
Efficacy and safety of alectinib in ALK-positive non-small cell lung cancer and blood markers for prognosis and efficacy: a retrospective cohort study
Introduction
According to the 2018 global cancer statistics, lung cancer has the highest incidence and mortality of all malignancies worldwide and in China (1). Non-small cell lung cancer (NSCLC) is the most common histological type of lung cancer. With advances in research, in addition to surgery, radiation therapy, chemotherapy and immunotherapy, targeted therapy has significantly improved the survival and quality of life of patients with NSCLC by precisely targeting mutation-related proteins or gene fragments (2). Li detected ALK gene of 1,042 patients with NSCLC by polymerase chain reaction (PCR), 3.84% of which had ALK rearrangements (3). Although the gene rearrangement rate was not high, ALK-tyrosine kinase inhibitors (ALK-TKIs) play a vital role in disease control in ALK-rearranged (ALK-positive) NSCLC. Crizotinib was the first targeted ALK inhibitor, and its efficacy and safety were significantly better than platinum combined with pemetrexed (4). However, the vast majority of patients with ALK-positive NSCLC treated with crizotinib develop resistance within 8–11 months. In addition, crizotinib is not effective in preventing disease progression of the central nervous system (CNS) because it is difficult for crizotinib to penetrate the blood brain barrier, and brain metastases account for 20% of progressive diseases that occur in patients without brain metastases before treatment (5).
Second-generation ALK inhibitors overcame most of the mutations related to crizotinib-resistant and achieved a significant increase in titer. Alectinib is a second generation of ALK-TKIs developed in recent years, which is more selective and 10 times more potent than crizotinib. A phase III study called J-ALEX compared the efficacy and safety of alectinib with crizotinib in patients with ALK-positive advanced or recurrent NSCLC in Japan, who had not previously received ALK-TKIs therapy (6). The latest report showed that the median progression-free survival (PFS) of patients receiving alectinib was 34.1 months, compared with 10.2 months in patients treated with crizotinib (7). In addition, the alectinib group had a higher proportion of patients who achieved an objective response compared with the crizotinib group (92% vs. 79%). These results were confirmed in the ALEX study, which showed that the median PFS reached 34.8 months in ALK-positive NSCLC patients treated with alectinib in first-line treatment (8). At the same time, this study also found that alectinib showed great advantages in efficacy of the CNS. Only 12% of patients in the alectinib group experienced CNS progression, compared with 45% in the crizotinib group (9). The J-ALEX trials have shown that alectinib has a better safety profile than crizotinib. The incidence of grade 3/4 adverse events was lower in patients treated with alectinib (26% vs. 52%) compared with the crizotinib group. Furthermore, the proportion of patients who stopped treatment in the alectinib group (9% vs. 20%) was also lower than that of the crizotinib group (6). Some ALK-positive NSCLC patients are unable to tolerate the adverse reactions of crizotinib, thus, requiring a switch to alectinib. A prospective study confirmed that such patients still experience significant therapeutic benefits (10).
Common hematological indicators, such as inflammatory factors, tumor markers and cell free DNA (cfDNA) have gradually been shown to play important roles in the microenvironment of NSCLC. There is considerable evidence regarding neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in NSCLC patients treated with immunotherapy (11-13) and targeted therapy (14,15). However, few studies have explored the significance of these indicators in the alectinib treatment of ALK-positive NSCLC (16). Tumor markers for determining the prognosis of alectinib treatment remain lacking. Thus, the aim was to assess the efficacy and safety of alectinib in ALK-positive NSCLC, and to explore potential prognostic factors. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-857/rc).
Methods
Patients
This study retrospectively analyzed 102 patients with ALK-positive NSCLC who were admitted to the Affiliated Cancer Hospital of Nanjing Medical University from October 2018 to October 2021. All patients satisfied the following inclusion criteria: (I) tissues from primary or metastatic lesions were diagnosed as NSCLC by pathology or cytology; (II) ALK-positivity was confirmed by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH); (III) clinical stage was unresectable stage III or IV; (IV) patients have been treated with alectinib for at least one month; and (V) patients were at least 18 years old. The following exclusion criteria were applied: (I) patients with severe hepatic and renal insufficiency; (II) patients with severe medical disease or acute infection; and (III) pregnant or lactating women. The sample size of this study was determined by the number of patients who met the inclusion and exclusion criteria. Patients who lost follow-up were not be included in the analysis.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital (No. 2022-031). Informed consent for this retrospective analysis was waived.
Data collection
The clinical data of patients were collated through our electronic medical records, including age, sex, body mass index (BMI), primary tumor location, degree of differentiation, tumor-node-metastasis (TNM), Eastern Cooperative Oncology Group (ECOG), whether bevacizumab combination therapy was used, treatment lines. Hematologic indicators before treatment and 3 months after treatment were collected, including blood count [leukocytes, hemoglobin (HGB), erythrocytes, platelets, neutrophils, lymphocytes, and monocytes], blood biochemistry [albumin (Alb) and lactate dehydrogenase (LDH)], coagulation indicators (fibrinogen and D-dimer), tumor markers [carcinoembryonic antigen (CEA), carbohydrate antigen (CA)125, CA199, neuron-specific enolase (NSE), cytokeratin 19, squamous cell carcinoma (SCC)], lymphocyte subset [lymphocyte ratio, CD3+ ratio, CD4+ ratio, CD8+ ratio, CD4+/CD8+, natural killer cell (NK) ratio, B cell ratio, Treg cell ratio], NLR, PLR, cytoplasmic thymidine kinase, thioredoxin reductase activity, and cfDNA concentration levels. The cfDNA concentration was measured by quantitative polymerase chain reaction (qPCR). Indicators lost before treatment or after 3-month treatment are excluded from statistical analysis. Survival and adverse reactions were followed up by telephone consultation and medical records.
