
Alectinib as first-line treatment for advanced ALK-positive non-small cell lung cancer in the real-world setting: preliminary analysis in a Chinese cohort
Highlight box
Key findings
• First-line alectinib showed substantial efficacy and an excellent safety profile in a real-world setting of Chinese NSCLC patients.
• Patients carrying shorter variants, with metastases in ≥3 distant organs and a tumor reduction rate ≤50% demonstrated more unfavorable mPFS than their counterparts.
What is known and what is new?
• Great efficacy and safety outcomes of alectinib were also substantiated in real-world setting. Multiple predictive factors to PFS of first-line alectinib were also established.
• This was the first research to comprehensively analyze the clinical outcomes of first-line alectinib in in a real-world setting of Chinese population.
What is the implication, and what should change now?
• Tumors with shorter EML4 fusion variants, more extensive metastases and less reduction in tumor lesions may require more aggressive management strategies.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide (1) and ≈85% of reported cases are non-small cell lung cancer (NSCLC), mostly of adenocarcinoma histology. In the past few decades, several oncogenic driver mutations have been identified in NSCLC, and this has revolutionized the treatment of NSCLC from traditional chemotherapy to targeted therapy according to the results of molecular screening. Anaplastic lymphoma kinase (ALK) rearrangements are regarded as a diamond mutation in NSCLC (2-7) and the EML4-ALK was the first fusion to be identified. The resultant abnormal signaling increases cell growth, proliferation and survival of tumor cells. Data from real-world studies have shown that median overall survival (OS) for advanced ALK+ NSCLC patients could be >7 years (8,9), with sequential tyrosine kinase inhibitor (TKI) therapy.
Alectinib as a second-generation ALK-TKI has demonstrated substantial efficacy as the first-line therapy in several clinical trials (J-ALEX, ALEX, ALESIA) (4,10,11), with significantly longer progression-free survival (PFS), and superior response and protective effects in the central nervous system (CNS) compared with crizotinib, which has prompted clinical experts to recommend alectinib as the preferred first-line option together with brigatinib and lorlatinib.
However, some real-world settings might not be well reflected in clinical trials due to their relatively stringent inclusion criteria and inflexible primary endpoints. For example, patients with symptomatic or active CNS metastases were excluded in all clinical trials of alectinib, and, furthermore, patients with Eastern Cooperative Oncology Group Performance Status (ECOG) ≥2 accounted for 5–10% of the overall population in the ALEX and ALESIA studies (4,11). Time-to-treatment failure (TTF) rather than PFS might be a more reasonable parameter to evaluate treatment duration and treatment benefits in real-world settings; for instance, patients who experience local or gradual progression might continue targeted therapy in combination with local radiation therapy or antiangiogenic agents, or in other circumstances, patients might completely discontinue the targeted therapy, based on severe adverse events or their own preference, without clear evidence of disease progression. Additionally, although the long-term benefits (i.e., TTF and OS) of first-line alectinib were substantiated in real-world settings, the predictive factors of PFS with first-line alectinib were not elaborated and analyzed in the WJOG 9516L study (9).
Since the identification of a fusion between EML4 and ALK in lung adenocarcinomas, at least 15 variants have been discovered (variant 1, 2, 3a/b are the most common EML4-ALK fusion variants), but data on the effects of these variants on the efficacy of alectinib as first-line treatment are limited (12,13). Therefore, we carried out this multicenter real-world study to more comprehensively evaluate the efficacy and safety of alectinib as first-line treatment by investigating the clinical outcomes of NSCLC patients with different EML4-ALK variants. We presented the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-803/rc).
Methods
Selection of patients
Our study was a single-arm observational real-world study. Patients diagnosed with locally advanced or metastatic ALK-positive (ALK+) NSCLC treated with first-line alectinib (dose from 300 to 600 mg b.i.d. based on clinician’s choice) were enrolled in 8 hospitals in China from September, 2018 to January, 2022. Patients with symptomatic or active CNS metastases were also included. All included patients were required to have a detailed radiological examination at baseline and undergo radiological evaluation at 2–3 monthly intervals during follow-up. Continuation of alectinib was permitted after local or gradual progression at the treating clinician’s discretion. There were no specific exclusion criteria. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 18-102/1680) and informed consent was taken from all the patients.
