
Immunotherapy with radiotherapy fails to improve prognosis of patients with stage IV non-small cell lung cancer: a retrospective cohort analysis of the THUNDER-2 study
Highlight box
Key findings
• The addition of external beam radiation therapy (EBRT) or radioactive particle implantation (RPI) to immunotherapy (IT) did not significantly improve patients’ overall survival (OS) in stage IV non-small cell lung cancer (NSCLC). Early combination IT after radiotherapy (RT) may benefit patients with long-term survival.
What is known and what is new?
• Previous studies showed that RT combined with IT can provide benefits to patients with advanced NSCLC.
• We found that the addition of RT to IT didn’t improve patients’ OS in stage IV NSCLC. In some specific populations, patients who underwent RPI to IT had a less favorable OS than those who underwent IT monotherapy.
What is the implication, and what should change now?
• This study may provide a basis for optimizing the combination strategy of IT. It suggests that EBRT should be given preference to relieve local symptoms in patients with stage IV NSCLC than RPI therapy. Appropriate combination modality and precise timing of combination could significantly prolong the long-term survival in stage IV NSCLC.
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 85–90% of all lung cancer types (1). Early diagnosis of disease is low due to the lack of typical clinical symptoms. About one third of lung cancer patients are already at stage IV at the time of initial diagnosis, leaving them beyond the optimal window for surgery.
Radiotherapy (RT) is considered an important treatment for patients with non- surgical NSCLC (2). Most patients with stage IV NSCLC have widespread disease. Palliative radiation therapy plays an integral part in relieving symptoms and improving quality of life (3). The clinical efficiency of RT has been attributed to its ability to induce DNA damage, which may lead to direct tumor cell death. Furthermore, the presence of radiation-induced antitumor immunity and its synergy with immunotherapy (IT) has been increasingly recognized as a potential therapeutic approach (4). RT affects the immune system in several ways, including alteration of the tumor microenvironment, release of cytokines and chemokines, infiltration of immune cells, and increased susceptibility of tumor cells to immunogenic cell death (3). Above all, the radiation treatment stimulates the expression of specific antigens in the irradiated tumor cells. These antigens can be recognized and phagocytosed by antigen-presenting cells (APCs) and presented to CD8 T cells. Together, these events promote anti-tumor T-cell immune responses. The aim of RT is evolving from direct tumor death to tumor immune microenvironment reconstitution and immune modulation.
Immune checkpoint inhibitors (ICIs), particularly those targeting programmed cell death protein-1 (PD-1) or programmed cell death ligand-1 (PD-L1), have shown durable efficacy in some NSCLC patients, and these agents have become the cornerstone of systemic antitumor therapy (5). By blocking inhibitory pathways that physiologically control the immune response, ICIs restore and maintain the immune system's attack on cancer cells (6). They have changed the treatment landscape for advanced/metastatic NSCLC and have been integrated into the second-line treatment of advanced NSCLC (7). Toripalimab and tislelizumab are novel humanized immunoglobulin monoclonal antibodies that targets PD-1. A few phases 1/3 clinical trials have shown that they have a favorable safety and anti-tumor activity in patients with NSCLC (8,9).
Patients with stage IV NSCLC may have larger tumors and are in poor physical condition with distant metastases, making larger tumors couldn’t reach the therapeutic dose. Systemic treatment is still recommended for patients with advanced lung cancer, IT has emerged as a new treatment option for lung cancer. However, only 20% of patients with non-small cell lung cancer respond to ICIs (10). Through time, most patients who initially respond to immunotherapy become resistant, thereby limiting the durability of immunotherapy (11).
The combination of RT and IT is an ongoing and promising area of research. The PACIFIC study showed that the median overall survival (OS) increased by 18.4 months in patients with durvalumab after concurrent chemoradiotherapy than patients with concurrent chemoradiotherapy, and 42.9% of patients survived for more than 5 years (12). Consolidation therapy with durvalumab after concurrent chemoradiotherapy has become the standard regimen for stage III unresectable NSCLC (13). The PEMBRO-RT phase 2 randomized clinical trial (RCT) indicated that stereotactic body radiation therapy (SBRT) followed by IT has a clear favorable trend over IT alone for metastatic NSCLC: the median progression-free survival (PFS) was 6.6 months versus 1.9 months (P=0.19), and the median OS was 15.9 months versus 7.6 months (P=0.16) (14). Although the results of the study were negative and did not statistically meet expectations, the study showed that RT combined with IT can provide real benefits to patients with metastatic NSCLC. For patients with advanced NSCLC, systemic therapy remains the treatment of choice, but in recent years RT is showing a possible role because of its immunomodulatory effects (15). A secondary analysis of the prospective study KEYNOTE-001 showed that patients with metastatic NSCLC who received RT prior to IT had longer PFS and OS than those who did not receive RT (16). In theory, RT combined with IT has the potential to overcome mutual disadvantages and improve prognosis. Meanwhile, there was no increase in adverse events (17), which may be related to the timing and modality of combination therapy. In clinical practice, the benefit of RT in stage IV NSCLC patients treated with IT is still unclear. Prior studies also failed to meet the criteria for statistically significant. The purpose of this study is aimed at evaluating the role of RT in these patients, defining any specific disease characteristics that can particularly benefit from the association of RT with IT. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-843/rc).
