
A new preoperative localization of pulmonary nodules guided by mixed reality: a pilot study of an animal model
Highlight box
Key findings
• MR-guided localization of pulmonary nodules is feasible and safe, which is worthy of further research and promotion.
What is known and what is new?
• The accuracy of MR-guided pulmonary nodule localization can meet clinical requirements.
• We invented a new method of localization of pulmonary nodules with MR-guided which is feasible and safe.
What is the implication, and what should change now?
• We used MR to locate pulmonary nodules successfully, which provided a new method for preoperative localization. and a new direction for the development of MR in clinical work. A future RCT will be needed to further validate its advantages.
Introduction
With the popularity of high-resolution computed tomography (HRCT), an increasing number pulmonary nodules are being found (1-3). As lung cancer has the highest incidence rate in the world, the high detection rate of pulmonary nodules increases the psychological burden of those affected. For high-risk pulmonary nodules, surgical resection is still the preferred diagnostic and treatment method recommended by the current guidelines of National Comprehensive Cancer Network (NCCN), European Society for Medical Oncology (ESMO) and Chinese Society of Clinical Oncology (CSCO). Video-assisted thoracoscopic surgery (VATS) has become the first choice for surgical treatment of pulmonary nodules. With the appearance of more and more ground glass nodules (GGN), the traditional localization methods such as surgeon’s palpation or observation of pleural changes cannot meet the clinical demands. Suzuki et al. reported that 54% of patients needed to switch from VATS to thoracotomy for successful resection due to the surgeon’s failure to see or palpate nodules (4). Therefore, the use of accurate preoperative localization is crucial for successful resection in VATS (5-7). Thus, many pulmonary nodule localization methods have emerged (3,8-11), such as hook-wire (12), micro-coil (7), spital coil, methylene blue (3), indocyanine green, lipiodol, barium, and so on. The earliest and most widely used method is computed tomography (CT)-guided hook-wire localization (10,13-17). However, this method has some disadvantages, such as over reliance on CT, excessive use of CT scans, large radiation dose, and so on. In addition, during and after hook-wire localization, patients may experience discomfort and anxiety in the procedure.
In recent years, with the development of information technology, the application of mixed reality (MR) in medicine has emerged (18,19). MR is a successor to virtual reality (VR) and augmented reality (AR) which is a new digital hologram technology. VR is a virtual environment that provides immersion experience to the user who feels completely simulated three-dimensional space, but cut off the real and realistic connection. AR is the projection of a virtual environment onto a real environment, but can’t provide the user immersion interactive experience. MR has the following main characteristics: (I) deep integration of realistic and virtual worlds; (II) accurate matching of three-dimension model and real world; (III) real-time interaction between the environment and users (19,20). Combined with MR, we have developed a new pulmonary nodule localization method. In order to further verify the feasibility and safety of this method, we conducted the following animal pilot study. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-884/rc).
Methods
Ethics
Experiments were performed under a project license (No. 2021SL036) granted by the Ethics Committee of Shanghai Changzheng Hospital, in compliance with institutional guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Experimental animals
A total of 30 male beagles, whose body type are more suitable for this study, aged 12 months and weighing 19.52–20.48 kg, were provided by the animal experiment center of the Naval Military Medical University, Shanghai, China.
Experimental reagent
Medical glue (0.3 mL; Beijing Compont Medical Instrument Co., Ltd., Beijing, China), sodium pentobarbital injection (100 mg; Shanghai Shangyao Xinya, Shanghai, China), and iohexol injection (50 mL; 15 g, Zhejiang Tianrui, Zhejiang, China).
Instruments and equipment
Multi row spiral CT scanner (Siemens, Erlangen, Germany, used for CT scan), hook-wire (Zhejiang Curaway Medical Technology Co., Ltd., Zhejiang, China; Figure 1A, used for localization), guider (Zhejiang Curaway Medical Technology Co., Ltd., China; Figure 1B, used to guide the puncture path), locator (the surface was covered with a reflective layer, which could be recognized by OptiTrack, Figure 1C, used for virtual and real world positioning), OptiTrack (motion capture device; Shenzhen Luster Technology Co., Ltd., Shenzhen, China; Figure 1D, used to capture the location information of the locators in the environment), computer (motive installed, Beijing virtual point Technology Co., Ltd., China, used to process data), HoloLens (Microsoft Corp., Redmond, WA, USA; Figure 1E, used for the presentation of MR effects).

Establishment of pulmonary nodule animal model
A 1:1 mixed solution of medical glue and iohexol injection was used to simulate pulmonary nodules requiring preoperative localization. The experimental animals were anesthetized by intraperitoneal injection of 3% pentobarbital at the rate of 1 mL/kg. After the anesthesia was stable, endotracheal intubation was performed, and the skin was prepared on the chest and abdomen of the experimental animals. Then, 0.1 mL of mixed solution was injected into the left or right lung of the experimental animal through any random intercostal by a researcher (RQ Wei), with a puncture depth of 3 cm (the thickness of chest wall was about 2 cm measured by CT scanning of the experimental animal in advance). A CT scan was performed on the experimental animal after being injected with the mixed solution. Then, 2 researchers (KN Huang, XY Wu) evaluated whether the modelling of the animals was appropriate (the nodules were located in the lung parenchyma, which better simulated the pulmonary nodules requiring preoperative localization) and the animals with whom the researchers were not satisfied were excluded from the trial. Finally, a total of 28 beagle models of pulmonary nodules were established after 1 was excluded due to the mixed solution having not been injected into the lung parenchyma, and another was excluded due to the mixed solution having remained in the muscle layer of the chest wall. Then, we continued to feed the animals for 30 days in sunny, flat cages sized 80 cm × 80 cm × 100 cm. The beagles were fed twice a day with special feed (Figure 2).