Data extraction
The demographic and clinical characteristics were recorded. The ALK fusion variant was identified at either baseline or at the time of progression based on the results of reverse transcription-polymerase chain reaction (RT-PCR) or next-generation sequencing (NGS) (ALK Ventana D5F3 was used to identify ALK positive cases for patients who didn’t choose NGS or RT-PCR at baseline). The authors reviewed the imaging data to evaluate the treatment response. Survival information and adverse events were obtained from clinical records or telephone follow-up by investigators at each center. The data cut-off date was 20, March, 2022. If a patient was lost to follow-up on 20, March, 2022, the former date of follow-up was regarded as the cut-off date.
Definitions, assessments and study endpoints
Metastases in symmetrical organs such adrenal glands, bones or non-regional draining lymph nodes were regarded as involving one distant organ. Longer EML4-ALK fusion variants contained EML4 fusions up to at least exon 13 and shorter variants included EML4 fusions up to exon 6. Radiological evaluation of intracranial and extracranial lesions was based on the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). Clinical activity endpoints of alectinib included objective response rate [ORR: complete response (CR) or partial response (PR)], tumor shrinkage rate, CNS-ORR (CR or PR for intracranial lesions), improvement of CNS-related symptoms, PFS, and CNS time-to-progression (CNS-TTP). More specifically, the tumor shrinkage rate was evaluated as the percentage of reduction of target lesions at the time of best response; improvement of CNS-related symptoms was a subjective report from the patient of significant improvement, moderate improvement, no improvement or deterioration; PFS was defined as the period between the start of alectinib and first documentation of radiological progression; CNS-TTP was evaluated only in patients with CNS metastases and was calculated from the start date of alectinib to the date of CNS progression. Safety endpoints included the description of adverse events. Dose interruption, dose adjustment and dose discontinuation due to adverse events were also recorded. Moreover, TTF as a parameter to evaluate the treatment duration of alectinib was defined as the period from the start of alectinib to complete discontinuation of alectinib due to any cause including disease progression, death, adverse events and patient’s preference.
Statistical analysis
Because of the study design, there was no statistical hypothesis. Statistical analyses were conducted with SPSS 26.0 statistical software (SPSS, Inc., Chicago, IL, USA). The distribution of patients and baseline demographic/clinical characteristics were presented using descriptive statistics. The ORR of intracranial and extracranial lesions was estimated with 95% confidence intervals (CIs) based on the exact binomial distribution. Differences between groups were compared using the Pearson’s χ2 test for categorical data, and t-tests for continuous data. Survival curves were estimated using the Kaplan-Meier method, and differences in the variables were calculated using the log-rank test. Cox’s proportional hazard model was used to estimate the hazard ratio (HR) and the corresponding 95% CI for the covariate of interest. The purpose of Cox regression analysis was to identify the predictive factors of PFS. A two-sided P value <0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 110 patients treated with first-line alectinib were included in our preliminary analysis. Table 1 shows their baseline characteristics. Patients with ECOG ≥2 accounted for almost 26.4% of the overall population (29 patients). Distant metastases were found in >85% of patients, of whom 24 had metastases in ≥3 distant organs, 29 patients were diagnosed with CNS metastases at baseline and 5 patients received local treatment for CNS lesions before initiation of alectinib, one of whom underwent total resection of the CNS lesion (CNS efficacy of alectinib was not evaluated in this patient); hence, 28 patients in total had intracranial metastases before the administration of alectinib, and 10/28 patients of these reported CNS-related symptoms at baseline.