Methods
Study cohort
A clinical practice analysis was carried out of patients with histologically- or cytologically-confirmed stage IV NSCLC in the THUNDER-2 study, including patients who had been treated with toripalimab or tislelizumab IT and had received RT including external beam radiation therapy (EBRT) or radioactive particle implantation (RPI) at 3 oncology centers in Shandong province between 2019 and 2021. The oncology centers participating in the study are Shandong Provincial Qianfoshan Hospital, Weifang People’s Hospital and Linyi Cancer Hospital. The primary lesion is not controlled or new distant metastases are present, so local RT is added to systemic therapy for regional control or palliative relief in these stage IV NSCLC. We enrolled patients diagnosed with NSCLC who had developed distant metastases by medical imaging or pathology, including widespread metastasis and oligometastasis. Meanwhile, subjects included also received IT with either toripalimab or tislelizumab. Patients with incomplete information and a previous history of severe systemic disease or autoimmune diseases were excluded. With a statistical test power of 90%, our sample size meets the requirement. We continuously collected and assessed the following patient information: basic personal details, distant metastasis condition, time of IT initiation, the number of lines of IT, Eastern Cooperative Oncology Group (ECOG) performance score, the number of chemotherapy cycles before IT, relative information of RT, and follow-up data. For patients who underwent EBRT, intensity modulated radiotherapy (IMRT) was used, and the median dose was 60 Gy (range, 25–100 Gy), delivered in the conventional fraction, 2–3 Gy/F, 5 F/W. The primary implanted particle in patients treated with RPI was 125I, and the median number of particle implantation was 1 time (range,1–4 times). Oligometastatic status was defined as a maximum of 5 metastases in 3 organs, but diffuse plasma membrane (meningeal, pleural, pericardial, and mesenteric) or bone marrow metastases were not included in this definition (18). Based on the peak timing of RT to promote tumor-specific antigen release, we preliminarily explored the optimal timing of immune drug intervention. Taking into account the experience of the PACIFIC study and clinical practice, we chose 1 month as the time limit for concurrent RT combined with IT. A further 2 treatment modalities, namely, concurrent radioimmunotherapy (the interval between RT and IT ≤1 month), and sequential radioimmunotherapy (the interval between RT and IT >1 months), were identified among those who had undergone RT according to the start and duration of the 2 treatments.
Follow-up and endpoints
Follow-up data was obtained retrospectively through the electronic medical records according to the standard practice of the disease. The physician follow-up included clinical assessments, thoracic computed tomography (CT) scans, abdomen B-ultrasound examination, brain magnetic resonance imaging (MRI) and other examinations as needed. Our experienced medical staff also conducted regular telephone follow-up every 3 months for the patients included. We kept a detailed record of the follow-up person, follow-up time, the survival status of the patients, the situation of disease progression, the time of death, cause of death and the assessment of the survivors in terms of physical status and complications during the follow-up process. The primary endpoint of our study was OS, which was measured from the initiation of IT until death or the last follow-up date.