Treatment
After the ALK translocation was confirmed by FISH or IHC, patients were administered alectinib (600 mg, bid) orally until intolerable adverse effects developed or until disease progression.
Grouping methods
Patients were divided into two groups according to the number of treatment lines. First-line treatment was defined as the absence of crizotinib or other second-generation ALK inhibitors before treatment with alectinib. Second-line treatment was defined as crizotinib resistance followed by alectinib treatment.
According to the indicators before and after treatment was normal or not, patients were divided into four groups. In group A, indicators were normal before treatment and normal after 3 months of treatment. In group B, indicators were normal before treatment and abnormal after 3 months of treatment. In group C, indicators were abnormal before treatment and normal after 3 months of treatment. In group D, indicators were abnormal before treatment and abnormal after 3 months of treatment.
Assessments
PFS was defined as the time from the first alectinib administration until disease progression or death from cancer. Indicators for the evaluation of adverse drug reactions (ADR) were assessed. Adverse reactions that occurred during treatment with alectinib were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) 5.0.
Statistical analysis
Demographic, clinical, and treatment characteristics and adverse events were summarized with descriptive statistics. The quantitative variables in this study included age, BMI, and hematologic indicators. The patients were further divided into 2 groups (i.e., <50 and ≥50 years old) based on age, as the number of new lung cancer cases and deaths peak in patients older than 50 years. Patients were further divided into 2 groups (i.e., <18.5 kg/m2 & >24 kg/m2 as abnormal, 18.5 to 24 kg/m2 as normal) based on the BMI. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff values for NLR and PLR in terms of their association with survival. Other hematological indicators were divided into normal and abnormal groups according to the critical values specified by the Affiliated Cancer Hospital of Nanjing Medical University. Chi-square test was used to compare categorical variables. The Kaplan-Meier method was used for survival analysis and PFS curves were plotted. The survival curves were compared between groups using the log-rank test. The log-rank test was used for univariate analysis and the Cox proportional-hazards model was used for multivariate analysis. The prognostic factors were initially screened using univariate analysis, and the test level was set to 0.05, that is, the factors with P<0.05 in the univariate analysis were included in the multivariate analysis to determine the independent prognostic factors of PFS. Subgroup analysis was conducted to detect the correlation between circulating tumor markers and therapeutic effect. All analyses were 2-sided, and significance was set at a P value of 0.05. The data were analyzed and plotted using statistical software such as SPSS 26.0, GraphPad Prism 8.0.2, and Origin 2021.
Results
Follow-up
As of November 16, 2022, the median follow-up time was 21.9 months [interquartile range (IQR), 10.0 to 29.9 months]. The longest follow-up time was 50.2 months. A total of 102 patients were included in the study, of which, 6 patients were lost to follow-up. In the remaining 96 ALK-positive patients, FISH was performed in 45 patients and IHC in 51 patients. A total of 59 patients (61.46%) received first-line therapy, and 36 patients (37.50%) received second-line therapy. One patient (1.04%) received third line therapy. Considering too small sample size of third-line treatment, the patient was not included in the efficacy and prognosis analysis, but included in the analysis of adverse events. Finally, 96 patients were admitted to this study for further safety analysis, and 95 patients for analysis of efficacy and prognosis of alectinib treatment (Figure 1).

Baseline patient characteristics
The median age of patients was 55.5 years; 95 patients included 42 males (44.21%) and 53 females (55.79%). There were 83 patients with an ECOG performance status of 0–1, and 12 patients with an ECOG performance status of 2. Within the study cohort, 81.05% of patients (77/95) had received pre-treatment prior to alectinib administration, of which 35.79% (34/95) had undergone surgery, 60.00% (57/95) had received chemotherapy, and 28.42% (27/95) had received radiation therapy. There were 21 patients who received combination alectinib and bevacizumab therapy (see Table 1 for comprehensive patient characteristics).
