD, Novartis, Merck, Otsuka, Novartis, Takeda; honoraria for seminars or talks to Industry from: Eli Lilly, Roche, Boehringer Ingelheim, AstraZeneca, Pierre Fabre, AMGEN, Celgene, BMS, MSD; research funding from: AstraZeneca, BMS, MSD. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Shanghai Pulmonary Hospital (No. K20-196Y), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet 1973;2:63-5. [Crossref] [PubMed]
- Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994;106:320S-3S. [Crossref] [PubMed]
- Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg 2005;129:64-72. [Crossref] [PubMed]
- Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267-71. [Crossref] [PubMed]
- Hanagiri T, Sugio K, Baba T, et al. Results of surgical treatment for patients with small cell lung cancer. J Thorac Oncol 2009;4:964-8. [Crossref] [PubMed]
- Badzio A, Kurowski K, Karnicka-Mlodkowska H, et al. A retrospective comparative study of surgery followed by chemotherapy vs. non-surgical management in limited-disease small cell lung cancer. Eur J Cardiothorac Surg 2004;26:183-8. [Crossref] [PubMed]
- Li S, Jin K, Pan Y, et al. Role of surgery in a case-control study of patients with clinical stage IIIA small cell lung cancer. J Thorac Dis 2021;13:2738-45. [Crossref] [PubMed]
- Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215-9. [Crossref] [PubMed]
- Weksler B, Nason KS, Shende M, et al. Surgical resection should be considered for stage I and II small cell carcinoma of the lung. Ann Thorac Surg 2012;94:889-93. [Crossref] [PubMed]
- Lüchtenborg M, Riaz SP, Lim E, et al. Survival of patients with small cell lung cancer undergoing lung resection in England, 1998-2009. Thorax 2014;69:269-73. [Crossref] [PubMed]
- Takei H, Kondo H, Miyaoka E, et al. Surgery for small cell lung cancer: a retrospective analysis of 243 patients from Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2014;9:1140-5. [Crossref] [PubMed]
- Combs SE, Hancock JG, Boffa DJ, et al. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. J Thorac Oncol 2015;10:316-23. [Crossref] [PubMed]
- Yang CJ, Chan DY, Shah SA, et al. Long-term Survival After Surgery Compared With Concurrent Chemoradiation for Node-negative Small Cell Lung Cancer. Ann Surg 2018;268:1105-12. [Crossref] [PubMed]
- Gao L, Shen L, Wang K, et al. Propensity score matched analysis for the role of surgery in stage III small cell lung cancer based on the eighth edition of the TNM classification: a population study of the US SEER database and a Chinese hospital. Lung Cancer 2021;162:54-60.
- Chen X, Zhu JL, Wang H, et al. Surgery and Surgery Approach Affect Survival of Patients With Stage I-IIA Small-Cell Lung Cancer: A Study Based SEER Database by Propensity Score Matching Analysis. Front Surg 2022;9:735102. [Crossref] [PubMed]
- National Comprehensive Cancer Network Guidelines for small cell lung cancer version 2.2022. [cited 2022 June 5]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- Rudin CM, Ismaila N, Hann CL, et al. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015;33:4106-11. [Crossref] [PubMed]
- Dingemans AC, Früh M, Ardizzoni A, et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol 2021;32:839-53. [Crossref] [PubMed]
- Kreisman H, Wolkove N, Quoix E. Small cell lung cancer presenting as a solitary pulmonary nodule. Chest 1992;101:225-31. [Crossref] [PubMed]
- Karrer K, Ulsperger E. Surgery for cure followed by chemotherapy in small cell carcinoma of the lung. Acta Oncologica 1995;34:899-906. [Crossref] [PubMed]
- Macchiarini P, Hardin M, Basolo F, et al. Surgery plus adjuvant chemotherapy for T1-3N0M0 small-cell lung cancer. Rationale for current approach. Am J Clin Oncol 1991;14:218-24. [Crossref] [PubMed]
- Rea F, Callegaro D, Favaretto A, et al. Long term results of surgery and chemotherapy in small cell lung cancer. Eur J Cardiothorac Surg 1998;14:398-402. [Crossref] [PubMed]
- Tsuchiya R, Suzuki K, Ichinose Y, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg 2005;129:977-83. [Crossref] [PubMed]
- Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [Crossref] [PubMed]
- Ignatius Ou SH, Zell JA. The applicability of the proposed IASLC staging revisions to small cell lung cancer (SCLC) with comparison to the current UICC 6th TNM Edition. J Thorac Oncol 2009;4:300-10. [Crossref] [PubMed]
- Urushiyama H, Jo T, Yasunaga H, et al. Adjuvant chemotherapy versus chemoradiotherapy for small cell lung cancer with lymph node metastasis: a retrospective observational study with use of a national database in Japan. BMC Cancer 2017;17:613. [Crossref] [PubMed]
- Wang TH, Hu YW, Hu YW. Benefit of Adjuvant Therapy in Postoperative Early-Stage Small-Cell Lung Cancer: Is There Sufficient Evidence? J Clin Oncol 2017;35:117. [Crossref] [PubMed]
- Wong AT, Rineer J, Schwartz D, et al. Assessing the Impact of Postoperative Radiation Therapy for Completely Resected Limited-Stage Small Cell Lung Cancer Using the National Cancer Database. J Thorac Oncol 2016;11:242-8. [Crossref] [PubMed]
- Yang Y, Zhang D, Zhou X, et al. Prophylactic cranial irradiation in resected small cell lung cancer: A systematic review with meta-analysis. J Cancer 2018;9:433-9. [Crossref] [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