Statistical analysis
Correlations between clinical baseline characteristics were assessed by Fisher’s exact chi-square test. If the baseline characteristics of the 3 groups are statistically significant, a 2 by 2 comparison were conducted. The OS of different combined modalities was evaluated by generating Kaplan–Meier curves and performing log rank tests. In investigative subgroup analyses, the effect of adding RPI or EBRT treatment to IT on OS was evaluated in predefined subgroups (age, gender, smoking history, histological features, distant metastatic status, liver metastasis, brain metastasis, ECOG performance score, IT stage, and cycles of previous chemotherapy) using the Cox proportional risk model. Forest plots were created to show the results of all subgroup analyses. In the entire sample, we performed univariate and multivariate Cox regression analyses to identify significantly predictive factors of OS. Multivariate Cox regression analyses were performed to determine whether there was a significant association between clinicopathological characteristics and patient outcome of IT in stage IV NSCLC. A Cox proportional hazards algorithm using the forward stepwise method was used in multivariate analyses. The P-values less than or equal to 0.1 in the univariable analysis were entered into the multivariable Cox regression analysis of total patients. Other statistical tests were 2-sided, and P≤0.05 was considered statistically significant. Sample size estimation using PASS V.15 (NCSS, Kaysville, Utah, USA). Statistical analyses were conducted using R (V.4.1.1; the R Foundation for Statistical Computing, Vienna, Austria) or SPSS 26.0 (IBM Corp., Armonk, NY, USA) software. A forest plot presenting the statistical parameters of each subgroup factor was drawn using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Shandong Provincial Qianfoshan Hospital review board (No. 2022-S005). Individual consent for this retrospective analysis was waived. The other two participating hospitals were informed and agreed the study.
Results
Patient characteristics
A total of 120 patients were enrolled in this retrospective study between February 2019 and August 2021. Of these patients, 76 patients received IT without RT, 11 received IT with RPI, and 33 received IT with EBRT. All of 44 patients were treated with RT prior to or concurrent with their first injection of ICIs. Of these, 15 patients received concurrent RT with IT and 29 patients received sequential RT with IT. The median interval time between RT and IT is 3.83 months (range, 0–90.50 months). The median follow-up for all patients was 15 months [95% confidence interval (CI): 7.831–22.169 months]. The baseline characteristics of the patients are summarized in Table 1. The number of IT lines in patients who had received RPI was mostly above the third line. IT without RT had a higher proportion of patients without brain metastases and there were more people using less than or equal to 4 cycles of chemotherapy prior to IT (Table 2).
Table 1
| Variable | No. (%) patients | P value | ||
|---|---|---|---|---|
| IT without RT (n=76) | IT with RT (n=44) | |||
| IT with RPI (n=11) | IT with EBRT (n=33) | |||
| Age, median [range] (years) | 64 [33–89] | 66 [48–85] | 63 [38–74] | 0.185 |
| <65 | 40 (52.6) | 5 (45.5) | 23 (69.7) | |
| ≥65 | 36 (47.4) | 6 (54.5) | 10 (30.3) | |
| Gender | 0.283 | |||
| Male | 51 (67.1) | 8 (72.7) | 27 (81.8) | |
| Female | 25 (32.9) | 3 (27.3) | 6 (18.2) | |
| Smoking status | 0.490 | |||
| No | 35 (46.1) | 3 (27.3) | 13 (39.4) | |
| Yes | 41 (53.9) | 8 (72.7) | 20 (60.6) | |
| Histological features | 0.810 | |||
| Squamous cell carcinomas | 34 (44.7) | 4 (36.4) | 13 (39.4) | |
| Non-squamous cell carcinomas | 42 (55.3) | 7 (63.6) | 20 (60.6) | |
| Distant metastases | 0.443 | |||
| Oligometastatic | 20 (26.3) | 2 (18.2) | 5 (15.2) | |
| Polymetastatic | 56 (73.7) | 9 (81.8) | 28 (84.8) | |
| Liver metastasis | 1.000 | |||
| No | 59 (77.6) | 9 (81.8) | 26 (78.8) | |
| Yes | 17 (22.4) | 2 (18.2) | 7 (21.2) | |
| Brain metastasis | 0.001 | |||
| No | 66 (86.8) | 9 (81.8) | 18 (54.5) | |
| Yes | 10 (13.2) | 2 (18.2) | 15 (45.5) | |
| ECOG performance score | 0.607 | |||
| ≤1 | 46 (60.5) | 5 (45.5) | 18 (54.5) | |
| ≥2 | 30 (39.5) | 6 (54.5) | 15 (45.5) | |
| IT stage | <0.001 | |||
| First-line and second-line | 71 (93.4) | 4 (36.4) | 14 (42.4) | |
| Third-line and more | 5 (6.6) | 7 (63.6) | 19 (57.6) | |
| Combined treatment model | 0.435 | |||
| Concurrent RT with IT (≤1 months) | – | 3 (27.3) | 12 (36.4) | |
| Sequential RT with IT (>1 months) | – | 8 (72.7) | 21 (63.6) | |
| Irradiated sites | 0.556 | |||
| Primary lesion | – | 5 (45.5) | 10 (30.3) | |
| Metastatic lesion | – | 3 (27.3) | 15 (45.5) | |
| Primary and metastatic lesions | – | 3 (27.3) | 8 (24.2) | |
| Cycles of previous chemotherapy | 0.047 | |||
| ≤4 | 49 (64.5) | 7 (63.6) | 13 (39.4) | |
| >4 | 27 (35.5) | 4 (36.4) | 20 (60.6) | |
IT, immunotherapy; RT, radiotherapy; RPI, radioactive particle implantation; EBRT, external beam radiation therapy; ECOG, Eastern Cooperative Oncology Group.