Table 1
| Characteristics | All (n=95), n (%) | First-line (n=59), n (%) | Second-line (n=36), n (%) |
|---|---|---|---|
| Gender | |||
| Male | 42 (44.21) | 31 (52.54) | 11 (30.56) |
| Female | 53 (55.79) | 28 (47.46) | 25 (69.44) |
| Age, years | |||
| <50 | 36 (37.89) | 24 (40.68) | 12 (33.33) |
| ≥50 | 59 (62.11) | 35 (59.32) | 24 (66.67) |
| BMI, kg/m2 | |||
| 18.5–24.9 | 58 (61.05) | 38 (64.41) | 20 (55.56) |
| <18.5 & >24.9 | 37 (38.95) | 21 (35.59) | 16 (44.44) |
| ECOG | |||
| 0–1 | 83 (87.37) | 54 (91.53) | 29 (80.56) |
| ≥2 | 12 (12.63) | 5 (8.47) | 7 (19.44) |
| Primary site | |||
| Central | 47 (49.47) | 29 (49.15) | 18 (50.00) |
| Peripheral | 48 (50.53) | 30 (50.85) | 18 (50.00) |
| Differentiation | |||
| I | 1 (1.05) | 1 (1.69) | 0 (0.00) |
| II | 24 (25.26) | 16 (27.12) | 8 (22.22) |
| III | 25 (26.32) | 13 (22.03) | 12 (33.33) |
| Unknown | 45 (47.37) | 29 (49.15) | 16 (44.44) |
| Stage | |||
| III | 14 (14.74) | 11 (18.64) | 3 (8.33) |
| IV | 81 (85.26) | 48 (81.36) | 33 (91.67) |
| Pre-treatment | |||
| Yes | 77 (81.05) | 46 (77.97) | 31 (86.11) |
| No | 18 (18.95) | 13 (22.03) | 5 (13.89) |
| Surgery | |||
| Yes | |||
| Radical | 29 (30.53) | 19 (32.20) | 10 (27.78) |
| Interventional | 5 (5.26) | 3 (5.08) | 2 (5.56) |
| No | 61 (64.21) | 37 (62.71) | 24 (66.67) |
| Chemotherapy | |||
| Yes | |||
| Cycles ≤4 | 31 (32.63) | 20 (33.90) | 11 (30.56) |
| Cycles >4 | 26 (27.37) | 11 (18.64) | 15 (41.67) |
| No | 38 (40.00) | 28 (47.46) | 10 (27.78) |
| Radiotherapy | |||
| Yes | |||
| Radical | 4 (4.21) | 3 (5.08) | 1 (2.78) |
| Preventive | 2 (2.11) | 1 (1.69) | 1 (2.78) |
| Palliative | 21 (22.11) | 10 (16.95) | 11 (30.56) |
| No | 68 (71.58) | 45 (76.27) | 23 (63.89) |
| Combined with bevacizumab | |||
| Yes | 21 (22.11) | 10 (16.95) | 11 (30.56) |
| No | 74 (77.89) | 49 (83.05) | 25 (69.44) |
| External metastasis site of primary tumor | |||
| Lymph node | 84 (88.42) | 50 (84.75) | 34 (94.44) |
| Intrapulmonary | 34 (35.79) | 20 (33.90) | 14 (38.89) |
| Brain | 26 (27.37) | 15 (25.42) | 11 (30.56) |
| Liver | 13 (13.68) | 6 (10.17) | 7 (19.44) |
| Bone | 39 (41.05) | 22 (37.29) | 17 (47.22) |
| Pleural | 44 (46.32) | 25 (42.37) | 19 (52.78) |
| Malignant pleural effusion | 35 (36.84) | 18 (30.51) | 17 (47.22) |
| Adrenal | 15 (15.79) | 10 (16.95) | 5 (13.89) |
| Other | 7 (7.37) | 2 (3.39) | 5 (13.89) |
| Number of transferred organs | |||
| <2 | 19 (20.00) | 15 (25.42) | 4 (11.11) |
| ≥2 | 76 (80.00) | 44 (74.58) | 32 (88.89) |
NSCLC, non-small cell lung cancer; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group.
PFS outcomes
All patients
At the data cutoff date, the median PFS of the overall cohort was 35.1 months [95% confidence interval (CI): not reached]. A total of 20 patients progressed after first-line treatment with alectinib, and 21 patients progressed following second-line treatment with alectinib. The median PFS was not achieved for first-line treatment and the median PFS for second-line treatment was 15.0 months (95% CI: 0.00–32.23; Figure 2).

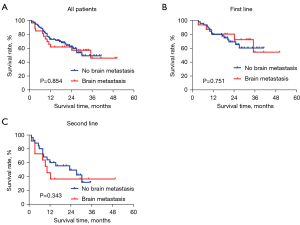
Subgroup analysis according to brain metastasis
This study further analyzed patients with or without brain metastases. There was a total of 15 patients with brain metastases in the first-line treatment group, of which 12 cases had brain metastases before treatment, and 3 patients had CNS progression during treatment. Among the 12 patients with brain metastases at baseline, the brain metastasis foci were reduced in 8 (66.7%) cases, unchanged in 2 (16.7%) cases, and enlarged in 2 (16.7%) cases after treatment with alectinib. In the second-line treatment group, there were 11 patients with brain metastases before alectinib treatment, of which 6 (54.5%) cases showed reduced brain metastases after treatment with alectinib, 3 (27.3%) cases had progressed, 1 (9.1%) case experienced stable disease, and 1 (9.1%) case was unknown response. There was also no difference in PFS between patients with and without brain metastases in subgroup analyses of the first-line treatment group and second-line treatment group (Table 2, Figure 3).
Table 2
| Treatment lines | New brain metastases | Primary brain metastases | PD, n (%) | SD, n (%) | PR, n (%) | CR, n (%) | Unknown response, n (%) |
|---|---|---|---|---|---|---|---|
| First-line | 3 | 12 | 2 (16.7) | 2 (16.7) | 6 (50.0) | 2 (16.7) | 0 |
| Second-line | 0 | 11 | 3 (27.3) | 1 (9.1) | 6 (54.5) | 0 | 1 (9.1) |
CNS, central nervous system; NSCLC, non-small cell lung cancer; PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response.
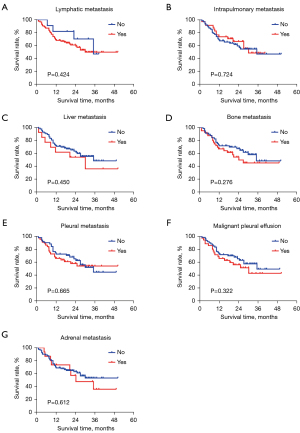
Subgroup analysis by other metastases
Subgroup analysis of metastases in other sites was performed and the results showed no significant correlation between metastases and PFS, with P values greater than 0.05 (Figure 4).