Table 1
| Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 42 (38.2) |
| Female | 68 (61.8) |
| Age (years) | |
| Median [range] | 54 [18–83] |
| <65 | 91 (82.7) |
| ≥65 | 19 (17.3) |
| ECOG | |
| 0–1 | 81 (73.6) |
| ≥2 | 29 (26.4) |
| Smoking history | |
| Never smoked | 92 (83.6) |
| Smoker | 18 (16.4) |
| Pathology | |
| Adenocarcinoma | 109 (99.1) |
| Non-adenocarcinoma | 1 (0.9) |
| Stage | |
| III or recurrence after surgery without distant metastases | 13 (11.8) |
| IV or recurrence after surgery with distant metastases | 97 (88.2) |
| Extrathoracic metastases | |
| Yes | 61 (55.5) |
| No | 49 (44.5) |
| CNS metastases | |
| Yes | 29 (26.4) |
| No | 81 (73.6) |
| Local therapy for CNS lesions before alectinib | |
| Yes | 5 (17.2)* |
| No | 24 (82.8) |
| CNS-related symptoms before alectinib# | |
| Yes | 10 (35.7) |
| No | 18 (64.3) |
| Distant organs involved | |
| 0 | 13 (11.8) |
| 1–2 | 73 (66.4) |
| ≥3 | 24 (21.8) |
| With target lesion | 87 (79.1) |
| Without target lesion | 23 (20.9) |
*, CNS lesion was completely removed in 1 patient; #, 1 patient with completely resected CNS lesion was excluded. ECOG, Eastern Cooperative Oncology Group Performance Status; CNS, central nervous system.
Efficacy
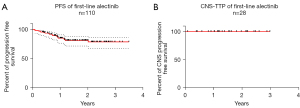
The ORR was 71.8% (95% CI: 62.4–80.0%) in the overall population and 88.5% of patients with target lesions demonstrated a radiological response (Table 2). Median tumor shrinkage rate was 60% (range, 0–100%), of which 60 (69.0%) and 22 (25.3%) patients had tumor reduction >50% and >75% respectively (Table 2). As for patients with CNS metastases, the CNS-ORR was 64.3% (95% CI: 44.1–81.4%); moreover, 92.9% (95% CI: 66.1–99.8%) of patients with measurable CNS metastases showed an intracranial response and 80% of patients (8/10) reported a significant improvement in symptoms attributable to CNS lesions (two patients with symptomatic CNS metastases received brain radiotherapy at baseline, one of them reported significant alleviation in CNS symptoms while the other reported moderate improvement) (Table 2). At the time of data cut-off, 19 patients showed disease progression: the 1- and 2-year PFS rates were 85.0% (95% CI: 76.3–90.7%) and 81.1% (95% CI: 71.5–87.7%), respectively, with a median follow-up of 18.3 months (range, 3.9–42.6 months) (Figure 1A). With a median follow-up of 19.6 months (range, 5.2–38.9 months), no CNS progression occurred in patients with CNS metastases [CNS-TTP: not reached (NR), 1- and 2-year CNS PFS rates were 100%] (Figure 1B).
Table 2
| Evaluation of first-line alectinib in different scenarios | Percentage/No. of patients |
|---|---|
| ORR in overall population (n=110) | 71.8% (95% CI: 62.4–80.0%) |
| 9 CR + 70 PR | |
| ORR in patients with target lesion (n=87) | 88.5% (95% CI: 79.9–94.3%) |
| 7 CR + 70 PR | |
| Median tumor shrinkage rate (n=87) | 60% (range, 0–100%) |
| Tumor shrinkage rate >50% | 69% (60/87) |
| Tumor shrinkage rate >75% | 25.3% (22/87) |
| CNS-ORR in patients with CNS metastases (n=28) | 64.3% (95% CI: 44.1–81.4%) |
| 8 CR + 10 PR | |
| CNS-ORR in patients with measurable CNS lesion (n=14) | 92.9% (95% CI: 66.1–99.8%) |
| 3 CR + 10 PR | |
| Improvement in CNS-related symptoms (n=10) | |
| Significant improvement | 8 (80.0%) |
| Moderate improvement | 2 (20.0%) |
| No improvement | 0 |
| Deterioration | 0 |
ORR, objective response rate; CI, confidence interval; CNS, central nervous system; CR, complete response; PR, partial response.
