
A multidisciplinary approach to the work up and management of pulmonary carcinoid tumors and DIPNECH: a narrative review
Introduction
Neuroendocrine tumors (NETs) of the lung represent a spectrum of disease. They range from the sometimes slow growing pulmonary carcinoid (PC) tumors (typical and atypical), to aggressive large cell neuroendocrine carcinoma (LCNEC) and small cell lung cancer (SCLC). PCs often have an indolent course and can sometimes be discovered incidentally. PCs represent only 2% of all lung cancers, however, embody approximately 25% of NETs. A premalignant condition known as diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) can sometimes co-occur in the setting of PCs but not all PCs have DIPNECH (1,2).
The incidence of all NETs is increasing (2,3). A Surveillance, Epidemiology, and End Results (SEER) analysis from 1973 to 2012 found that incidence rates of NETs increased 6.4-fold between 1973 and 2012 (3); this increase was most pronounced in PCs which had the highest incidence. Conversely, the incidence of all malignancies is decreasing including lung cancers (2). Among the most significant contributors to the increase in NETs is superior and more frequent use of imaging and increased awareness.
Survival of PCs vary by stage and mitotic index-whether they are typical carcinoid (TC) or atypical carcinoid (AC), with the median survival of all distant stage PCs being 24 months (3). PCs are staged according to the American Joint Commission on Cancer 8th Edition which was designed for staging of non-small cell lung cancer (NSCLC). Studies have questioned this staging system owning that it may not reflect the biologic behavior of PCs and alternatives have been offered (4,5).
While the presentation, work up, diagnosis and treatment overlap with NSCLC, PCs have several nuances that require different methods and modalities. This manuscript will provide a multidisciplinary overview to approaching patients with DIPNECH and PCs. We present the following article in accordance with the Narrative Review reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-415/rc).
Methods
A review of the literature pertaining to DIPNECH and PCs was conducted with the last search being April 15, 2022. Table 1 describes the search strategy.
Table 1
| Items | Specification |
|---|---|
| Date of search | Last search April 15, 2022 |
| Databases and other sources used | Web of Science, PubMed |
| Search terms used | lung carcinoid, DIPNECH, lung neuroendocrine and bronchopulmonary carcinoid |
| Timeframe | Relevant literature until April 15, 2022 |
| Inclusion and exclusion criteria | Given the rarity of this disease, we included all study types, including clinical trials, case series, expert guidelines and some case reports |
| Selection criteria | All authors were available to select items to report. When information overlapped the most pertinent manuscript was selected |
DIPNECH, diffuse idiopathic pulmonary neuroendocrine cell hyperplasia.
Workup, diagnosis and management of PCs
Presentation
PCs are often clinically silent and symptom development depends on the location of the tumor. Up to 90% of patients with centralized tumors develop symptoms, whereas peripheral tumors are generally found incidentally (6). If symptoms occur, they are generally non-specific. The most common symptoms are coughing, wheezing and consequences of airway narrowing, such as dyspnea, stridor or post-obstructive pneumonia (7,8).
Carcinoid syndrome (CS) occurs due to the systemic release of vasoactive amines and polypeptides, such as serotonin, histamine, and kallikrein (9). The development of CS is uncommon in PCs (10). In fact, CS has been found to occur in only 8% of patients with PCs (11). PCs rarely over produce adrenocorticotropic hormone and growth hormone releasing hormone can lead to Cushing’s syndrome and acromegaly, respectively (8,12,13).
Imaging
Imaging is required to determine location, tumor stage and assess overall burden of disease. A chest radiograph can identify PCs in 40% of situations (14,15), however, multiphasic contrast-enhanced computed tomography (CT) provides cross-section imaging that can better characterize abnormalities and anatomic relationships and is the gold standard (15,16). The characteristics of PCs can mimic other lung cancers but can show endoluminal involvement or can abut the airway and be associated with bronchiectasis, mucoid impaction, atelectasis and air trapping (17).
The majority of PCs overexpress somatostatin receptors (SSTR) and SSTR based imaging is required for all PCs (1). Radiotracers have been developed, comprising a somatostatin analog (SSA) chelated to a radioisotope. Variations in the SSA, chelator, and choice of isotope have been shown in preclinical models to alter the affinity to SSTRs (18,19). From a clinical standpoint, the type of SSTR based imaging is sometimes geographically and cost dependent. Not all institutions have access to imaging with DOTATATE PET, however, it has been that this modality is more cost effective and results in superior imaging compared with indium based SPECT/CT (20).
Imaging of SSTR in NETs can be accomplished by γ-cameras utilizing either planar or SPECT technique using In-111-diethylenetriaminepentaacetic acid (DTPA)-octreotide (111In-DPTA) to localize PCs. 111In-DPTA scintigraphy has been shown to be sensitive for detecting NETs and is superior to CT and MR imaging in uncovering metastatic disease (21). More recently, octreotide derivatives such as DOTATATE, DOTANOC, or DOTATOC, have been linked to position emitting isotopes, such as gallium-68 (68Ga DOTATATE, 68Ga DOTANOC or 68Ga -DOTATOC) and cooper-64 (64Cu DOTATATE), which can be evaluated by PET/CT. Not only can these modalities provide information on disease location and burden, they also can influence treatment decisions. Imaging using a PET tracer, either 68Ga or 64Cu is superior to SPECT and is preferred (22).
Diagnosis
Bronchoscopy is a diagnostic tool for the evaluation of PCs. In the majority of cases, bronchoscopy reveals budding, highly vascularized, well circumscribed lesions that are reddish pink in color (23). This diagnostic modality allows for visual inspection of a proximal or endobronchial lesion as well as for biopsy either under direct vision or using navigational or imaging technology for peripheral lesions. While concern exists for hemorrhage during the biopsy, the incidence of serious bleeding is low (24-27). A biopsy is superior to bronchial washing studies, as the latter has shown a diagnostic yield of 23% (28). Approximately 10% of PCs are in peripheral locations and require a navigational bronchoscopy or transthoracic needle biopsy under CT guidance (23). The primary concern with this approach is pneumothorax, which occurs with an average of 20% of cases (29).
Pathology
TCs and ACs are well-differentiated NETs in terms of architecture and cytological features. Clinically, TCs are low-grade and ACs are intermediate-grade (4). Morphologically, PCs may have organoid, trabecular, rosette, insular, pseudoglandular, or solid growth patterns. Oncocytic, clear cell, and melanin-laden carcinoids, although rare, can occur. Tumor cells are uniform, featuring round to oval or spindled nuclei with finely granular nuclear chromatin, moderate to abundant eosinophilic cytoplasm, and inconspicuous nucleoli. Per the 2021 World Health Organization (WHO) Classification of Thoracic Tumours (28) TCs have a mitotic count of <2 mitoses/2 mm2 and lack necrosis. ACs have 2–10 mitoses/2 mm2 and/or may have necrosis, which is usually focal and punctate (30). Figure 1 shows the histological features of TC and AC.
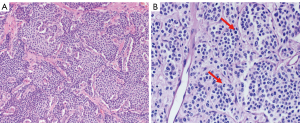
By immunohistochemistry, PCs are positive for low-molecular-weight cytokeratins. They are strongly reactive for neuroendocrine markers such as chromogranin A, synaptophysin, CD56, and INSM1 (31). Insulinoma-Associated Protein 1 Is a Crucial Regulator of Neuroendocrine Differentiation in Lung Cancer (31). TTF1 tends to be positive in peripheral but negative in central tumors. The Ki-67 index, a marker of cell proliferation, can aid in differentiating low-grade PCs from high-grade NETs, such as LCNEC and SCLC, especially in a small biopsy with crush artifact. Commonly, TCs will have a Ki-67 expression of <5%, whereas ACs have a Ki-67 of 5–30%. A specific Ki-67 index is not a requirement for the classification of either PC, as there is significant overlap between the Ki-67 index in these groups, and thus, the Ki-67 index is not recommended to be used for distinguishing between these tumor subtypes (10,32). Additionally, it has also been reported that adding Ki-67 index does not provide a prognostic value to PCs, as a histological diagnosis has been shown to be a stronger predictor of overall survival than Ki-67 index (32). As such, reporting Ki-67% index is not mandatory for PCs.