Prognostic analysis
Prognostic-related factors were analyzed in all patients. According to ROC analysis, the optimal cutoff values were 4.05 for NLR and 127 for PLR (Figure 5). Univariate analysis revealed that treatment lines, NLR, CEA, LDH, CA199, and cfDNA may be associated with PFS (P<0.05) (Table 3). In multivariate analysis, treatment lines and NLR were independent prognostic factors for PFS (P<0.05) (Table 4, Figure 6).
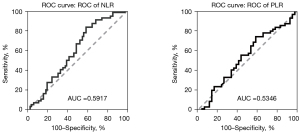
Table 3
| Characteristics | P |
|---|---|
| Gender | |
| Male & female | 0.422 |
| Age (years) | |
| <50 & ≥50 | 0.267 |
| BMI | |
| Normal & abnormal | 0.906 |
| ECOG | |
| 0–1 & ≥2 | 0.144 |
| Hypertension or diabetes | |
| Yes & no | 0.308 |
| Primary site | |
| Central & peripheral | 0.678 |
| Differentiation | |
| I & II & III | 0.595 |
| Stage | |
| III & IV | 0.212 |
| Pre-treatment | |
| Yes & no | 0.279 |
| Surgery | |
| Yes & no | 0.173 |
| Radical & interventional | 0.153 |
| Chemotherapy | |
| Yes & no | 0.603 |
| Cycles ≤4 & >4 | 0.564 |
| Radiotherapy | |
| Yes & no | 0.553 |
| Radical & preventive & palliative | 0.118 |
| Bevacizumab | |
| With & without | 0.486 |
| Lymph node metastasis | |
| Yes & no | 0.423 |
| Intrapulmonary metastases | |
| Yes & no | 0.729 |
| Brain metastases | |
| Yes & no | 0.857 |
| Liver metastases | |
| Yes & no | 0.452 |
| Bone metastases | |
| Yes & no | 0.278 |
| Pleural metastases | |
| Yes & no | 0.668 |
| Malignant pleural effusion | |
| Yes & no | 0.324 |
| Adrenal metastases | |
| Yes & no | 0.614 |
| Number of transferred organs | |
| <2 & ≥2 | 0.307 |
| Number of treatment lines | |
| First-line & second-line | 0.004 |
| Blood count | |
| Leucocyte | |
| Normal & abnormal | 0.348 |
| Hemoglobin | |
| Normal & abnormal | 0.108 |
| Erythrocyte | |
| Normal & abnormal | 0.142 |
| Platelet | |
| Normal & abnormal | 0.380 |
| Neutrophil | |
| Normal & abnormal | 0.102 |
| Lymphocyte | |
| Normal & abnormal | 0.603 |
| NLR | |
| <4.05 & ≥4.05 | 0.005 |
| PLR | |
| <127 & ≥127 | 0.150 |
| Blood biochemistry | |
| Monocyte | |
| Normal & abnormal | 0.083 |
| Alb | |
| Normal & abnormal | 0.342 |
| Coagulation | |
| LDH | |
| Normal & abnormal | <0.001 |
| Fibrinogen | |
| Normal & abnormal | 0.149 |
| Tumor markers | |
| D-dimer | |
| Normal & abnormal | 0.065 |
| CEA | |
| Normal & abnormal | 0.029 |
| CA125 | |
| Normal & Abnormal | 0.923 |
| CA199 | |
| Normal & abnormal | 0.002 |
| NSE | |
| Normal & abnormal | 0.080 |
| Keratin 19 | |
| Normal & abnormal | 0.482 |
| Lymphocyte subset | |
| Squamous cell carcinoma antigen | |
| Normal & abnormal | 0.245 |
| Lymphocyte (%) | |
| Normal & abnormal | 0.957 |
| CD3+ T cell (%) | |
| Normal & abnormal | 0.717 |
| CD4+ T cell (%) | |
| Normal & abnormal | 0.577 |
| CD8+ T cell (%) | |
| Normal & abnormal | 0.654 |
| CD4/CD8 | |
| Normal & abnormal | 0.847 |
| NK (%) | |
| Normal & abnormal | 0.842 |
| B cell (%) | |
| Normal & abnormal | 0.793 |
| Others | |
| Regulatory T cell (%) | |
| Normal & abnormal | 0.491 |
| Thioredoxin reductase | |
| Normal & abnormal | 0.730 |
| cfDNA | |
| Normal & abnormal | 0.008 |
PFS, progression-free survival; NSCLC, non-small cell lung cancer; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; Alb, albumin; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen; CA, carbohydrate antigen; NSE, neuron-specific enolase; NK, natural killer cell; cfDNA, cell free DNA.
Table 4
| Projects | P | HR | 95% CI |
|---|---|---|---|
| Number of treatment lines | 0.042 | 3.213 | 1.042–9.907 |
| NLR | 0.034 | 3.058 | 1.091–8.575 |
| LDH | 0.971 | 0.979 | 0.310–3.096 |
| CEA | 0.335 | 1.754 | 0.559–5.502 |
| CA199 | 0.059 | 3.277 | 0.957–11.224 |
| cfDNA | 0.792 | 1.214 | 0.288–5.127 |
PFS, progression-free survival; NSCLC, non-small cell lung cancer; HR, hazard ratio; CI, confidence interval; NLR, neutrophil-lymphocyte ratio; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen; CA, carbohydrate antigen; cfDNA, cell free DNA.