Progression pattern, repeat biopsy and subsequent therapy
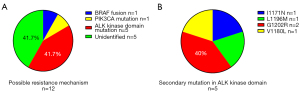
A total of 17 patients experienced extracranial progression, 1 patient had CNS progression and 1 had both extracranial and intracranial progression (Table 3). Repeat biopsy was conducted in 14 patients with progression events, among whom 2 patients were found to have no tumor cells in the samples (Figure S1). Secondary mutation in the ALK kinase domain was identified in 5 patients (Figure 2A,2B), and another 2 patients were found to have a BRAF fusion and a PIK3CA mutation, respectively (Figure 2A). Of the patients with local or gradual progression, 8 continued alectinib treatment in combination with local therapy (including radiation therapy and local ablative therapy) or antiangiogenic agent. At the time of data cut-off, 19 patients had completely discontinued alectinib treatment, 16 patients received ≥1 line of subsequent therapy and subsequent treatment with other ALK-TKIs were given to 15 patients (Table 3).
Table 3
| Progression pattern, reason for discontinuation of alectinib and subsequent therapy | No. of patients |
|---|---|
| Progression events at the time of data cut-off | 19 |
| Intracranial or extracranial progression | |
| Intracranial progression | 1 (5.3%) |
| Extracranial progression | 17 (89.5%) |
| Intracranial and extracranial progression | 1 (5.3%) |
| Permanent discontinuation of alectinib at the time of data cut-off | 19 |
| Reason for discontinuation of alectinib | |
| Progression events | 17 (89.5%) |
| Severe adverse events | 2 (10.5%) |
| Patient’s preference | 0 |
| Subsequent therapy for patients with progression events | 19 |
| Continuation of alectinib at the time of data cut-off | 1 (5.3%) |
| No subsequent therapy | 2 (10.5%) |
| ≥1 line of subsequent therapy | 16 (84.2%) |
| ≥1 line of other ALK-TKI | 15 (78.9%) |
ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor.
Safety
In total 109 patients had recorded adverse events. The common adverse events are described in Tables 4,5; most patients experienced grade 1–2 adverse events and only 6.4% of patients had grade 3–4 adverse events. No symptomatic bradycardia or ≥ grade 2 elongation of the QTc interval was reported. Elevated total bilirubin was the most common reason for dose interruption and reduction: 18 patients had at least one dose interruption and dose reduction was also reported in 18 patients. Only 2 patients (1.8%) permanently discontinued alectinib because of severe adverse events: 1 patient experienced repeated grade 3 diarrhea and the other had a grade 3 event of elevated aminotransferase and a grade 2 event of elevated total bilirubin.
Table 4
| Adverse events | Grade 1–2 (%) | Grade 3–4 (%) |
|---|---|---|
| Constipation | 52.3 | 0 |
| Fatigue | 33.9 | 0 |
| Edema | 21.1 | 0 |
| Musculoskeletal pain | 26.6 | 0 |
| Aminotransferase increased | 24.8 | 3.7 |
| Total bilirubin increased | 28.4 | 0.9 |
| Rash | 11.9 | 0 |
| Weight gain | 13.8 | 0.9 |
Table 5
| Outcomes of adverse events | No. of patients |
|---|---|
| Grade 3–4 adverse events | 7 (6.4%) |
| Dose interruption | 18 (16.5%) |
| Common adverse event led to dose interruption | Grade 2–3 total bilirubin increased (n=10) |
| Grade 2–3 aminotransferase increased (n=7) | |
| Dose reduction | 18 (16.5%) |
| Common adverse event led to dose reduction | Grade 2–3 total bilirubin increased (n=7) |
| Grade 3 aminotransferase increased (n=4) | |
| Permanent discontinuation due to adverse events | 2 (1.8%) |
| Severe adverse event led to permanent discontinuation of alectinib | Grade 3 diarrhea (n=1) |
| Grade 3 aminotransferase increased + Grade 2 total bilirubin increased (n=1) |
TTF
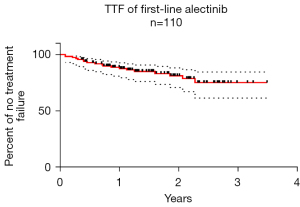
At the time of data cut-off, 91 patients were continuing alectinib treatment and 19 had completely discontinued due to disease progression in 17 cases and severe adverse events in 2 cases (Table 3). The median TTF was not reached; the 1-year and estimated 2-year treatment failure-free rates were 88.6% (95% CI: 80.8–93.4%) and 81.0% (95% CI: 70.6–88.0%) respectively with a median follow-up of 18.3 months (range, 3.9–42.6 months) (Figure 3).