Table 2
| Variable | IT without RT vs. IT with RPI | IT without RT vs. IT with EBRT | IT with RPI vs. IT with EBRT | |||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | P value | No. (%) | P value | No. (%) | P value | |||
| Brain metastasis | 0.645 | <0.001 | 0.158 | |||||
| No | 66 (86.8)/9 (81.8) | 66 (86.8)/18 (54.5) | 9 (81.8)/18 (54.5) | |||||
| Yes | 10 (13.2)/2 (18.2) | 10 (13.2)/15 (45.5) | 2 (18.2)/15 (45.5) | |||||
| IT stage | <0.001 | <0.001 | 1.000 | |||||
| First-line and second-line | 71 (93.4)/4 (36.4) | 71 (93.4)/14 (42.4) | 4 (36.4)/14 (42.4) | |||||
| Third-line and more | 5 (6.6)/7 (63.6) | 5 (6.6)/19 (57.6) | 7 (63.6)/19 (57.6) | |||||
| Cycles of previous chemotherapy | 1.000 | 0.021 | 0.185 | |||||
| ≤4 | 49 (64.5)/7 (63.6) | 49 (64.5)/13 (39.4) | 7 (63.6)/13 (39.4) | |||||
| >4 | 27 (35.5)/4 (36.4) | 27 (35.5)/20 (60.6) | 4 (36.4)/20 (60.6) | |||||
IT, immunotherapy; RT, radiotherapy; RPI, radioactive particle implantation; EBRT, external beam radiation therapy.
Varied prognostic trends in the overall population with different IT combination modalities
IT combined with RPI had the least favorable prognostic trend (median survival: 2 months; 95% CI: 0–4.158 months). The median survival time were 9 months in IT without RT (95% CI: 4.739–13.207 months) and 10 months in IT with EBRT (95% CI: 6.489–13.511 months), respectively, the difference was not significant (P=0.148) (Figure 1).

Prognostic effects of adding RT in different populations
In a subgroup analysis of OS (Figure 2), the addition of EBRT to IT was not associated with a significantly superior effect in patients with stage IV NSCLC (Figure 2A). IT with RPI seems to result in a worse prognosis in male patients [hazard ratio (HR) =2.433, 95% CI: 1.084–5.459, P=0.031], non-squamous carcinoma pathologies (HR =2.680, 95% CI: 1.079–6.659, P=0.034), patients with oligometastases (HR =7.967, 95% CI: 1.318–48.156, P=0.024), patients with liver metastases (HR =10.808, 95% CI: 1.728–67.601, P=0.011) or brain metastases (HR =20.087, 95% CI: 2.487–162.203, P=0.005), and those with ECOG performance score greater than or equal to 2 (HR =2.769, 95% CI: 1.031–7.440, P=0.043) (Figure 2B).
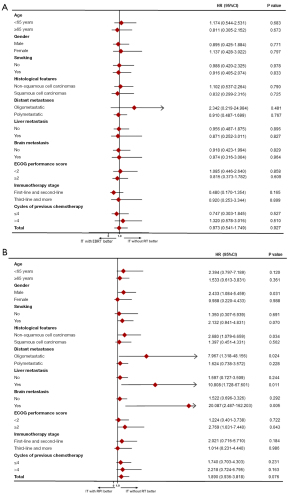
Prognostic analysis of the overall population
To investigate the prognostic value of different characteristics on patients’ OS in the overall population, we performed a Cox regression analysis. In univariate analyses, gender (female, HR =1.554, 95% CI: 0.921–2.621, P=0.099), ECOG performance score ≥2 (HR =2.576, 95% CI: 1.559–4.256, P<0.001), IT stage (third-line and more, HR =1.863, 95% CI: 1.099–3.158, P=0.021), and treatment group (IT with RPI, HR =1.890, 95% CI: 0.936–3.818, P=0.076) were significantly associated with poor prognosis in the total population, as shown in Table 3. Multivariate Cox analysis of prognostic elements revealed that ECOG score and IT stage were the independent prognostic factors. ECOG scores ≥2 (HR =2.701, 95% CI: 1.630–4.475, P<0.001) and third-line and more IT (HR =2.032, 95% CI: 1.189–3.471, P=0.009) were shown to be independently associated with poor prognosis. IT with RPI and IT with EBRT did not improve outcomes in the general population (Table 3).