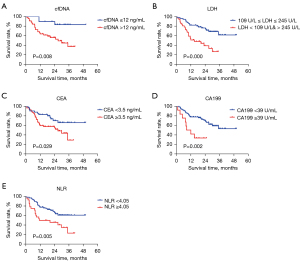
Efficacy indicators
The relationship between hematological indexes and prognosis was further analyzed, and the following results were obtained: Group A vs. Group C: for these parameters (LDH, CEA, CA199, NLR), there was no significant difference in PFS between Group A and Group C (P>0.05); Group C vs. Group D: for LDH, CEA, CA199, and NLR, PFS in Group C was significantly longer than that in Group D, respectively (P<0.05); Group A + Group C vs. Group B + Group D: for CEA, CA199, and NLR, PFS was significantly longer in patients with normal indicator than in those with abnormal indicator after 3 months of treatment with aletinib, respectively (P<0.05) (Table 5, Figure 7).
Table 5
| Projects | Subgroups | cfDNA | LDH | CEA | CA199 | NLR |
|---|---|---|---|---|---|---|
| Number (proportion) | Group A | 4 (25.0%) | 19 (35.2%) | 18 (34.6%) | 31 (79.5%) | 37 (71.2%) |
| Group B | 2 (12.5%) | 12 (22.2%) | 0 (0.0%) | 0 (0.0%) | 1 (1.9%) | |
| Group C | 2 (12.5%) | 9 (16.7%) | 12 (23.1%) | 5 (12.8%) | 10 (19.2%) | |
| Group D | 8 (50.0%) | 14 (25.9%) | 22 (42.3%) | 3 (7.7%) | 4 (7.7%) | |
| P | A vs. C | – | 0.177 | 0.407 | 0.483 | 0.599 |
| A vs. D | – | 0.003 | 0.035 | <0.001 | 0.007 | |
| C vs. D | – | 0.037 | 0.024 | 0.004 | 0.035 | |
| A + C vs. B + D | 0.254 | 0.108 | 0.021 | <0.001 | 0.001 |
Group A: indicators were normal before treatment and normal after 3 months of treatment. Group B: indicators were normal before treatment and abnormal after 3 months of treatment. Group C: indicators were abnormal before treatment and normal after 3 months of treatment. Group D: indicators were abnormal before treatment and abnormal after 3 months of treatment. NSCLC, non-small cell lung cancer; cfDNA, cell free DNA; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen; CA, carbohydrate antigen; NLR, neutrophil-lymphocyte ratio.

Adverse reactions
The most common adverse reactions were anemia, liver dysfunction, fatigue, constipation, and rash. Six patients (6.25%) experienced grade III and above adverse reactions. These patients improved after symptomatic treatment and continued to take the original dose of alectinib (Table 6, Figure 8).
Table 6
| Adverse reactions | All | Proportion | Grade I | Proportion | Grade II | Proportion | Grade III | Proportion | Grade IV | Proportion |
|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhea | 1 | 1.04% | 1 | 1.04% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% |
| Vomiting | 2 | 2.08% | 1 | 1.04% | 0 | 0.00% | 1 | 1.04% | 0 | 0.00% |
| Weight gain | 4 | 4.17% | 2 | 2.08% | 1 | 1.04% | 1 | 1.04% | 0 | 0.00% |
| Nausea | 4 | 4.17% | 3 | 3.13% | 1 | 1.04% | 0 | 0.00% | 0 | 0.00% |
| Edema | 6 | 6.25% | 4 | 4.17% | 2 | 2.08% | 0 | 0.00% | 0 | 0.00% |
| Elevated serum creatinine | 9 | 9.38% | 8 | 8.33% | 1 | 1.04% | 0 | 0.00% | 0 | 0.00% |
| Myalgia | 10 | 10.42% | 10 | 10.42% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% |
| Rash | 10 | 10.42% | 7 | 7.29% | 3 | 3.13% | 0 | 0.00% | 0 | 0.00% |
| Fatigue | 17 | 17.71% | 16 | 16.67% | 1 | 1.04% | 0 | 0.00% | 0 | 0.00% |
| Constipation | 20 | 20.83% | 18 | 18.75% | 2 | 2.08% | 0 | 0.00% | 0 | 0.00% |
| Anemia | 36 | 37.50% | 19 | 19.79% | 13 | 13.54% | 3 | 3.13% | 1 | 1.04% |
| Abnormal liver function | 36 | 37.50% | 36 | 37.50% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% |
NSCLC, non-small cell lung cancer.