Univariate and multivariate analyses of patients with target lesions
Table 6 shows the results of univariate and multivariate analyses of patients with target lesions (n=87). Covariates with P<0.05 in the univariate analysis were included in the multivariate model. Patients with metastases involving ≥3 distant organs were found to have worse PFS than their counterparts (HR =4.1, 95% CI: 1.2–13.9, P=0.023). More favorable PFS occurred in patients with tumor reduction >50% compared with those without (HR =0.25, 95% CI: 0.08–0.79, P=0.018).
Table 6
| Variable | Univariable analysis (P) | Multivariable analysis | ||
|---|---|---|---|---|
| Hazard ratio | 95% CI | P | ||
| Age (<65 vs. ≥65 years) | 0.108 | – | – | – |
| Sex (male vs. female) | 0.790 | – | – | – |
| ECOG (≥2 vs. 0–1) | 0.04 | 1.7 | 0.58–5.1 | 0.326 |
| Smoking history (smoker vs. never smoked) | 0.887 | – | – | – |
| Stage (III or recurrence without distant metastases vs. IV or recurrence with distant metastases) | 0.332 | – | – | – |
| Extrathoracic metastases (yes vs. no) | 0.261 | – | – | – |
| CNS metastases (yes vs. no) | 0.476 | – | – | – |
| Distant organs involved (≥3 vs. ≤2) | 0.022 | 4.1 | 1.2–13.9 | 0.023 |
| Tumor reduction (>50% vs. ≤50%) | 0.045 | 0.25 | 0.08–0.79 | 0.018 |
PFS, progression-free survival; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group Performance Status; CNS, central nervous system.
PFS with different ALK fusion variants
The ALK fusion variant was identified in 61 patients, of whom 49 carried EML4-ALK variants. The distribution of ALK fusion variants is shown in Figure 4A. There was no significant difference in PFS between EML4 and non-EML4 fusion variants (NR vs NR, HR =1.26, 95% CI: 0.30–5.2, P=0.76, Figure 4B), however, patients with longer variants demonstrated significantly more favorable PFS than those with shorter variants (NR vs. 24.2 months, HR =0.17, 95% CI: 0.04–0.68, P=0.004, Figure 4C) (baseline patient characteristics were balanced between longer and shorter variants, Table S1).

Discussion
Several generations of ALK-TKIs have been established as standard treatment for patients with advanced ALK+ NSCLC (2-7). As a second-generation ALK-TKI, alectinib has demonstrated robust efficacy in clinical trials with both ORR and CNS-ORR >80%. Furthermore, the long-term benefits of first-line alectinib have been substantiated in clinical trials and real-world settings, with PFS of almost 3 years and a 5-year OS rate >60% (4,9,10,14). This has resulted into the fact that alectinib is the preferred first-line treatment in most countries. However, more information is required; for example, data on the efficacy of alectinib in patients with symptomatic CNS metastases are limited, and the predictive factors of PFS with first-line alectinib are also not well established.
Compared with clinical trials, our real-world study reflected some situations in clinical practice; for example, patients with ECOG ≥2 accounted for almost 25% of the overall population and patients with symptomatic CNS metastases were also included. Under these circumstances, our results revealed 1- and 2-year PFS rates >80%, which further substantiates the efficacy of first-line alectinib. Our findings also indicated robust efficacy of alectinib for CNS lesions in a real-world setting, in line with the study conducted by Lin et al. (15). We found excellent safety profiles and tolerability of alectinib, also consistent with previous reports (4,11), and adverse events such as nausea, diarrhea, and vomiting, which can negatively affect treatment compliance, were rarely reported.