Table 3
| Covariate | Univariable model | Multivariable model | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age, years | – | – | – | ||||
| <65 | 1 | – | – | – | |||
| ≥65 | 1.237 | 0.754–2.030 | 0.400 | – | – | – | |
| Gender | * | – | – | ||||
| Male | 1 | – | – | – | |||
| Female | 1.554 | 0.921–2.621 | 0.099 | – | – | – | |
| Smoking | – | – | – | ||||
| No | 1 | – | – | – | |||
| Yes | 0.990 | 0.598–1.638 | 0.969 | – | – | – | |
| Histological features | – | – | – | ||||
| Non-squamous cell carcinomas | 1 | – | – | – | |||
| Squamous cell carcinomas | 0.796 | 0.476–1.330 | 0.384 | – | – | – | |
| Distant metastases | – | – | – | ||||
| Oligometastatic | 1 | – | – | – | |||
| Polymetastatic | 1.438 | 0.748–2.766 | 0.277 | – | – | – | |
| Liver metastasis | – | – | – | ||||
| No | 1 | – | – | – | |||
| Yes | 1.236 | 0.679–2.250 | 0.487 | – | – | – | |
| Brain metastasis | – | – | – | ||||
| No | 1 | – | – | – | |||
| Yes | 1.036 | 0.580–1.852 | 0.904 | – | – | – | |
| ECOG performance score | |||||||
| ≤1 | 1 | 1 | |||||
| ≥2 | 2.576 | 1.559-4.256 | <0.001 | 2.701 | 1.630–4.475 | <0.001 | |
| IT stage | |||||||
| First-line and second-line | 1 | 1 | |||||
| Third-line and more | 1.863 | 1.099–3.158 | 0.021 | 2.032 | 1.189–3.471 | 0.009 | |
| Combined treatment model | – | – | – | ||||
| IT without RT | 1 | – | – | – | |||
| Concurrent radioimmunotherapy (≤1 month) | 0.764 | 0.339–1.724 | 0.517 | – | – | – | |
| Sequential radioimmunotherapy (>1 months) | 1.502 | 0.862–2.616 | 0.151 | – | – | – | |
| Cycles of previous chemotherapy | – | – | – | ||||
| ≤4 | 1 | – | – | – | |||
| >4 | 1.068 | 0.649–1.756 | 0.796 | – | – | – | |
| Treatment group | * | – | – | ||||
| IT without RT | 1 | – | – | – | |||
| IT with RPI | 1.890 | 0.936–3.818 | 0.076 | – | – | – | |
| IT with EBRT | 0.973 | 0.541–1.749 | 0.927 | – | – | – | |
*, not in the final step of the multivariable analysis. HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; IT, immunotherapy; RT, radiotherapy; RPI, radioactive particle implantation; EBRT, external beam radiation therapy.
Scheduling of combined IT after RT affects patients’ long-term outcomes
We further analyzed whether the temporal layout in the RT combined with IT modality affects the efficacy. In the Kaplan-Meier curve, we found a trend of benefit for concurrent IT with RT in general population with OS over nearly 8 months (P=0.189) (Figure 3A). Meanwhile, similar benefit potential was also observed in the group treated with concurrent IT and EBRT (P=0.202) (Figure 3B). In Cox analysis, concurrent radioimmunotherapy (HR =0.764, 95% CI: 0.339–1.724, P=0.517) had a development potential to improve prognosis, and prognostic tendency remained favorable in simultaneous IT with EBRT (HR =0.516, 95% CI: 0.183–1.454, P=0.211). Although the P-values were not statistically significant, we observed a trend that concurrent radioimmunotherapy (≤1 month) might improve the long-term prognosis of patients in the above two groups. Due to the comparatively small population of RPI, it was not possible to analyze the impact of timing in RPI patients.