Discussion
ALK is a transmembrane receptor tyrosine kinase that catalyzes the transfer of γ-phosphoric acid on adenosine-triphosphate to a protein tyrosine residue to activate tyrosine, thereby activating downstream signaling pathways. Mutations in the ALK gene cause downstream signaling pathway activation and dysregulation, allowing tumor cells expressing these proteins to proliferate and differentiate and inhibit apoptosis (17). Approximately 3% to 5% of patients with NSCLC have ALK rearrangement, and these patients are effectively treated with ALK-TKIs. In recent year, alectinib has attracted much attention for its efficacy and safety in ALK-positive NSCLC (9,18). In this study, the median PFS of the overall patient was 35.1 months. The median PFS was not achieved in first-line treatment and the median PFS in second-line treatment was 15.0 months. A Study have shown that alectinib is highly active in patients with advanced, crizotinib-refractory ALK-positive NSCLC, which can still deliver 8.9-month PFS. PFS for second-line treatment in our study was longer than previously reported, possibly due to lower brain metastasis rates before alectinib treatment (30% vs. 61%) (18). In this study, the incidence of CNS progression in first-line therapy was 8.5%, consistent with 8.7% reported in the J-ALEX study, while the rate of CNS progression in the crizotinib group in this study was up to 25.0% (19). Alectinib is significantly superior to crizotinib in reducing CNS progression and controlling brain metastases. It penetrates the blood-brain barrier more easily than crizotinib, thus gaining a significant advantage in the treatment of ALK-positive NSCLC brain metastases, which has been demonstrated in several other studies (17,20-22). According to ALEX study, for alectinib vs. for crizotinib, the median PFS times in patients with baseline CNS metastases were 27.7 and 7.4 months, respectively, and for patients without baseline CNS metastases, the median PFS times were 34.8 and 14.7 months, respectively (23). In second-line treatment, alectinib is still effective in patients who develop CNS progression after previous treatment with crizotinib. Overall, alectinib has shown significant efficacy on the CNS, regardless of whether or not the patient had previously received crizotinib treatment. Alectinib shows superiority in PFS, but whether PFS can be converted into survival benefits remains to be explored. J-ALEX recently announced a 5-year overall survival rate, the result did not show superiority of alectinib to crizotinib (24).
Although ALK inhibitors are widely used in advanced or recurrent NSCLC, to date, there are no identifiable biomarkers that can predict the efficacy of ALK inhibitors in ALK-positive NSCLC. In our investigation, LDH, circulating immune cells, cfDNA concentration, and plasma tumor markers in the peripheral blood before and after alectinib treatment were analyzed. First, the study explored whether the common hematological indicators at baseline could be markers for the prognosis of advanced NSCLC. In the univariate analysis, LDH, CEA, CA199, cfDNA, and NLR all showed statistical significance. In the multivariate analysis, only NLR were shown to be independent prognostic factors. At baseline, patients with abnormal levels of these biomarkers tended to have a poorer prognosis. Second, this study explored the clinical efficacy of certain tumor markers, NLR and cfDNA after 3 months of alectinib treatment. Considering the lack of reliable predictors of alectinib efficacy and the enormous challenges of obtaining enough tumor tissue for molecular testing in advanced patients, predictive biomarkers of peripheral blood, such as tumor markers and inflammatory factors, may be potential indicators that can be dynamically monitored and used for efficacy analysis. The results herein demonstrated that patients with normal pretreatment indicators had significantly higher PFS than patients with abnormal pretreatment indicators. However, whether indicators were normal or not before treatment, patients whose indicators reached the normal range after 3 months of treatment showed no statistical difference in PFS. Patients with CEA, CA199, NLR, and LDH in the normal range after 3 months of alectinib treatment had significantly better PFS than those who remained abnormal after 3 months of treatment. This suggested that different ALK-positive NSCLC patients have differences in sensitivity to alectinib treatment, and CEA, CA199, NLR, and LDH after 3 months of treatment can effectively predict the therapeutic effect of alectinib.
CEA is a serum glycoprotein, which is currently the most widely used marker for colorectal cancer, breast cancer, and lung cancer. CA199 can be used as an indicator for monitoring and predicting recurrence of digestive system tumors, such as pancreatic cancer, gallbladder cancer, and lung adenocarcinoma. CEA and CA199 are nonspecific tumor markers that are overexpressed in many malignant tumors, including NSCLC. They are easily detected in blood samples, which makes them valuable for prognosis and follow-up evaluation of NSCLC. A previous study confirmed that high baseline CEA levels are associated with poor survival in patients with stage III–IV NSCLC (25). This was consistent with our conclusions herein. Another study found that serum CEA levels may be an independent prognostic factor for predicting the efficacy of epidermal growth factor receptor (EGFR) TKIs in patients with advanced NSCLC, however, the correlation between changes in CEA levels and the efficacy of ALK inhibitors remains unconfirmed (26). In previous reports of tumor markers, CA199 has shown relatively low sensitivity and specificity. It is often used in combination with other tumor markers for the diagnosis of lung cancer and the evaluation of the efficacy of chemotherapy (27,28). In our study, CA199 was found to be an independent prognostic factor in univariate, which may be associated with ALK rearrangement. In a research on the relationship between gene mutation and tumor markers in lung adenocarcinoma, the baseline levels of CA199 in samples with ALK rearrangement were higher than those observed in samples with EGFR mutations, KRAS mutations, and ROS1 rearrangement (29). It remains unclear whether there is any