Preliminary analysis disclosed some predictive factors of PFS with first-line alectinib, such as the number of distant organs involved, the extent of tumor reduction and the type of EML4-ALK fusion variant. Patients with metastases in ≥3 distant organs demonstrated worse PFS, which may be explained by the theory of tumor heterogeneity, because greater extent of distant dissemination results in higher tumor heterogeneity with a heterogeneous response by different subclones. Also, previous studies have linked presence of the unfavourable EML4-ALK variant V3 with a greater tumor cell migratory potential and a higher total number of metastatic sites (16), however, we didn’t observe this phenomenon in our research (Table S1), this probably due to limited sample size in our study. A further finding was that, patients with tumor reduction >50% had more favorable PFS compared with their counterparts, possibly because of a higher proportion of subclones sensitive to the targeted therapy in the entire tumor, which would lead to a longer duration of treatment response. Similar data (17) about the relationship of deeper responses with longer survival were recently reported also for brigatinib in the prospective randomized phase 3 study ALTA-1L , which underlines the validity and potential clinical utility of the results presented here. Regarding the effect of different fusion variants on the efficacy of ALK-TKIs, accumulating data suggest that longer EML4-ALK variants, like V1 (E13:A20) and V2 (E20:A20) are associated with better outcome than the shorter V3 (E6:A20) under different ALK TKI (18): in previous retrospective studies, tumor variants with more invasive biology (V3) showed less response to crizotinib (16,19), while V1 was associated with longer PFS of crizotinib compared with non-V1 (20), and another one study indicated that V2 showed the best outcome under crizotinib among all EML4-ALKvariants (21). Investigations of the effect of ALK variants on the efficacy of second-generation ALK-TKIs revealed contradictory results: no significant difference in PFS between V1 and V3 in the ALEX and BFAST studies (12,13), but the ALTA-1L study results suggested that patients with V3 had inferior PFS compared with those with V1 in both the brigatinib and crizotinib arms (22). Therefore, it has been suggested that the lack of a PFS difference between V1 and V3 in the ALEX and BFAST studies for both alectinib and crizotinib is most likely a technical artifact due to the use of DNA NGS, which is less sensitive than RNA-based methods (like RT-PCR and RNA-NGS) and could bias the results by missing more favourable V1 cases with lower allelic frequencies (23). In further support of this notion, our molecular workup included RT-PCR and our results demonstrate that V3 is associated with inferior outcome under first-line alectinib in the real-world setting. Interestingly, one real-world study has shown that the intracranial PFS is also shorter for TKI-treated patients with V3 compared to longer EML4-ALK variants (24).
Our study had some limitations and many questions still need to be resolved in the future. Firstly, the lack of a comparative group means that some selection bias cannot be excluded. Also, long-term efficacy and survival outcomes will mature further required with a longer duration of follow-up, especially for progression patterns and resistance mechanisms to first-line alectinib. Additionally, although patients with symptomatic CNS metastases were included in our study and our results indeed revealed excellent intracranial efficacy of alectinib, we still could not draw a definitive conclusion on whether alectinib could lower or defer the need for cranial radiotherapy. In addition, the evaluation of CNS-related symptoms was mainly based on the patient’s subjective assessment, which might give rise to less accurate results. Moreover, we did not exclude the influence of co-mutations such as TP53 which impair patient outcomes synergistically with EML4-ALK V3 (25) when analyzing the effects of the different variants on PFS, because the identification of the ALK variants was partly based on the results of RT-PCR or NGS in a small panel under which circumstance no co-mutation could be confirmed in the baseline. Also, we had a relatively small sample of known ALK fusion variants, which would also introduce some selection bias. Last but not least, although the extent of tumor reduction was relevant to the efficacy of alectinib, some patients in clinical practice who have moderate tumor shrinkage can still experience a very long duration of stable disease, and the underlying mechanism of this needs further investigation, as does the relationship between the clearance of circulating tumor DNA (ctDNA) and the extent of tumor reduction.
Conclusions
On the whole, alectinib showed substantial efficacy and a good safety profile in a real-world setting. Clinical outcomes and long-term survival evaluations require a longer follow-up for conclusive results. Patients with more extensive metastases, less reduction in tumor lesions and carrying shorter fusion variants might need more intensive treatment strategies.