Combining RT with first-line or second-line IT trended toward to improved survival
First-line and second-line IT significantly improved patient survival and was an independent protective factor in multivariate Cox analysis of the overall population (Table 3). Therefore, we further explored whether the first-line and second-line IT combined with RT could better improve patient prognosis. In Kaplan-Meier curve analysis, first-line and second-line IT combined with RT trended toward a benefit for patients with OS over approximately 7 months, however, the P values (P=0.224) were not statistically significant in the 3 groups of patients (Figure 4A). First-line and second-line IT with EBRT improved the prognosis direction in patients with OS beyond almost 4 months, although the P value (P=0.054) was marginal it was not statistically different (Figure 4B). In Cox analysis, first-line and second-line IT with RT (HR =0.539, 95% CI: 0.190–1.528, P=0.245) had a possibility to improve outcome, and prognostic tendency was promising in first-line and second-line IT with EBRT (HR =0.184, 95% CI: 0.025–1.347, P=0.096). The time interval between RT and IT ranged from 0 to 3 months. Although IT with RPI or EBRT therapy did not improve prognosis in the total population, we observed a trend toward a benefit in the long-term survival when IT was advanced to first-line or second-line therapeutic regimens and combined with RT.

Discussion
The primary findings of this study suggest that IT combined with EBRT or RPI did not improve prognosis in any subgroup of stage IV NSCLC. As far as we know, this study is the first to ascertain in the clinical practice setting that RPI does not induce the tumor immune microenvironment and enhances the efficacy of IT in stage IV NSCLC. In some specific populations, patients who underwent RPI to IT had a less favorable OS than those who underwent IT monotherapy.
Prior animal investigations suggest that low-dose continuous radiation from radioactive particles can alter the immune phenotype of tumors, thereby reducing the incidence of tumor metastasis (19). It was shown that 125I RPI combined with cytokine-induced killer cell therapy significantly inhibits the in vivo growth of mouse liver cancer cells and improves animal survival time by promoting anti-tumor immunity. 125I RPI upregulated the expression of major histocompatibility complex (MHC) class I chain-related gene A in hepatocellular carcinoma cells and enhanced cytokine-induced killer cell-mediated apoptosis through activation of caspase-3. In addition, cytokine-induced killer cells provide immune substrates to induce a strong immune response in the body after 125I RPI treatment (20). However, in our study, the overall prognosis of RPI was poor, and in a subgroup analysis, particle implantation was generally associated with a negative prognosis in specific populations. This may be related to the small sample size of patients treated with RPI in this study (n=11), the utilization of IT in most patients necessitating third-line therapy, and the late intervention of IT (63.6% for third-line IT, P<0.001), which also influenced the statistical results to some extent. Whether the efficacy of RPI therapy improves immune status needs to be further explored.
The effect of dose setting and fractionation pattern on the antitumor immune response remain to be explored. A couple of immunogenic mice studies have shown a more pronounced immunomodulatory effect of large fractionated radiation therapy compared to single-dose radiation therapy (21-23). Large fractionated RT modality minimizes the toxic effects of additional radiation therapy. High-dose RT activates the cGAS-STING signaling pathway and promotes acute exposure of interferon in the body to effectively induce anti-tumor immunity response (24-26). Chronic interferon exposure from low-dose RT often results in a state of immunosuppression. It has also been shown that tumor burden is negatively correlated with the efficacy of IT (27). RT on multiple sites of metastases releases a large amount of antigen while reducing the tumor load, thus stimulating the immunogenicity of the tumor. This, in the context of IT, could lead to consider local treatment on all metastatic sites (28).
An analysis from the National Cancer Database revealed that patients who treated with hypofractionated RT had improved 1-year OS (59.0%) compared with those who did not receive RT (55.7%), but this was not statistically significant (HR =0.9, 95% CI: 0.8–1.1, P=0.22); however, patients receiving CFRT had significantly less favorable OS relative to those receiving no RT (HR =1.3, 95% CI: 1.3–1.4, P<0.001) (29). In our study, people receiving EBRT were treated with conventional fractionation, and there was no significant improvement in patient prognosis. The optimal fractionation of EBRT combined with IT requires further study.
A systematic review and meta-analysis including 19 studies showed that OS, intracranial local control rate, and intracranial distant control rate were better with concurrent RT combined with IT than with RT sequential IT in patients of lung cancer with brain metastases (30). Although we did not obtain the same results in our study, the survival curves suggest that there may be a survival benefit in terms of trend for long-term survival in patients treated with concurrent RT with IT.
In the 2022 American Society of Clinical Oncology (ASCO) report, the addition of RT to first-line IT combination chemotherapy improved the prognosis of patients with advanced NSCLC, and its toxicity was well-tolerated. Due to the limitation of sample size, our enrolled patients had no history of EBRT in first-line IT, so we integrated patients with first- and second-line IT for analysis, and found that this can benefit patients with long-term survival. This inspired the strategic importance of early combination IT after RT.