Treatment for PCs
Surgical treatment
Surgery is the treatment of choice for localized PCs and has shown excellent five-year survival rates—94% for TC and 67% for AC (33,34). After diagnosis of a PC, a meticulous pre-operative evaluation should be performed. Work-up should include history and physical examination, assessment of functional status, pulmonary function tests (PFTs), a nutritional assessment, and testing related to pertinent co-morbid conditions. While smoking has not been associated with PCs, smokers should be counselled to stop as this reduces peri-operative pulmonary complications but this should not delay or prevent curative intent surgery (35,36).
Patients undergoing resection may have co-existing conditions and exposures which can perturb pulmonary function. Underlying DIPNECH commonly manifests with shortness of breath in a third of patients with the majority of patients demonstrating an obstructive pattern on spirometry. Spirometry and measurement of forced expiratory volume (FEV1) and diffusion capacity for carbon monoxide (DLCO) is recommended for all patients undergoing pulmonary resection (37). A pre-operative predicted FEV1 or DLCO of 60% or less has been shown to be an important in predicting respiratory complications and increased peri-operative morbidity and thus further testing should be recommended (38). Options for secondary testing include a shuttle walk test, stair-climbing, exercise stress test or a ventilation-perfusion scan. The former are more subjective, while later are more quantitative and have thresholds associated with surgical risk (39). The location of tumor is also important when examining pulmonary function. For patients with a central, or endobronchial tumors with obstruction and atelectasis, a resection may in fact ameliorate ventilation and perfusion matching and post-operative lung function.
The majority of patients with localized PCs are considered for resection. The surgical approach depends on the size and location of the tumor. Tumors are considered central if they are included in the first third of the lung and these are more likely to be visible during bronchoscopy. Peripheral tumors are located outside of the medial third of the lung.
Peripheral tumors
Lobectomy remains the most commonly performed surgery for PCs (38). For patients in whom a sublobar resection may be anatomically considered, controversy remains as to whom this should be offered. Sublobar resections comprehend both wedge resections and anatomical segmentectomies. The literature evaluating resection of PC’s, in particular related to sublobar resections is heterogeneous, and composed predominantly of single institution, retrospective cohort studies. SEER database studies performed by Fox and colleagues in 2013 concluded sublobar resection to be non-inferior for patients with TC while Afoke and colleagues concluded sublobar resection can be considered for patients with typical PCs, 2 cm or less in diameter (40,41). Further studies are needed in this area. Based on currently available data, segmentectomy for small (<2 cm) TCs should be considered when anatomically feasible.
Central tumors
Small tumors located centrally within the tracheobronchial tree with intraluminal extent of disease may be effectively treated with endobronchial therapy (EBT) alone (40). For patients in whom EBT does not eradicate disease, subsequent surgery can be performed safely and is not associated with increased morbidity (40). Larger, central tumors with significant extraluminal extent or positive nodes should be referred for surgical resection (30). Ultimately, a pneumonectomy may be required, however, all patients should be assessed for parenchymal sparing options such a sleeve whenever possible as this approach has been shown to have improved short and long term outcomes and improved overall survival compared with pneumonectomy (39,42).
With the advent of more advanced EBT options there is an appeal to coupling endobronchial and surgical treatment to enhance the ability to perform parenchymal sparing operations. Reports of success thus far remain anecdotal and larger scale data has not corroborated that EBT reduces the extent of subsequent surgical resection (39).
Surgical approach
Surgery for PCs, like NSCLC, may be performed either minimally invasive or open. Due to carcinoids representing a small percentage of lung cancer, data on outcomes by approach is often combined with or extrapolated from NSCLC data (38). Segmentectomy, bronchoplasty, sleeve lobectomy (Figure 2) are technically more complex, but are performed with low morbidity and mortality using minimally invasive approaches by experienced thoracic surgeons. (40,43).
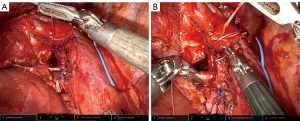
Tumor histology, size and nodal status are important prognostic factors for PCs (38).
Lymph node assessment not only accurately defines pathologic stage, it informs treatment selection, as patients with nodal involvement should be relegated to surgical resection over endobronchial therapies (41). The commission on cancer released guidelines (Standard 5.8) recommending that any curative intent lung resection, including PCs, should have at least 1 separately submitted N1 lymph node station and nodes from at least three distinct mediastinal nodal stations (44).
Adjuvant therapy for locally advanced PCs
There is no clear role for adjuvant treatment. Unlike NSCLC, there are no large prospective trials showing benefit. Guidelines are mixed, with some advocating the consideration of adjuvant treatment for AC with N2 positivity, despite acknowledging the lack of data (45). Because of this lack of data, other guidelines advocate against the use of adjuvant treatment regardless of the stage (46).
Endobronchial treatment for carcinoid tumors
While surgical resection is the gold standard for treatment for PCs, not all patients with resectable tumors are good surgical candidates (47). In addition, lung preserving resections can be considered in selected patients by identifying the origin of the tumor and reducing the disease burden through bronchoscopic interventions. Additionally, PCs are commonly located centrally, where they can be visualized during flexible bronchoscopy and predominantly endobronchial tumors without invasion of adjacent tissues (48,49). This leads to the feasibility of using EBTs, such as cryotherapy and laser which can also be complementary to surgery. EBT is most appropriate for patients with small, centrally located, intraluminal PCs, without evidence of distant metastasis. Studies have shown that even in larger tumor lesions with some extraluminal tumor growth, EBT may still provide symptomatic relief and limit the advancement of the disease (50,51).
One option of EBT is cryotherapy which utilizes a liquefied gas (cryogen) to flow and expand through a nozzle located at the tip of the cryoprobe, causing a Joule-Thompson effect (51,52). The temperature drop from the cryogen during expansion allows ice ball formation, which freezes the free intracellular and extracellular molecules around and, in the tumor, causing cellular destruction. Mucosal-based vascular tumors such as PCs are cryosensitive. Cryotherapy is routinely performed under general anesthesia with either flexible bronchoscopy through a rigid bronchoscope or flexible bronchoscopy via an endotracheal tube or laryngeal mask airway. Recently, Perikleous et al. provided data on the safety and efficacy of cryotherapy in for PCs, where complete remission was achieved in 33% when the maximum tumor length was less than 20 mm and allowed 35% of the patients with larger tumors to undergo parenchymal sparing resections (53). They also demonstrated that cryotherapy provided significant symptomatic relief in 92% of patients. The median number of cryotherapy applications performed was three (53), compared to an average of five in an older study (54). Bertoletti et al. showed that cryotherapy was a safe and effective adjunct therapy in addition to resection of TCs, with only one recurrence seven years after initial EBT (55). Figure 3 shows a representation of treatment with cryotherapy.
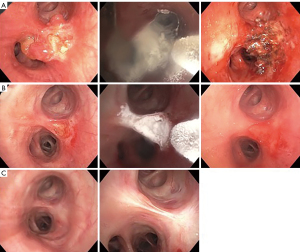
Another EBT is thermal therapy such as laser ablation, commonly the neodymium-doped yttrium aluminum garnet (Nd:YAG) laser. Laser therapy allows vaporization of tumors for immediate restoration of airway patency. Laser therapy is performed under general anesthesia with flexible bronchoscopy via rigid bronchoscope or endotracheal tube (56). Neyman et al. treated 25 patients with Nd:YAG laser with curative intent, and 16 patients (64%) had successful treatment without surgery, while five patients underwent lobectomy following EBT (57). Cavaliere et al. demonstrated that the use of Nd:YAG laser is curative in selected TCs. Among the 38 of 150 TCs (25%) that were treated with a curative intent based on criteria (small volume (<4–5 cm3), pedunculated or limited implantation base (<1.5 cm2), and minor wall infiltration), all patients remained in remission with follow-ups from one to 198 months (58).