association between CA199 levels and ALK rearrangement. LDH is an enzyme that plays an important role in anaerobic glycolysis (30). Higher levels of LDH promote tumor invasion and metastasis, and thus, inhibition of LDH will lead to reduced cell proliferation. Therefore, higher levels of LDH suggest a lower overall survival rate in NSCLC patients (31). NSE is an acidic protein specific to neurons and neuroendocrine cells, and is thus a specific marker of neuroendocrine tumors. NSE is more common in, but not unique to, small cell lung cancers. Some patients with NSCLC have increased levels of NSE. The univariate survival analysis of our study showed that NSE-negative patients had better PFS than those who were positive, but it was not an independent prognostic factor in multivariate analysis. NLR is a novel inflammatory index, which is closely related to the body’s immunity. In the process of tumorigenesis and development, inflammation plays an important role in tumor proliferation, apoptosis, migration, invasion, and metastasis. Research on the NLR and the efficacy of immune checkpoint inhibitors are currently in full swing, however few studies have analyzed the correlation of NLR and efficacy of ALK inhibitors. A study of the Chinese Academy of Medical Sciences confirmed that in ALK-positive NSCLC patients treated with crizotinib, the trend of NLR, derived NLR [dNLR = neutrophil count/(white blood cell count-neutrophil count)], PLR, and HGB can be used to assess a patient’s progress (15). A Japanese study noted that immunological and nutritional markers could be useful in predicting the outcomes of first-line treatment with alectinib (32). In our study, NLR was not only an independent prognostic factor in univariate and multivariate analysis, but also changes in NLR before and after treatment can reflect the efficacy and be used to further predict survival. However, PLR and HGB did not show a correlation with survival, which may be related to different cut-off values for indicators selected by research institutions. cfDNA refers to DNA that appears in a free state outside the cell and is widely present in the body’s plasma, serum, and urine. In the plasma of healthy people, cfDNA comes from apoptotic cells, not from necrotic cells. cfDNA in the plasma of cancer patients not only come from apoptotic cells, but also from the DNA that is actively released by tumor cells and DNA released by tumor cell necrolysis. The concentration of cfDNA in the blood of cancer patients is higher than that of normal healthy people, and there is tumor-specific information on fragment distribution, fragmented information, and terminal sequences. Therefore, cfDNA is also often used as a marker for tumor surveillance and has been proposed as a prognostic factor in NSCLC patients under targeted therapies (33) or immunotherapy (34). Recently, ALEX research proposed that plasma cfDNA concentration may have prognostic value in advanced ALK-positive NSCLC (16).
A meta-analysis of 8 clinical trials showed that the most common adverse events were constipation (29%), anemia (25%), myalgia (18%), peripheral edema (18%), taste disturbances (18%), and elevated creatine phosphokinase (18%) (35). The side effect profile of alectinib in our study was in keeping with that reported in prospective trials. The most common adverse reactions in our study were abnormal liver function (37.50%), anemia (37.50%), constipation (20.83%), fatigue (17.71%), rash (10.42%), myalgia (10.42%), elevated serum creatinine (9.38%), and edema (6.25%). Six patients (6.25%) who experienced grade III or above adverse reactions all improved after symptomatic treatment and continuation at the original dose. Overall, patients showed good tolerance to alectinib, and no patients discontinued the drug due to side effects, reflecting the advantages of this drug in terms of safety.
The foremost advantage of our study is that the identification of biomarkers that can predict the efficacy of ALK inhibitor. As far as we know, our study appears as the first one that reported a such evaluation. The real-world population reflects the true state of the patients in the clinical setting, which supports the universality of this report. However, there were some limitations to this work. It was a retrospective study without a control group or a head-to-head comparison with crizotinib, and thus lacks a more intuitive and detailed comparisons. A retrospective study compared the survival of NSCLC patients with ALK rearrangement undergoing sequential crizotinib treatment with alectinib or single therapy with alectinib (36). Our study lacked comparison of the efficacy of alectinib as sequential therapy and single therapy, and failed to further clarify the best method for the clinical application of alectinib.
Conclusions
Alectinib as a first-line or second-line treatment for ALK-positive NSCLC effectively prolonged the PFS, and led to good tolerance to ADRs. Detection of serum tumor markers, such as CEA, CA199, NLR, and LDH, can indicate therapeutic success and predict prognosis in patients treated with alectinib. In addition, in order to have more robust results, larger samples of circulating tumor marker before and after alectinib treatment are warranted for future investigations.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81802277) and the Jiangsu Health Commission (No. Y20180808).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-857/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-857/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-857/coif). AR declares consulting fees form AstraZeneca, participation on advisory board (Takeda), supporting for attending to meetings form Thermofisher and BMS. BGMH declares Advisory Board of Merck, Sharpe and Dohme, Eisai, Pfizer, Sanofi and Takeda. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital (No. 2022-031). Informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Noor ZS, Cummings AL, Johnson MM, et al. Targeted Therapy for Non-Small Cell Lung Cancer. Semin Respir Crit Care Med 2020;41:409-34. [Crossref] [PubMed]
- Li H. Correlation between ALK,ROS1 and BRAF-V600E gene mutations and clinical features in patients with non-small cell lung carcinoma. Journal of Molecular Diagnostics and Therapy 2020;12:701-4,719.