The impact of PD-L1 expression level on RT combined with IT is a very interesting but unresolved issue. In the PEMBRO-RT trial, PD-L1-negative patients treated with pembrolizumab plus RT had a much-improved prognosis (14). In a pooled analysis of the PEMBRO-RT and MDACC randomized trials, it was not determined whether there was a meaningful association between PD-L1 status and the benefit of pembrolizumab plus RT (31). Due to the small sample size for detecting PD-L1 expression status in this data, this subgroup was not included in the analysis.
This study showed a trend toward better outcomes in the many subgroups of IT with EBRT compared to IT without RT, but certain pre-specified criteria for meaningful clinical benefit remained unmet. Therefore, a larger sample size is needed to more accurately detect the prognostic impact of adding RT to IT on patient prognosis.
There is a trend towards combination therapy with ICIs. In the era of IT, the addition of RT has achieved new breakthroughs; our study suggests that EBRT should be given preference as local palliative treatment to relieve local symptoms in patients with stage IV NSCLC than RPI therapy. Appropriate combination modality, suitable RT dose, and precise timing of combination could significantly prolong survival in advanced NSCLC (32). In future, if traditional combination modalities alone, such as RT, chemotherapy, and targeted therapy, fail to achieve significant OS benefit, new combination modalities will hold great promise, such as the addition of cytokines to RT combined with IT (33). Based on the existing studies, exploring new combination models to better improve the survival of patients with advanced lung cancer will be the focus of our next research.
Conclusions
While the robustness of the present conclusions is limited by relatively small sample size and retrospective nature of this research, the results suggest that combining EBRT or RPI with IT did not significantly improve patients’ OS in stage IV NSCLC. Concurrent IT with RT could improve long-term therapeutic outcome. In patients with a history of RT, early follow-up combined IT, such as advancing IT to the first and second line or current combined with RT, might confer a significantly beneficial trend among patients with a longer survival period. These results warrant confirmation in large sample population.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group. The authors also appreciate the Ethics Committee of Shandong Provincial Qianfoshan Hospital, Shandong University.
Funding: This study was funded by the National Natural Science Foundation of China (No. 81803043) and Shandong Natural Science Foundation (Nos. ZR2021LSW023 and ZR2021QH356).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-843/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-843/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-843/coif). AWK is the member of Advisory board in Astra Zeneca and Medtronic, the member of Steering committee for clinical trials in Roche Genentech. AWK receives compensation for time in preparatory work, travel, lodging from Roche Genentech; receives compensation in form of honorarium for time from Astra Zeneca; receives compensation for time in work, travel, lodging from Medtronic. RS receives travel grants from BHS, HSD. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Shandong Provincial Qianfoshan Hospital review board (No. 2022-S005). The other two participating hospitals were informed and agreed the study. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brzezniak C, Satram-Hoang S, Goertz HP, et al. Survival and Racial Differences of Non-Small Cell Lung Cancer in the United States Military. J Gen Intern Med 2015;30:1406-12. [Crossref] [PubMed]
- Ko EC, Raben D, Formenti SC. The Integration of Radiotherapy with Immunotherapy for the Treatment of Non-Small Cell Lung Cancer. Clin Cancer Res 2018;24:5792-806. [Crossref] [PubMed]
- Vinod SK, Hau E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020;25:61-71. [Crossref] [PubMed]
- Herrera FG, Irving M, Kandalaft LE, et al. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. The Lancet Oncology 2019;20:e417-33. [Crossref] [PubMed]
- Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol 2021;18:625-44. [Crossref] [PubMed]
- Proto C, Ferrara R, Signorelli D, et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev 2019;75:39-51. [Crossref] [PubMed]
- Jiang T, Wang P, Zhang J, et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: a multicenter phase-II trial. Signal Transduct Target Ther 2021;6:355. [Crossref] [PubMed]