Brokx et al. demonstrated that EBT made surgery unnecessary in 42% of the cases with a minimum of a five-year cohort study. Thirty-three percent of the patients had complete remission following the first EBT. Extraluminal involvement had a higher recurrence rate, leading to the need for surgical resection (59). Reuling et al. reported that the predictive success rate for EBT is endoluminal tumors with a diameter of less than 15 mm and purely intraluminal tumor growth as evident on CT chest. Other vital criteria include small base attachment (<1.5 cm2), without lymph node involvement or suspected locoregional or distant metastasis (50). In most EBT studies, a multi-modality approach (use of thermal and cold therapies) has been used in combination to achieve complete remission. In Reuling et al., 61 out of 125 patients (49%) had successful EBT. Ten patients (8%) had residual disease of recurrence during follow-up, with four patients (3%) who had evidence of residual disease ≤2 years after EBT, and six patients (5%) showed recurrence >2 years after the first EBT (50). Overall, EBT is a safe procedure, with complications such as bleeding, bronchospasm, broken tooth, and vocal cord paralysis related to using rigid bronchoscopy for the latter two (50,55)
Van Boxem et al. reported using Nd:YAG laser, photodynamic therapy, and brachytherapy in 19 patients with resectable endoluminal TCs. Successful EBT was achieved in 14 patients (74%) with a median follow-up of 29 months, with one patient undergoing sleeve resection due to stenosis of the treated airway (60).
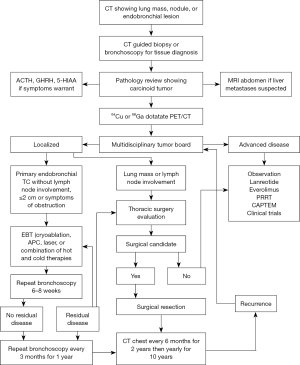
When EBT is performed, though there is no robust data to suggest the optimal surveillance schedule for bronchoscopic follow-up, most studies perform a bronchoscopy at six to eight weeks from the first EBT to assess response to treatment and provide additional treatment if required. Other follow-ups following the second bronchoscopy are dependent on response to therapy but would require continued surveillance with CT chest and/or bronchoscopy at least annually. Figure 4 shows a suggested algorithm for work up and management of PCs, including EBT.
Medical treatments for advanced disease
In advanced disease, the goal of treatment is to control tumor growth and manage symptoms. Given the rare nature of PCs, there have been few prospective trials. Treatment principals often are based on the experience of treating gastrointestinal (GI) or pancreatic NETs.
Since PCs frequently express SSTRs, SSAs, such as octreotide or lanreotide, are recommended for advanced disease (45,46,61). SSAs anti-proliferative effects occur through both direct and indirect mechanisms (62). Direct anti-tumor effects of SSAs require SSTR expression on tumor cells and direct binding to SSTR subtypes. This causes regulation of these multiple complex pathways resulting in cell cycle arrest, inhibition of growth factors, and cell apoptosis. Indirect anti-tumor mechanisms occur through immunomodulatory effects and inhibition of growth factors that regulate tumor growth through hormonal pathways, which subsequently cause the inhibition of cell proliferation and cell death.
For patients with TCs, asymptomatic disease and a low tumor burden, observation can be employed based on guidelines, but this remains controversial due to evidence that SSAs promote disease stabilization (63,64). Most evidence of SSAs comes from non-PC studies such as the phase III CLARINET trial that showed when compared to placebo, treatment with lanreotide in patients with gastroenteropancreatic NETs resulted in significantly increased progression-free survival (PFS) (median NR vs. 18 months; HR, 0.47) with a manageable toxicity profile (65).
The SPINET trial (NCT02683941) was a phase III, randomized, placebo-controlled trial that aimed to evaluate the safety and efficacy of lanreotide in patients with PCs but was stopped early due to insufficient enrollment. Results have been presented on the 77 enrolled patients with the primary outcome of PFS. In the intention to treat population, median PFS was 16.6 months in the lanreotide arm (n=56) vs. 13.6 months for placebo (n=21) [HR, 0.90; 95% CI: 0.46–1.88) (66)]. When stratifying patients based on TC or AC, the median PFS was 21.9 months in the lanreotide arm and 13.9 months for placebo for those with TC. In patients with AC, median PFS was 13.8 months and 11.0 months for lanreotide and placebo, respectively. Treatment related adverse events occurred in 74.5% in the lanreotide arm and 53.8% in the placebo arm and were mostly grade 1 and 2 (66). With the lack of large prospective clinical trials, these results support the guidelines recommended using SSAs in the first-line setting, particularly those with TC.
The mammalian target of rapamycin (mTOR) inhibitor, everolimus, is approved for the treatment of unresectable, locally advanced, or metastatic PCs based on the results of the RADIANT-2 and RADIANT-4 trials. mTOR is part of the family of PI3K-related protein kinases and regulates cell proliferation, growth and survival signaling. By inhibiting mTOR, everolimus prevents subsequent phosphorylation of substrates blocking mRNA translation resulting in inhibition of protein synthesis and tumor shrinkage (62).
The RADIANT-2 and RADIANT-4 trials were phase III studies evaluating the efficacy of everolimus in patients with advanced, well-differentiated, nonfunctional NETs of both GI and pulmonary origin. In the 44 patients with PCs in RADIANT-2, the PFS was 13.6 months in the everolimus plus octreotide arm and 5.6 months in the placebo plus octreotide arm (67). RADIANT-4 compared everolimus vs. placebo alone and enrolled 302 patients, including 90 patients with PCs (68). In a subgroup analysis of PCs, the PFS was 9.2 months in the everolimus arm vs. 3.6 months in the placebo arm (HR, 0.50; 95% CI, 0.28–0.88) (64). In both trials, adverse events with everolimus were mostly grade 1 and 2 (67-69).
Studies with chemotherapy in patients with advanced PCs are limited. The most commonly used regimens include a platinum agent plus etoposide or temozolomide plus or minus capecitabine (45). Due to the low proliferative rates of PCs, cytotoxic chemotherapy has limited efficacy but can provide benefit in patients who have more aggressive disease and tumors with a higher Ki-67 proliferation rate or low SSTR expression. The use of a platinum agent plus etoposide in these patients has been extrapolated from the treatment of SCLC (70). Retrospective studies have shown a response rate (RR) of 20% to 25% in patients with PCs treated with cisplatin plus etoposide (71-73). When used as monotherapy temozolomide has shown a RR ranging from 14% to 30% and stable disease ranging from 42% to 53% in retrospective studies of patients with PCs (72,74). In 33 patients with PCs who received temozolomide plus capecitabine (CAPTEM), 6 patients (18.2%) had a partial response and 19 patients (57.6%) had stable disease with a median PFS of 9 months (75). Oxaliplatin and streptozocin based chemotherapies have also shown some benefit in these patients and are recommended by some guidelines to consider (46,61). Cytotoxic chemotherapy can be considered for first line treatment in patients with ACs or aggressive disease based on consensus guidelines (45,46,61).
Immunotherapy has largely been disappointing for treatment of patients with NETs. In the phase Ib KEYNOTE-028 trial, which looked at the use of pembrolizumab in patients with advanced solid tumors, the objective response rate (ORR) was 12% in the 25 patients with carcinoid tumors with the majority of patients having stable disease (76). Spartalizumab was studied in a phase II trial in patients with metastatic carcinoid tumors and showed a RR of 7.4%. However, in patients with PCs (n=30), ORR was 16.7% while stable disease was achieved in 56.7% of patients (77). Of note, all five patients who had a partial response had AC. Two phase II studies, CA209-538 and DART SWOG 1609, have looked at the PD-L1 inhibitor nivolumab in combination with CTLA-4 inhibitor ipilimumab in patients with advanced NETs. CA209-538 included 11 patients with PCs (n=29) and showed an ORR of 24% (78). Responses were only seen in patients with intermediate or high-grade tumors. Similarly, DART SWOG 1609 included six patients with PCs (n=32) and showed an ORR of 25% with responses only being seen in patients with high grade tumors (79). There continues to be ongoing trials using immunotherapy including NCT03420521 which is a phase 2 trial using nivolumab with ipilimumab in subjects with advanced NETs including PCs (80). Another ongoing trial is NCT04579757 which surufatinib in combination with tislelizumab in patients with advanced solid tumors including a NET cohort (81). Table 2 shows considerations for management for advanced disease.