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol 2016;27:iii42-50. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Nakagawa K, Hida T, Nokihara H, et al. Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2020;139:195-9. [Crossref] [PubMed]
- Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatmentIe anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 2018;29:2214-22. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Pan Y, Xiao W, Ye F, et al. Outcomes of switching from crizotinib to alectinib in patients with advanced non-small cell lung cancer with anaplastic lymphoma kinase fusion. Ann Transl Med 2021;9:1014. [Crossref] [PubMed]
- Fujimoto A, Toyokawa G, Koutake Y, et al. Association between pretreatment neutrophil-to-lymphocyte ratio and immune-related adverse events due to immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac Cancer 2021;12:2198-204. [Crossref] [PubMed]
- Platini H, Ferdinand E, Kohar K, et al. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Markers for Advanced Non-Small-Cell Lung Cancer Treated with Immunotherapy: A Systematic Review and Meta-Analysis. Medicina (Kaunas) 2022;58:1069. [Crossref] [PubMed]
- Bryant AK, Sankar K, Strohbehn GW, et al. Prognostic and predictive value of neutrophil-to-lymphocyte ratio with adjuvant immunotherapy in stage III non-small-cell lung cancer. Lung Cancer 2022;163:35-41. [Crossref] [PubMed]
- Cho A, Kranawetter B, Untersteiner H, et al. Neutrophil-to-Lymphocyte Ratio Is Superior to Other Leukocyte-Based Ratios as a Prognostic Predictor in Non-Small Cell Lung Cancer Patients with Radiosurgically Treated Brain Metastases Under Immunotherapy or Targeted Therapy. World Neurosurg 2021;151:e324-31. [Crossref] [PubMed]
- Yang Y, Xu H, Yang G, et al. The value of blood biomarkers of progression and prognosis in ALK-positive patients with non-small cell lung cancer treated with crizotinib. Asia Pac J Clin Oncol 2020;16:63-9. [Crossref] [PubMed]
- Dziadziuszko R, Peters S, Mok T, et al. Circulating Cell-free DNA as a Prognostic Biomarker in Patients with Advanced ALK+ Non-small Cell Lung Cancer in the Global Phase III ALEX Trial. Clin Cancer Res 2022;28:1800-8. [Crossref] [PubMed]
- Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer 2013;13:685-700. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Nishio M, Nakagawa K, Mitsudomi T, et al. Analysis of central nervous system efficacy in the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2018;121:37-40. [Crossref] [PubMed]
- Zou Z, Xing P, Hao X, et al. Intracranial efficacy of alectinib in ALK-positive NSCLC patients with CNS metastases-a multicenter retrospective study. BMC Med 2022;20:12. [Crossref] [PubMed]
- Sakamoto H, Yanagitani N, Manabe R, et al. Characteristics of central nervous system progression in non-small cell lung cancer treated with crizotinib or alectinib. Cancer Rep (Hoboken) 2021;4:e1414. [Crossref] [PubMed]
- Tang H, Jin L, Zhang Z, et al. Comparison of Clinical Efficacy of Alectinib Versus Crizotinib in ALK-Positive Non-Small Cell Lung Cancer: A Meta-Analysis. Front Oncol 2021;11:646526. [Crossref] [PubMed]
- Camidge DR, Dziadziuszko R, Peters S, et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac Oncol 2019;14:1233-43. [Crossref] [PubMed]
- Hotta K, Hida T, Nokihara H, et al. Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naïve Japanese patients with ALK-positive non-small-cell lung cancer. ESMO Open 2022;7:100527. [Crossref] [PubMed]
- Cedrés S, Nuñez I, Longo M, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer 2011;12:172-9. [Crossref] [PubMed]
- Zhao XM, Zhao J, Xing KL, et al. Prognostic and predictive value of serum carcinoembryonic antigen levels in advanced non-small cell lung cancer patients with epidermal growth factor receptor sensitive mutations and receiving tyrosine kinase inhibitors. Oncotarget 2017;8:70865-73. [Crossref] [PubMed]
- Zhu Y, Yang Y, Wang Y, et al. Role of serum CA125 and CA199 concentration in diagnosis and prognosis evaluation of lung cancer patients. Int J Clin Exp Pathol 2016;9:5388-96.
- Liao Y, Yang D, Liu W, et al. The diagnostic effect and clinical treatment guidance value of CA125 and CA199 combined with NSE in patients with primary lung cancer. Basic & Clinical Pharmacology & Toxicology 2020;127:117.
- Noonan SA, Patil T, Gao D, et al. Baseline and On-Treatment Characteristics of Serum Tumor Markers in Stage IV Oncogene-Addicted Adenocarcinoma of the Lung. J Thorac Oncol 2018;13:134-8. [Crossref] [PubMed]
- Yeung C, Gibson AE, Issaq SH, et al. Targeting Glycolysis through Inhibition of Lactate Dehydrogenase Impairs Tumor Growth in Preclinical Models of Ewing Sarcoma. Cancer Res 2019;79:5060-73. [Crossref] [PubMed]
- Jin L, Chun J, Pan C, et al. Phosphorylation-mediated activation of LDHA promotes cancer cell invasion and tumour metastasis. Oncogene 2017;36:3797-806. [Crossref] [PubMed]
- Takeda T, Yamada T, Tanimura K, et al. Prognostic Markers of Survival among Japanese Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer Receiving First-Line Alectinib. Diagnostics (Basel) 2021;11:2170. [Crossref] [PubMed]
- Provencio M, Serna-Blasco R, Franco F, et al. Analysis of circulating tumour DNA to identify patients with epidermal growth factor receptor-positive non-small cell lung cancer who might benefit from sequential tyrosine kinase inhibitor treatment. Eur J Cancer 2021;149:61-72. [Crossref] [PubMed]
- Provencio M, Serna-Blasco R, Nadal E, et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol 2022;40:2924-33. [Crossref] [PubMed]
- Fan J, Xia Z, Zhang X, et al. The efficacy and safety of alectinib in the treatment of ALK+ NSCLC: a systematic review and meta-analysis. Onco Targets Ther 2018;11:1105-15. [Crossref] [PubMed]
- Ito K, Yamanaka T, Hayashi H, et al. Sequential therapy of crizotinib followed by alectinib for non-small cell lung cancer harbouring anaplastic lymphoma kinase rearrangement (WJOG9516L): A multicenter retrospective cohort study. Eur J Cancer 2021;145:183-93. [Crossref] [PubMed]