- Wang Z, Ying J, Xu J, et al. Safety, Antitumor Activity, and Pharmacokinetics of Toripalimab, a Programmed Cell Death 1 Inhibitor, in Patients With Advanced Non-Small Cell Lung Cancer: A Phase 1 Trial. JAMA Netw Open 2020;3:e2013770. [Crossref] [PubMed]
- Lu S, Wang J, Yu Y, et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J Thorac Oncol 2021;16:1512-22. [Crossref] [PubMed]
- Tamminga M, de Wit S, Hiltermann TJN, et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immunother Cancer 2019;7:173. [Crossref] [PubMed]
- Pathak R, Pharaon RR, Mohanty A, et al. Acquired Resistance to PD-1/PD-L1 Blockade in Lung Cancer: Mechanisms and Patterns of Failure. Cancers (Basel) 2020;12:3851. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. [Crossref] [PubMed]
- Song P, Zhang J, Shang C, et al. Real-world evidenceand clinical observations of the treatment of advanced non-small cell lung cancer with PD-1/PD-L1 inhibitors. Sci Rep 2019;9:4278. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. The Lancet Oncology 2017;18:895-903. [Crossref] [PubMed]
- Anscher MS, Arora S, Weinstock C, et al. Association of Radiation Therapy With Risk of Adverse Events in Patients Receiving Immunotherapy: A Pooled Analysis of Trials in the US Food and Drug Administration Database. JAMA Oncol 2022;8:232-40. [Crossref] [PubMed]
- Mentink JF, Paats MS, Dumoulin DW, et al. Defining oligometastatic non-small cell lung cancer: concept versus biology, a literature review. Transl Lung Cancer Res 2021;10:3329-38. [Crossref] [PubMed]
- Zhao GS, Liu S, Yang L, et al. Evaluation of radioactive (125)I seed implantation for the treatment of refractory malignant tumours based on a CT-guided 3D template-assisted technique: efficacy and safety. BMC Cancer 2020;20:718. [Crossref] [PubMed]
- Zhang J, Wu N, Lian Z, et al. The Combined Antitumor Effects of (125)I Radioactive Particle Implantation and Cytokine-Induced Killer Cell Therapy on Xenograft Hepatocellular Carcinoma in a Mouse Model. Technol Cancer Res Treat 2017;16:1083-91. [Crossref] [PubMed]
- Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618. [Crossref] [PubMed]
- Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol 2012;2:153. [Crossref] [PubMed]
- Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306-10. [Crossref] [PubMed]
- Iyengar P, Zhang-Velten E, Court L, et al. Accelerated Hypofractionated Image-Guided vs Conventional Radiotherapy for Patients With Stage II/III Non-Small Cell Lung Cancer and Poor Performance Status: A Randomized Clinical Trial. JAMA Oncol 2021;7:1497-505. [Crossref] [PubMed]
- Yin L, Xue J, Li R, et al. Effect of Low-Dose Radiation Therapy on Abscopal Responses to Hypofractionated Radiation Therapy and Anti-PD1 in Mice and Patients With Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2020;108:212-24. [Crossref] [PubMed]
- Kho VM, Mekers VE, Span PN, et al. Radiotherapy and cGAS/STING signaling: Impact on MDSCs in the tumor microenvironment. Cell Immunol 2021;362:104298. [Crossref] [PubMed]
- Kim SI, Cassella CR, Byrne KT. Tumor Burden and Immunotherapy: Impact on Immune Infiltration and Therapeutic Outcomes. Front Immunol 2021;11:629722. [Crossref] [PubMed]
- Schubert P, Rutzner S, Eckstein M, et al. Prospective Evaluation of All-lesion Versus Single-lesion Radiotherapy in Combination With PD-1/PD-L1 Immune Checkpoint Inhibitors. Front Oncol 2020;10:576643. [Crossref] [PubMed]
- Bates JE, Morris CG, Milano MT, et al. Immunotherapy with hypofractionated radiotherapy in metastatic non-small cell lung cancer: An analysis of the National Cancer Database. Radiother Oncol 2019;138:75-9. [Crossref] [PubMed]
- Yang Y, Deng L, Yang Y, et al. Efficacy and Safety of Combined Brain Radiotherapy and Immunotherapy in Non-Small-Cell Lung Cancer With Brain Metastases: A Systematic Review and Meta-Analysis. Clin Lung Cancer 2022;23:95-107. [Crossref] [PubMed]
- Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. The Lancet Respiratory Medicine 2021;9:467-75. [Crossref] [PubMed]
- Liu L, Bai H, Wang C, et al. Efficacy and Safety of First-Line Immunotherapy Combinations for Advanced NSCLC: A Systematic Review and Network Meta-Analysis. J Thorac Oncol 2021;16:1099-117. [Crossref] [PubMed]
- Su X, Xu Y, Fox GC, et al. Breast cancer-derived GM-CSF regulates arginase 1 in myeloid cells to promote an immunosuppressive microenvironment. J Clin Invest 2021;131:e145296. [Crossref] [PubMed]
(English Language Editor: J. Jones)