Table 2
| Clinical presentation | Consideration | Alternatives |
|---|---|---|
| Metastatic, asymptomatic, low volume, TC | Observation | Lanreotide if SSTR positive on functional imaging, Everolimus |
| Metastatic, asymptomatic, low volume, AC | Observation (especially with documented stability) | Everolimus, Lanreotide if SSTR positive on functional imaging |
| Metastatic, symptomatic, low volume disease, TC or AC | Lanreotide if SSTR positive on functional imaging | Everolimus |
| Metastatic, symptomatic, high volume disease, TC or AC | CAPTEM | PRRT |
| Metastatic, oligoprogressive disease within lungs, TC or AC | SBRT | Change in systemic treatment |
| Metastatic, progressive on lanreotide without local therapy options, TC or AC | PRRT | Everolimus, CAPTEM |
| All patients should be considered for clinical trials if available regardless of presentation | ||
TC, typical carcinoid; AC, atypical carcinoid; SSTR, somatostatin receptor; CAPTEM, capecitabine/temozolomide; SBRT, stereotactic body radiotherapy; PRRT, peptide receptor radionuclide therapy.
Radionuclide therapy
Peptide receptor radionuclide therapy (PRRT) has been a transformative therapy for patients with SSTR positive NETs (82,83). Though several types of PRRT have been tested in the past, the type of PRRT which has garnered regulatory licensure is 177Lutetium(177Lu)-Dotatate (84). Although currently only approved for patients with GEPNETs, the therapy has also demonstrated anti-tumor activity for patients with other SSTR positive NETs (85-87) (Table 3). Randomized evidence with 177Lu-Dotatate remains lacking in patients with PCs however both prospective and retrospective exist.
Table 3
| Study, year | Radioisotope | Total (N) | PC, N (%) | Median PFS, months | Median OS, months | RR |
|---|---|---|---|---|---|---|
| Brabander et al., 2017 (86) | 177Lu-Dotatate | 610 | 23 (3.8) | 20 | 52 | 30% |
| Ianniello et al., 2016 (88) | 177Lu-Dotatate | 34; 15 TC; 19 AC | 34 (100.0) | 18.5 overall; 20.1 TC; 15.7 AC | 48.6 overall; 48.6 TC; 37 AC | 33% TC; 0% AC |
| Mirvis et al., 2020 (89) | 177Lu-Dotatate or 90Yttrium Dotatate | 25; 11 TC; 11 AC; 3 unknown | 25 (100.0) | 17 (177Lu-Dotatate); 12 (90Yttrium; Dotatate) | 42 | 30% |
| Lim et al., 2020 (90) | 177Lu-Dotatate | 48; 15 TC; 32 AC; 1 unknown | 48 (100.0) | NR | 49 | 33% |
| Zidan et al., 2022 (91) | 177Lu-Dotatate | 48; 5 TC; 43 AC | 48 (100.0) | 23 | 59 | 20% |
| Mariniello et al., 2016 (92) | 177Lu-Dotatate and/or 90Yttrium Dotatoc | 114 | 114 (100.0) | 28 | 58.8 | 26.5% |
| Sabet et al., 2017 (93) | 177Lu-Dotatate | 22 | 22 (100.0) | 27 | 42 | 27% |
PC, pulmonary carcinoid; PFS, progression free survival; OS, overall survival; RR, response rate; TC, typical carcinoid; AC, atypical carcinoid.
The prognostic factors which appear to be associated with shorter benefit from PRRT for patients with PCs include FDG avid tumors and higher tumor Ki-67 index (88,89). A recent publication suggests patients who are heavily pretreated and possess greater baseline disease bulk have reduced benefit from PRRT (94). Though rare, one of the long-term concerns with PRRT is the risk of myelodysplasia and acute myeloid leukemia which occur in approximately 2% of patients (95,96).
A crucial prospective randomized trial of 177Lu-Dotatate has recently opened to accrual [NCT04665739]. In this phase II study, 108 patients with SSTR positive PCs (TC or AC) will be randomized to 4 cycles of 177Lu-Dotatate or everolimus. If this study meets its primary endpoint of PFS, it is likely that 177Lu-Dotatate will garner regulatory licensure for PCs.
Several ongoing trials are exploring novel forms of PRRT such as targeted alpha therapy (TAT) in patients with PCs. One type of TAT is 212Lead-DOTAMTATE which is being tested in an ongoing phase I study in PRRT naïve patients with SSTR expression [NCT03466216]. From the preliminary results, among 10 patients treated at the highest dose level (4 with PCs), the ORR was 80% (97). Another TAT which has been tested and demonstrated clinical promise in patients with GEPNETs, but not yet in PC, is 225Actinium-DOTATOC (98).
Radiation therapy
Stereotactic body radiation therapy (SBRT) has become increasingly used as a form of definitive therapy for patients with PCs based on data from NSCLC where it shows better local control than conventional radiation therapy (99,100). The initial case series describing the experience with SBRT in PCs was from a single institution (101). In this series, 4 patients (2 TC, 2 AC) with unresectable disease were treated; 3 patients underwent treatment to a single lesion while 1 patient underwent treatment to 2 lesions. Patients were treated with either 54 Gy in 3 fractions or 50 Gy in 5 fractions. All patients achieved disease control of the treated lesions however 1 patient developed distant disease progression within 2 months. Another single institution analysis reported on the experience of 10 patients with inoperable PCs (9 TC, 1 AC), with no extra-thoracic disease, treated with SBRT (102). The median dose was 50 Gy in 5–10 fractions. After a median follow up period of 25 months, 4 patients were still alive. Median OS was 27.1 months. Another series of 7 patients with PCs (6 TC, 1 AC; 5 with distant extra-thoracic disease in the liver), were treated with doses of 42–60 Gy in 5–8 fractions (103). Among the patients with distant disease, SBRT was utilized for a growing primary tumor. After a median follow up period of 13.8 months, local control was achieved in all 7 patients (ORR of 85.7%) with 5 patients still alive at last follow-up.
A national cancer database analysis evaluated outcomes in patients with TCs treated with SBRT and conventionally fractionated therapy (104). Of 154 included patients, 84 received SBRT (median dose of 50 Gy in 4 fractions) while 70 received conventional therapy (median dose of 54 Gy in 24 fractions). The OS of patients, after propensity matching, was 66 months in the SBRT arm vs. 58 months in the conventional group (P=0.034). Though prospective experience with external beam radiation is needed for patients with PCs, it appears that radiation therapy is a reasonable approach to achieve local control in patients with inoperable disease or growing oligoprogressive disease. SBRT is more convenient due to reduced dose fractionation and appears to be at least equally effective.
Follow up and surveillance
Patients should be surveilled after definitive treatment. Both TC and AC patients will generally be followed with CT imaging at 6-month intervals for at least 2 years and then yearly for at least 10 years. There is not currently a role to surveil with Dotatate PET/CT. Figure 4 shows a suggested workup and treatment algorithm.
DIPNECH
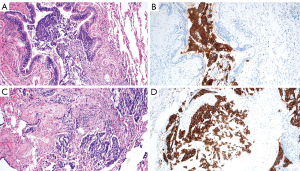
Multifocal hyperplasia of pulmonary neuroendocrine cells (PNEC) was first described by Aguayo et al. in 1992 in six patients had multifocal PNEC hyperplasia associated with peribronchiolar fibrosis (105). PNECs are found throughout the lungs, and it represents <1% of the cells in adult human lungs (99). The 2001 WHO classification of lung tumors termed this entity DIPNECH (106). DIPNECH is characterized by proliferation of PNEC, believed to occur in response to a lung injury (hypoxia, chronic obstructive pulmonary disease) or idiopathic (105,107). DIPNECH is characterized by proliferation of neuroendocrine cells and/or carcinoid tumorlets involving small airways (Figure 5A,5B). The neuroendocrine cells have round, oval, or spindle-shaped nuclei with speckled chromatin and a moderate amount of amphophilic or eosinophilic cytoplasm. In hyperplasia, neuroendocrine cells are limited to the bronchiolar mucosa. Nodular proliferations of neuroendocrine cells that invade beyond the bronchiolar wall measuring <5 mm are classified as tumorlets, whereas those that are 5 mm or larger are categorized as a carcinoid tumor (108-110). Marchevsky et al. suggested that DIPNECH be defined by the presence of multifocal PNEC hyperplasia associated with ≥3 small airways (111). The neuroendocrine cells generally stain positive by immunohistochemistry for chromogranin, synaptophysin, CD56, and INSM1 (112,113). In DIPNECH syndrome, bronchiolar fibrosis with luminal narrowing or constrictive bronchiolitis is present (Figure 5C,5D). NSCLC may also be found in DIPNECH, emphasizing the importance of histologic examination of growing nodules (110). DIPNECH is more common in females, with reports of a female to male ratio of 10:1 (35,111,114,115). Patients are usually non-smokers in their fifth to the sixth decade of (35,109,115-117).