Acknowledgments
We thank all the patients and their family members for participating in the study. The authors also appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-803/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-803/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-803/coif). PC has received research funding from AstraZeneca, Amgen, Merck, Novartis, Roche, and Takeda, speaker’s honoraria from AstraZeneca, Pfizer, Novartis, Roche, and Takeda, support for attending meetings from AstraZeneca, Daiichi Sankyo, Eli Lilly, Gilead, Janssen, Novartis, and Takeda, and personal fees for participating to advisory boards from Boehringer Ingelheim, Chugai, Pfizer and Roche, all outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 18-102/1680) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J Clin Oncol 2020;38:3592-603. [Crossref] [PubMed]
- Horn L, Wang Z, Wu G, et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: a randomized clinical trial. JAMA Oncol 2021;7:1617-25. [Crossref] [PubMed]
- Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 2020;383:2018-29. [Crossref] [PubMed]
- Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget 2017;8:21903-17. [Crossref] [PubMed]
- Ito K, Yamanaka T, Hayashi H, et al. Sequential therapy of crizotinib followed by alectinib for non-small cell lung cancer harbouring anaplastic lymphoma kinase rearrangement (WJOG9516L): A multicenter retrospective cohort study. Eur J Cancer 2021;145:183-93. [Crossref] [PubMed]
- Nakagawa K, Hida T, Nokihara H, et al. Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2020;139:195-9. [Crossref] [PubMed]
- Zhou C, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med 2019;7:437-46. [Crossref] [PubMed]
- Camidge DR, Dziadziuszko R, Peters S, et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac Oncol 2019;14:1233-43. [Crossref] [PubMed]
- Dziadziuszko R, Mok T, Peters S, et al. Blood First Assay Screening Trial (BFAST) in Treatment-Naive Advanced or Metastatic NSCLC: Initial Results of the Phase 2 ALK-Positive Cohort. J Thorac Oncol 2021;16:2040-50. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Lin JJ, Jiang GY, Joshipura N, et al. Efficacy of Alectinib in Patients with ALK-Positive NSCLC and Symptomatic or Large CNS Metastases. J Thorac Oncol 2019;14:683-90. [Crossref] [PubMed]
- Christopoulos P, Endris V, Bozorgmehr F, et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ non-small cell lung cancer. Int J Cancer 2018;142:2589-98. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Association of depth of target lesion response to brigatinib with outcomes in patients with ALK inhibitor-naive ALK+ NSCLC in ALTA-1L. J Clin Oncol 2022;40:abstr 9072.
- Christopoulos P, Kirchner M, Endris V, et al. EML4-ALK V3, treatment resistance, and survival: refining the diagnosis of ALK+ NSCLC. J Thorac Dis 2018;10:S1989-91. [Crossref] [PubMed]
- Woo CG, Seo S, Kim SW, et al. Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann Oncol 2017;28:791-7. [Crossref] [PubMed]
- Yoshida T, Oya Y, Tanaka K, et al. Differential Crizotinib Response Duration Among ALK Fusion Variants in ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3383-9. [Crossref] [PubMed]
- Li Y, Zhang T, Zhang J, et al. Response to crizotinib in advanced ALK-rearranged non-small cell lung cancers with different ALK-fusion variants. Lung Cancer 2018;118:128-33. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus Crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J Thorac Oncol 2021;16:2091-108. [Crossref] [PubMed]
- Elsayed M, Christopoulos P. Therapeutic Sequencing in ALK+ NSCLC. Pharmaceuticals (Basel) 2021;14:80. [Crossref] [PubMed]
- El Shafie RA, Seidensaal K, Bozorgmehr F, et al. Effect of timing, technique and molecular features on brain control with local therapies in oncogene-driven lung cancer. ESMO Open 2021;6:100161. [Crossref] [PubMed]
- Christopoulos P, Kirchner M, Bozorgmehr F, et al. Identification of a highly lethal V3+ TP53+ subset in ALK+ lung adenocarcinoma. Int J Cancer 2019;144:190-9. [Crossref] [PubMed]
(English Language Editor: K. Brown)