The common symptoms of DIPNECH are chronic nonproductive cough, dyspnea on exertion, and wheezing (35,56,105,109,115). Approximately 45% to 60% of patients have been diagnosed with another lung disease due to the rarity of this disease and lack of recognition (114,115,118). PFTs often demonstrate obstructive or mixed obstructive with restrictive ventilatory abnormality, whereas a normal or entirely restrictive pattern is uncommon (109,114,115). The clinical course is often long-term stability or slow progressive functional decline or changes in PFTs. There have been reports of rapid worsening of severe obstruction, usually due to constrictive bronchiolitis (35,114,115). Studies have demonstrated that as nodules increase in number and size, there is a parallel decrease in FEV1 and forced vital capacity (112,114).
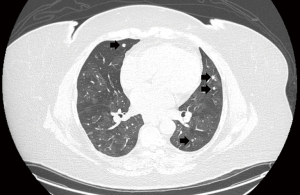
High suspicion of DIPNECH can be made by CT of the chest followed by confirmation via lung biopsy. Common CT findings are a constrictive bronchiolitis pattern with scattered pulmonary nodules, mosaic attenuation with air trapping, patchy ground-glass opacities, or endobronchial wall thickening (35,113,114,115,119). Sazonova et al. proposed that the most significant predictor of DIPNECH on CT are pulmonary nodules and mosaicism (119). The nodules may be of varying sizes, round to ovoid with a solid density, usually multiple, associated with one or more dominant lesions, which is often a carcinoid tumor (110,120). Most of the nodules are in a centrilobular distribution along with small bronchovascular bundles and are lower lobe predominant (112). SSTR expression through functional imaging can be seen; however, uptake may be limited due to the small nodules (113,118). Figure 6 shows a CT image of a patient with DIPNECH.
Surgical lung biopsy or resection remain the gold standard for histologic diagnosis of DIPNECH; a definitive pathological diagnosis cannot be made on small biopsies (109,115). It is vital to provide adequate tissue specimens to ascertain any associated neuroendocrine cell proliferation (82,106,112,121). Progression of DIPNECH to PC with associated metastases is low but has been reported (111,122). Therefore, routine CT surveillance every 12 to 24 months is recommended (123).
Most proposed medical treatments are based on reports of clinical experience and retrospective data. As most patients have a varying degree of respiratory symptoms with some abnormalities on PFTs, a few reports described the potential benefits of systemic steroids (SS) and inhaled corticosteroids (ICS). There is no clear evidence to demonstrate the efficacy of SS or ICS. Nassar et al. reported that four out of seven patients who received SS had a clinical benefit and two remained stable (35). Thus, the use of SS is controversial and patients should have close follow-up to evaluate efficacy.
The National Comprehensive Cancer Network (NCCN) NET Guidelines recommend SSAs such as octreotide or lanreotide for respiratory symptom control (45). Al-Toubah et al. reviewed 42 patients with DIPNECH; 11 received SSAs as monotherapy, 31 received SSAs in combination with other therapies (bronchodilators, steroids, and benzonatate). Thirty-three out of 42 patients experienced symptomatic improvement. Among patients who had pre-and post-treatment PFT, 14 out of 15 patients (93%) demonstrated improved FEV1 (118). These results are similar to other smaller studies (113,124). Another potential treatment is targeted therapy using an mTOR inhibitor such as everolimus or sirolimus, as DIPNECH has been found to exhibit activation of the mTOR pathway (125). There is no substantial data on the efficacy of mTOR inhibitors to advocate its use with DIPNECH as studies have not demonstrated symptomatic improvement or changes of FEV1 other than improved radiographic findings (117,126). Lastly, there are case reports utilizing lung transplantation as primary treatment for DIPNECHs (116,127).
The long-term outcomes for patients with DIPNECH are limited. Most patients remain stable while approximately 30% experience clinical or radiographic progression, and 11% demonstrate clinical or radiologic improvement. Deteriorating PFTs are often related to worsening obliterative bronchiolitis and/or other fibrotic lung changes. Mortality in patients with DIPNECH has been attributed to transplant-associated complications and, rarely, progression of DIPNECH (105,114,116). Table 4 lists selected studies in DIPNECH.
Table 4
| Author (year) | N | Main symptoms [%] | PFTs [%] | CT chest findings [%] | Other imaging [%] | Treatment [%] | Clinical outcomes [%] |
|---|---|---|---|---|---|---|---|
| Almquist et al., 2021 (56) | 59 | Cough [71] | Obstructive physiology [most common] | Bilateral nodules [93] | N/A | ICS + inhaled bronchodilator [80] | Symptomatic improvement: |
| Dyspnea on exertion [44] | Mosaic attenuation [70] | Systemic steroid [49] | • General treatment group [18] | ||||
| Dyspnea [34] | SSA [15] | • SSA group [56] | |||||
| Improvement in PFT in SSA group [33] | |||||||
| Carr et al., 2015 (114) | 30 | Chronic cough [27] | Obstructive [87] | Pulmonary nodules [100] | N/A | Oral steroids [46] | Improved cough with octreotide [27] |
| Dyspnea [20] | Mixed [13] | Air trapping [96] | ICS [67] | ||||
| Both chronic cough and dyspnea [43] | Mosaic attenuation [84] | Octreotide long-acting release [37] | |||||
| Bronchial wall thickening [80.1] | |||||||
| Samhouri et al., 2020 (115) | 44 | Cough [68] | Obstructive [52] | Bilateral nodules [95] | N/A | Observation [25] | Stable [77] |
| Dyspnea [30] | Restrictive [11] | Mosaic attenuation [77] | Inhaled bronchodilator [55] | Symptomatic improvement [20] | |||
| Mixed [9] | ICS [41] | Symptomatic progression [3] | |||||
| Nonspecific [23] | Systemic steroids [2] | ||||||
| Normal [5] | Octreotide [32] | ||||||
| Myint et al., 2018 (117) | 13 | Cough [69] | N/A | Pulmonary nodules [85] | N/A | SSA and azithromycin [31] | Improved cough [54] |
| Dyspnea [62] | Diffuse ground glass [15] | Octreotide alone [23] | Improved dyspnea [8] | ||||
| Mosaicism [8] | Azithromycin alone [23] | ||||||
| Air trapping [8] | Octreotide and everolimus [8] | ||||||
| Nodular opacities [8] | Observation [15] | ||||||
| Al-Toubah et al., 2020 (118) | 42 | Cough [80.9] | Mild impairment [53] | Pulmonary nodules [100] | 111In-DPTA scintigraphy or 68Ga-DOTATATE PET/CT [45] | SSA | Mild symptoms improvement [36] |
| Dyspnea [64.2] | Moderate impairment [20] | Some level of positive uptake [63] | Octreotide [83] | Moderate symptoms improvement [14] | |||
| Severe impairment [7] | Lanreotide [17] | Significant symptoms improvement [26] | |||||
| Normal [20] | Concomitant inhalers, systemic steroids, benzonatate [74] | No improvement [24] | |||||
| Improvement in PFTs [93] | |||||||
| Chauhan et al. 2015 (124) | 5 | Cough [100] | N/A | N/A | N/A | Octreotide [80] | Improved cough [80] |
| Sun et al., 2022 (128) | 33 | Cough [66.7] | Obstructive [31] | Bilateral lung nodules [80] | N/A | Beta-adrenergic and/or ICS [36.4] | Symptomatic improvement [88.9], including all who received SSA therapy |
| Dyspnea [54.5] | Restrictive [13.8] | Mosaic attenuation [50] | SSA [24.2] | ||||
| Asymptomatic [21.2] | Mixed [10.3] | Air trapping [26.7] | Systemic steroids [12.1] | ||||
| Bronchial wall thickening [23.3] | Active imaging surveillance [12.1] |
PFTs, pulmonary function tests; CT, computed tomography; SSA, somatostatin analog; 111In-DPTA, In-111-diethylenetriaminepentaacetic acid (DTPA)-octreotide; PET, positron emission tomography; ICS, inhaled corticosteroids.
Conclusions
PCs represent a challenge to thoracic and neuroendocrine practitioners. In the early stage, surgical resection remains the standard of care for definitive treatment. Local therapies, including EBT and SBRT, continue to evolve but should be considered reasonable alternatives in non-surgical candidates. In advanced disease, approved options continue to be limited, however, many strategies are promising, including PRRT. DIPNECH remains a commonly misdiagnosed entity but is garnering more awareness and treatment strategies. Ultimately, the goal to move this field forward is dependent on increasing awareness of these rare diseases and clinical trial development. Wherever possible, we recommend that patients be evaluated in a multidisciplinary setting at a high-volume NET center.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-415/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-415/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-415/coif). The authors have no conflicts of interest to declare. RAR serves as an unpaid editorial board member of Translational Lung Cancer Research from January 2022 to December 2023. RAR has been a consultant for Amgen, Ipsen Biopharmaceuticals, Novartis, Advanced Accelerator Applications, Curium Pharma, EMD Serono, Astra-Zeneca. He has also received research funding (to institution) from Merck & Co and Aadi Biosciences. He is also on the speaker bureaus for Ipsen Biopharmaceuticals and Astra-Zeneca. He is a member of the North American Neuroendocrine Tumor Society-Board of Directors. SD has been a consultant for Ipsen Biopharmaceuticals, Novartis, and TerSera. EAG has been a consultant for Astra-Zeneca and is on the speaker bureau for Intuitive Surgical and ASCO. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine Tumors of the Lung: Current Challenges and Advances in the Diagnosis and Management of Well-Differentiated Disease. J Thorac Oncol 2017;12:425-36. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. Erratum in: CA Cancer J Clin 2021;71:359. [Crossref] [PubMed]
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Yoon JY, Sigel K, Martin J, et al. Evaluation of the Prognostic Significance of TNM Staging Guidelines in Lung Carcinoid Tumors. J Thorac Oncol 2019;14:184-92. [Crossref] [PubMed]
- Abdel-Rahman O. Modified staging system for pulmonary carcinoids on the basis of lung cancer TNM system. Clin Transl Oncol 2018;20:670-7. [Crossref] [PubMed]
- Öberg K, Hellman P, Ferolla P, et al. Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii120-3. [Crossref] [PubMed]
- Phan AT, Oberg K, Choi J, et al. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas 2010;39:784-98. [Crossref] [PubMed]
- Gosain R, Mukherjee S, Yendamuri SS, et al. Management of Typical and Atypical Pulmonary Carcinoids Based on Different Established Guidelines. Cancers (Basel) 2018;10:510. [Crossref] [PubMed]
- Rubin de Celis Ferrari AC, Glasberg J, Riechelmann RP. Carcinoid syndrome: update on the pathophysiology and treatment. Clinics (Sao Paulo) 2018;73:e490s. [Crossref] [PubMed]
- Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. [Crossref] [PubMed]
- Halperin DM, Shen C, Dasari A, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol 2017;18:525-34. [Crossref] [PubMed]
- Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, et al. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion: the University of Texas MD Anderson Cancer Center Experience. Cancer 2011;117:4381-9. [Crossref] [PubMed]
- Fainstein Day P, Frohman L, Garcia Rivello H, et al. Ectopic growth hormone-releasing hormone secretion by a metastatic bronchial carcinoid tumor: a case with a non hypophysial intracranial tumor that shrank during long acting octreotide treatment. Pituitary 2007;10:311-9. [Crossref] [PubMed]
- Limaiem F, Tariq MA, Wallen JM. Lung Carcinoid Tumors. 2022 Oct 11. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
- Carter BW, Benveniste MF, Betancourt SL, et al. Imaging Evaluation of Malignant Chest Wall Neoplasms. Radiographics 2016;36:1285-306. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Breast Cancer. Version2.2022. © National Comprehensive Cancer Network, Inc. 2022.
- Benson RE, Rosado-de-Christenson ML, Martínez-Jiménez S, et al. Spectrum of pulmonary neuroendocrine proliferations and neoplasms. Radiographics 2013;33:1631-49. [Crossref] [PubMed]
- Krausz Y, Freedman N, Rubinstein R, et al. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: comparison with 111In-DTPA-octreotide (OctreoScan®). Mol Imaging Biol 2011;13:583-93. [Crossref] [PubMed]
- Reubi JC, Schär JC, Waser B, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 2000;27:273-82. [Crossref] [PubMed]
- Froelich MF, Schnitzer ML, Holzgreve A, et al. Cost-Effectiveness Analysis of 68Ga DOTA-TATE PET/CT, 111In-Pentetreotide SPECT/CT and CT for Diagnostic Workup of Neuroendocrine Tumors. Diagnostics (Basel) 2021;11:334. [Crossref] [PubMed]
- Shi W, Johnston CF, Buchanan KD, et al. Localization of neuroendocrine tumours with [111In] DTPA-octreotide scintigraphy (Octreoscan): a comparative study with CT and MR imaging. QJM 1998;91:295-301. [Crossref] [PubMed]
- Pfeifer A, Knigge U, Binderup T, et al. 64Cu-DOTATATE PET for Neuroendocrine Tumors: A Prospective Head-to-Head Comparison with 111In-DTPA-Octreotide in 112 Patients. J Nucl Med 2015;56:847-54. [Crossref] [PubMed]
- Papaporfyriou A, Domayer J, Meilinger M, et al. Bronchoscopic diagnosis and treatment of endobronchial carcinoid: case report and review of the literature. Eur Respir Rev 2021;30:200115. [Crossref] [PubMed]
- Todd TR, Cooper JD, Weissberg D, et al. Bronchial carcinoid tumors: twenty years' experience. J Thorac Cardiovasc Surg 1980;79:532-6. [Crossref] [PubMed]
- Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001;119:1647-51. [Crossref] [PubMed]
- Divisi D, Crisci R. Carcinoid tumors of the lung and multimodal therapy. Thorac Cardiovasc Surg 2005;53:168-72. [Crossref] [PubMed]
- Kurul IC, Topçu S, Taştepe I, et al. Surgery in bronchial carcinoids: experience with 83 patients. Eur J Cardiothorac Surg 2002;21:883-7. [Crossref] [PubMed]
- Aron M, Kapila K, Verma K. Carcinoid tumors of the lung: a diagnostic challenge in bronchial washings. Diagn Cytopathol 2004;30:62-6. [Crossref] [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6:S99-S107. [PubMed]
- Papotti M, Brambille E, Dingemans AC, et al. WHO Classification of Tumours: Thoracic Tumors. 5th ed. Lyon, France: WHO Press; 2021.
- Fujino K, Motooka Y, Hassan WA, et al. Insulinoma-Associated Protein 1 Is a Crucial Regulator of Neuroendocrine Differentiation in Lung Cancer. Am J Pathol 2015;185:3164-77. [Crossref] [PubMed]
- Walts AE, Ines D, Marchevsky AM. Limited role of Ki-67 proliferative index in predicting overall short-term survival in patients with typical and atypical pulmonary carcinoid tumors. Mod Pathol 2012;25:1258-64. [Crossref] [PubMed]
- Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol 2015;26:1604-20. [Crossref] [PubMed]
- Filosso PL, Rena O, Donati G, et al. Bronchial carcinoid tumors: surgical management and long-term outcome. J Thorac Cardiovasc Surg 2002;123:303-9. [Crossref] [PubMed]
- Nassar AA, Jaroszewski DE, Helmers RA, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: a systematic overview. Am J Respir Crit Care Med 2011;184:8-16. [Crossref] [PubMed]
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg 2011;142:1412-7. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [Crossref] [PubMed]
- Fox M, Van Berkel V, Bousamra M 2nd, et al. Surgical management of pulmonary carcinoid tumors: sublobar resection versus lobectomy. Am J Surg 2013;205:200-8. [Crossref] [PubMed]
- Afoke J, Tan C, Hunt I, et al. Is sublobar resection equivalent to lobectomy for surgical management of peripheral carcinoid? Interact Cardiovasc Thorac Surg 2013;16:858-63. [Crossref] [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Yang CF, D'Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [Crossref] [PubMed]
- Katz MHG, Francescatti AB, Hunt KK, et al. Technical Standards for Cancer Surgery: Commission on Cancer Standards 5.3-5.8. Ann Surg Oncol 2022;29:6549-58. [Crossref] [PubMed]
- Shah MH, Goldner WS, Benson AB, et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:839-68. [Crossref] [PubMed]
- Singh S, Bergsland EK, Card CM, et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients With Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines. J Thorac Oncol 2020;15:1577-98. [Crossref] [PubMed]
- Hilal T. Current understanding and approach to well differentiated lung neuroendocrine tumors: an update on classification and management. Ther Adv Med Oncol 2017;9:189-99. [Crossref] [PubMed]
- Aydin E, Yazici U, Gulgosteren M, et al. Long-term outcomes and prognostic factors of patients with surgically treated pulmonary carcinoid: our institutional experience with 104 patients. Eur J Cardiothorac Surg 2011;39:549-54. [Crossref] [PubMed]
- Bini A, Brandolini J, Cassanelli N, et al. Typical and atypical pulmonary carcinoids: our institutional experience. Interact Cardiovasc Thorac Surg 2008;7:415-8. [Crossref] [PubMed]
- Reuling EMBP, Dickhoff C, Plaisier PW, et al. Endobronchial Treatment for Bronchial Carcinoid: Patient Selection and Predictors of Outcome. Respiration 2018;95:220-7. [Crossref] [PubMed]
- DiBardino DM, Lanfranco AR, Haas AR. Bronchoscopic Cryotherapy. Clinical Applications of the Cryoprobe, Cryospray, and Cryoadhesion. Ann Am Thorac Soc 2016;13:1405-15. [Crossref] [PubMed]
- Amoils SP. The Joule Thomson cryoprobe. Arch Ophthalmol 1967;78:201-7. [Crossref] [PubMed]
- Perikleous P, Mayer N, Finch J, et al. Treatment of Pulmonary Carcinoid Tumors With Bronchoscopic Cryotherapy: A 28-Year Single-center Experience. J Bronchology Interv Pulmonol 2022;29:71-82. [Crossref] [PubMed]
- Luckraz H, Amer K, Thomas L, et al. Long-term outcome of bronchoscopically resected endobronchial typical carcinoid tumors. J Thorac Cardiovasc Surg 2006;132:113-5. [Crossref] [PubMed]
- Bertoletti L, Elleuch R, Kaczmarek D, et al. Bronchoscopic cryotherapy treatment of isolated endoluminal typical carcinoid tumor. Chest 2006;130:1405-11. [Crossref] [PubMed]
- Almquist DR, Sonbol MB, Ross HJ, et al. Clinical Characteristics of Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia: A Retrospective Analysis. Chest 2021;159:432-4. [Crossref] [PubMed]
- Neyman K, Sundset A, Naalsund A, et al. Endoscopic treatment of bronchial carcinoids in comparison to surgical resection: a retrospective study. J Bronchology Interv Pulmonol 2012;19:29-34. [Crossref] [PubMed]
- Cavaliere S, Foccoli P, Toninelli C. Curative bronchoscopic laser therapy for surgically resectable tracheobronchial tumors: personal experience. J Bronchol 2002;9:90-5. [Crossref]
- Brokx HA, Paul MA, Postmus PE, et al. Long-term follow-up after first-line bronchoscopic therapy in patients with bronchial carcinoids. Thorax 2015;70:468-72. [Crossref] [PubMed]
- van Boxem TJ, Venmans BJ, van Mourik JC, et al. Bronchoscopic treatment of intraluminal typical carcinoid: a pilot study. J Thorac Cardiovasc Surg 1998;116:402-6. [Crossref] [PubMed]
- Baudin E, Caplin M, Garcia-Carbonero R, et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2021;32:439-51. Erratum in: Ann Oncol 2021;32:1453-5. [Crossref] [PubMed]
- Bousquet C, Lasfargues C, Chalabi M, et al. Clinical review: Current scientific rationale for the use of somatostatin analogs and mTOR inhibitors in neuroendocrine tumor therapy. J Clin Endocrinol Metab 2012;97:727-37. [Crossref] [PubMed]
- Aparicio T, Ducreux M, Baudin E, et al. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer 2001;37:1014-9. [Crossref] [PubMed]
- Ducreux M, Ruszniewski P, Chayvialle JA, et al. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am J Gastroenterol 2000;95:3276-81. [Crossref] [PubMed]
- Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224-33. [Crossref] [PubMed]
- Reidy-Lagunes D, Kulke M, Wolin E, et al. Tumor growth rate (TGR) in intestinal/pancreatic neuroendocrine tumors: post hoc SPINET: a randomized, double-blind, placebo-controlled phase III study of lanreotide autogel/depot (LAN) in patients with advanced lung neuroendocrine tumors. Presented at: the 2016 North American Neuroendocrine Tumor Society meeting; September 30-October 1; Jackson, Wyoming. Abstract 156.
- Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011;378:2005-12. [Crossref] [PubMed]
- Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016;387:968-77. [Crossref] [PubMed]
- Fazio N, Buzzoni R, Delle Fave G, et al. Everolimus in advanced, progressive, well-differentiated, non-functional neuroendocrine tumors: RADIANT-4 lung subgroup analysis. Cancer Sci 2018;109:174-181. [Crossref] [PubMed]
- Pusceddu S, Lo Russo G, Macerelli M, et al. Diagnosis and management of typical and atypical lung carcinoids. Crit Rev Oncol Hematol 2016;100:167-76. [Crossref] [PubMed]
- Forde PM, Hooker CM, Boikos SA, et al. Systemic therapy, clinical outcomes, and overall survival in locally advanced or metastatic pulmonary carcinoid: a brief report. J Thorac Oncol 2014;9:414-8. [Crossref] [PubMed]
- Chong CR, Wirth LJ, Nishino M, et al. Chemotherapy for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer 2014;86:241-6. [Crossref] [PubMed]
- Wirth LJ, Carter MR, Jänne PA, et al. Outcome of patients with pulmonary carcinoid tumors receiving chemotherapy or chemoradiotherapy. Lung Cancer 2004;44:213-20. [Crossref] [PubMed]
- Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res 2007;13:2986-91. [Crossref] [PubMed]
- Papaxoinis G, Kordatou Z, McCallum L, et al. Capecitabine and Temozolomide in Patients with Advanced Pulmonary Carcinoid Tumours. Neuroendocrinology 2020;110:413-21. [Crossref] [PubMed]
- Ott PA, Bang YJ, Piha-Paul SA, et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J Clin Oncol 2019;37:318-27. [Crossref] [PubMed]
- Yao JC, Strosberg J, Fazio N, et al. Spartalizumab in metastatic, well/poorly differentiated neuroendocrine neoplasms. Endocr Relat Cancer 2021;28:161-72. [Crossref] [PubMed]
- Klein O, Kee D, Markman B, et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin Cancer Res 2020;26:4454-9. [Crossref] [PubMed]
- Patel SP, Othus M, Chae YK, et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin Cancer Res 2020;26:2290-6. [Crossref] [PubMed]
- Scott SC, De Jesus-Acosta A, Hu C, et al. Dual immune checkpoint blockade in advanced well-differentiated neuroendocrine tumors, a phase II clinical trial. J Clin Oncol 2021;39:e16201. [Crossref]
- Dasari A, Kauh JS, Tucci C, et al. An open-label phase 1 b/2 study of surufatinib in combination with tislelizumab in subjects with advanced solid tumors. J Clin Oncol 2021; [Crossref]
- Das S, Al-Toubah T, El-Haddad G, et al. 177Lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev Gastroenterol Hepatol 2019;13:1023-31. [Crossref] [PubMed]
- Haider M, Das S, Al-Toubah T, et al. Somatostatin receptor radionuclide therapy in neuroendocrine tumors. Endocr Relat Cancer 2021;28:R81-93. [Crossref] [PubMed]
- Food and Drug Administration. Highlights of Prescribing Information. Accessed 3/31/2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208700s000lbl.pdf
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125-35. [Crossref] [PubMed]
- Brabander T, van der Zwan WA, Teunissen JJM, et al. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res 2017;23:4617-24. [Crossref] [PubMed]
- Vyakaranam AR, Crona J, Norlén O, et al. Favorable Outcome in Patients with Pheochromocytoma and Paraganglioma Treated with 177Lu-DOTATATE. Cancers (Basel) 2019;11:909. [Crossref] [PubMed]
- Ianniello A, Sansovini M, Severi S, et al. Peptide receptor radionuclide therapy with (177)Lu-DOTATATE in advanced bronchial carcinoids: prognostic role of thyroid transcription factor 1 and (18)F-FDG PET. Eur J Nucl Med Mol Imaging 2016;43:1040-6. [Crossref] [PubMed]
- Mirvis E, Toumpanakis C, Mandair D, et al. Efficacy and tolerability of peptide receptor radionuclide therapy (PRRT) in advanced metastatic bronchial neuroendocrine tumours (NETs). Lung Cancer 2020;150:70-5. [Crossref] [PubMed]
- Lim LE, Chan DL, Thomas D, et al. Australian experience of peptide receptor radionuclide therapy in lung neuroendocrine tumours. Oncotarget 2020;11:2636-46. [Crossref] [PubMed]
- Zidan L, Iravani A, Oleinikov K, et al. Efficacy and Safety of 177Lu-DOTATATE in Lung Neuroendocrine Tumors: A Bicenter study. J Nucl Med 2022;63:218-25. [Crossref] [PubMed]
- Mariniello A, Bodei L, Tinelli C, et al. Long-term results of PRRT in advanced bronchopulmonary carcinoid. Eur J Nucl Med Mol Imaging 2016;43:441-52. [Crossref] [PubMed]
- Sabet A, Haug AR, Eiden C, et al. Efficacy of peptide receptor radionuclide therapy with 177Lu-octreotate in metastatic pulmonary neuroendocrine tumors: a dual-centre analysis. Am J Nucl Med Mol Imaging 2017;7:74-83. [PubMed]
- Das S, Chauhan A, Du L, et al. External Validation of a Clinical Score for Patients With Neuroendocrine Tumors Under Consideration for Peptide Receptor Radionuclide Therapy. JAMA Netw Open 2022;5:e2144170. [Crossref] [PubMed]
- Sonbol MB, Halfdanarson TR, Hilal T. Assessment of Therapy-Related Myeloid Neoplasms in Patients With Neuroendocrine Tumors After Peptide Receptor Radionuclide Therapy: A Systematic Review. JAMA Oncol 2020;6:1086-92. [Crossref] [PubMed]
- Das S, Berlin J, Savona M. Pancytopenia in a Patient With Metastatic Well-Differentiated Neuroendocrine Tumor After Peptide Receptor Radionuclide Therapy. JAMA Oncol 2021;7:1060-1. [Crossref] [PubMed]
- Delpassand ES, Tworowska I, Esfandiari R, et al. Targeted α-Emitter Therapy with 212Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial. J Nucl Med 2022;63:1326-33. [Crossref] [PubMed]
- Ballal S, Yadav MP, Bal C, et al. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging 2020;47:934-46. [Crossref] [PubMed]
- Haque W, Verma V, Polamraju P, et al. Stereotactic body radiation therapy versus conventionally fractionated radiation therapy for early stage non-small cell lung cancer. Radiother Oncol 2018;129:264-9. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Colaco RJ, Decker RH. Stereotactic radiotherapy in the treatment of primary bronchial carcinoid tumor. Clin Lung Cancer 2015;16:e11-4. [Crossref] [PubMed]
- Singh DP, Cummings MA, Chen Y, et al. Inoperable Pulmonary Carcinoid Tumors: Local control rates with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2019;104:253. [Crossref] [PubMed]
- Thomas K, Smith C, Marsala A, et al. The use of stereotactic body radiotherapy in pulmonary carcinoid tumors: a case series. Precis Cancer Med 2021;4:11. [Crossref]
- Wegner RE, Abel S, Horne ZD, et al. Stereotactic body radiation therapy versus fractionated radiation therapy for early-stage bronchopulmonary carcinoid. Lung Cancer Manag 2019;8:LMT14. [Crossref] [PubMed]
- Aguayo SM, Miller YE, Waldron JA Jr, et al. Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med 1992;327:1285-8. [Crossref] [PubMed]
- Brambilla E, Travis WD, Colby TV, et al. The new World Health Organization classification of lung tumours. Eur Respir J 2001;18:1059-68. [Crossref] [PubMed]
- Gould VE, Linnoila RI, Memoli VA, et al. Neuroendocrine components of the bronchopulmonary tract: hyperplasias, dysplasias, and neoplasms. Lab Invest 1983;49:519-37. [PubMed]
- Baniak NM, Wilde B, Kanthan R. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH)--An uncommon precursor of a common cancer?. Pathol Res Pract 2016;212:125-9. [Crossref] [PubMed]
- Davies SJ, Gosney JR, Hansell DM, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: an under-recognised spectrum of disease. Thorax 2007;62:248-52. [Crossref] [PubMed]
- Rossi G, Cavazza A, Spagnolo P, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia syndrome. Eur Respir J 2016;47:1829-41. [Crossref] [PubMed]
- Marchevsky AM, Wirtschafter E, Walts AE. The spectrum of changes in adults with multifocal pulmonary neuroendocrine proliferations: what is the minimum set of pathologic criteria to diagnose DIPNECH? Hum Pathol 2015;46:176-81. [Crossref] [PubMed]
- Little BP, Junn JC, Zheng KS, et al. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia: Imaging and Clinical Features of a Frequently Delayed Diagnosis. AJR Am J Roentgenol 2020;215:1312-20. [Crossref] [PubMed]
- Gorshtein A, Gross DJ, Barak D, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia and the associated lung neuroendocrine tumors: clinical experience with a rare entity. Cancer 2012;118:612-9. [Crossref] [PubMed]
- Carr LL, Chung JH, Duarte Achcar R, et al. The clinical course of diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. Chest 2015;147:415-22. [Crossref] [PubMed]
- Samhouri BF, Azadeh N, Halfdanarson TR, et al. Constrictive bronchiolitis in diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. ERJ Open Res 2020;6:e00527-2020. [Crossref] [PubMed]
- Wirtschafter E, Walts AE, Liu ST, et al. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia of the Lung (DIPNECH): Current Best Evidence. Lung 2015;193:659-67. [Crossref] [PubMed]
- Myint ZW, McCormick J, Chauhan A, et al. Management of Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia: Review and a Single Center Experience. Lung 2018;196:577-81. [Crossref] [PubMed]
- Al-Toubah T, Strosberg J, Halfdanarson TR, et al. Somatostatin Analogs Improve Respiratory Symptoms in Patients With Diffuse Idiopathic Neuroendocrine Cell Hyperplasia. Chest 2020;158:401-5. [Crossref] [PubMed]
- Sazonova O, Manem V, Béland C, et al. Development and Validation of Diffuse Idiopathic Pulmonary Neuroendocrine Hyperplasia Diagnostic Criteria. JTO Clin Res Rep 2020;1:100078. [Crossref] [PubMed]
- Lee JS, Brown KK, Cool C, et al. Diffuse pulmonary neuroendocrine cell hyperplasia: radiologic and clinical features. J Comput Assist Tomogr 2002;26:180-4. [Crossref] [PubMed]
- Timmerman RD, Hu C, Michalski JM, et al. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:1287-8. [Crossref] [PubMed]
- Flint K, Ye C, Henry TL. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia (DIPNECH) with liver metastases. BMJ Case Rep 2019;12:228536. [Crossref] [PubMed]
- Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw 2018;16:693-702. [Crossref] [PubMed]
- Chauhan A, Ramirez RA. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia (DIPNECH) and the Role of Somatostatin analogs: A Case Series. Lung 2015;193:653-7. [Crossref] [PubMed]
- Rossi G, Cavazza A, Graziano P, et al. mTOR/p70S6K in diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. Am J Respir Crit Care Med 2012;185:341-author reply 341-2. [Crossref] [PubMed]
- Russier M, Plantier L, Derot G, et al. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia Syndrome Treated With Sirolimus. Ann Intern Med 2018;169:197-8. [Crossref] [PubMed]
- Zhou H, Ge Y, Janssen B, et al. Double lung transplantation for diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. J Bronchology Interv Pulmonol 2014;21:342-5. [Crossref] [PubMed]
- Sun TY, Hwang G, Pancirer D, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: clinical characteristics and progression to carcinoid tumour. Eur Respir J 2022;59:2101058. [Crossref] [PubMed]