Localization progress
Anesthesia and skin preparation were carried out according to the above methods. On the basis of the nodule position of the experimental animal, it was positioned properly and fixed on the CT scan bed. OptiTrack was installed and the computer was used to bridge the HoloLens, Motive, and OptiTrack.
Researcher N Xin pasted 3 MR locators on the body surface of the experimental animal (the 3 locators needed to be completely exposed to OptiTrack’s field of vision, and they could be in the same straight line). Then, the CT scan was performed to obtain the DICOM data of imaging. Researcher N Xin performed 3-dimensional (3D) reconstruction of the chest wall, pulmonary nodules, and 3 locators of the model animals. Meanwhile, the puncture path was designed, and the puncture depth was measured by Mimics 20.0 (Materialise, Plymouth, MI, USA) (Figure 3A). Finally, data were imported into HoloLens.
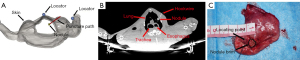
The CT scan bed was taken as the localization bed. Researcher N Xin wore HoloLens to confirm that the MR image matched the experimental animal. After routine disinfection and sheet laying, the animal was subjected to local anesthesia with 2% lidocaine. The path of the disposable guider was set to coincide with the MR image path. Then, a hook-wire was used to puncture along the path of the disposable guider to the depth measured previously, the hook-wire was released and properly fixed. Afterwards, CT scan was performed to obtain DICOM data for verification (Figure 3B). Next, the model animal was killed and dissected for further verification (Figure 3C). A total of 28 beagles were localized accordingly.
Outcome indicators
Primary outcome indicators
Localization deviation, which refers to the deviation distance between the actual location point and the edge of the nearest pulmonary nodule measured by imaging.
Secondary outcome indicators
Procedural duration (the time from the placement of experimental animals to the end of localization), insertion attempts (number of times the experimental animals were punctured by hook-wire), complications (pneumothorax, hemorrhage, localizer dislodgement, and other related complications caused by localization).
Statistical analysis
The software SPSS 20.0 (IBM Corp., Armonk, NY, USA) was used for data analysis. The measurement data were analyzed according to the mean ± standard deviation () description and counting data were described by frequency. T-test and χ2-test were used to analyze and compare groups of data. The difference was considered statistically significant when P<0.05.
Results
Outcome indicators of localization under MR-guidance
The results of this trial showed that deviation of MR-guided localization was 5.71±2.59 mm, localization time was 8.07±1.44 min, and insertion attempts was 1. Pneumothorax and localizer dislodgement occurred in 1 case, respectively.
Accuracy of localization in subgroup under MR-guidance
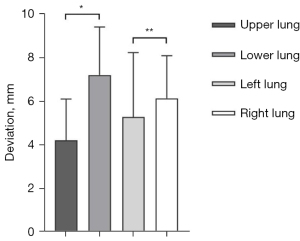
In our study, the accuracy of upper lung localization was significantly better than that of the lower lung (4.21±1.90 vs. 7.21±1.21 mm, P=0.0007, t=3.855). However, there was no statistical difference between the left and right lung (5.29±2.96 vs. 6.14±1.96 mm, P=0.37, t=0.9031) (Figure 4).
Discussion
With the popularity of HRCT, an increasing number of pulmonary nodules are being found. In order to perform the operation more accurately, the localization of pulmonary nodules has always been the focus of thoracic surgeons. Accurate preoperative localization can not only guide surgeons, but also help pathologists to find lesions in resection specimens (21,22). With the increasing demand for localization, thoracic surgeons have employed various methods for preoperative and intraoperative localization of pulmonary nodules. With the development of the information age, the application field of artificial intelligence is becoming increasingly vast. MR, as a kind of artificial intelligence technology, has just begun to be developed in the medical field (23,24). Peng et al. (22) tried to use MR to locate pulmonary nodules before operation, but their study was just a case report. Meanwhile, the localization accuracy and process need to be improved. Thus, combined with MR, we developed a new pulmonary nodule localization method. In order to further verify the feasibility and safety of this method, we conducted this animal trial.
We used a 1:1 mixed solution of medical glue and iohexol injection to simulate pulmonary nodules. On the one hand, iohexol can be well imaged under CT. On the other hand, medical glue can quickly coagulate and form round-like nodules after being injected into lung parenchyma. Care should be taken that the solution should be used immediately after mixing as it is prone to condense rapidly because of the medical glue. The injection should be fast, or the needle will also be blocked by the condensation of medical glue. In addition, the thickness of the chest wall should be measured in advance to determine the puncture depth, so as to prevent too shallow an injection to enter the lung parenchyma or excessively deep injection into the heart or blood vessels. We conducted the localization 30 days after the establishment of the animal model. On the one hand, we considered that the bleeding at the puncture point on the skin surface of the animal may affect the judgment of the MR-guided localization. On the other hand, we also confirmed the short-term stability of the simulated pulmonary nodule. Otherwise, due to the serious CT artifacts caused by the large respiratory movement of the beagles, we often administered excessive anesthetic drugs to inhibit animal’s respiration, so as to obtain better imaging data and more stable localization.
Our results of this animal trial showed that the deviation of MR-guided localization was 5.71±2.59 mm, which can meet the needs of pulmonary nodule localization in clinical work (22,24). The mean localization time was 8.07±1.44 min, which adhered to the needs in real clinical work. Meanwhile, only one puncture was used for each pulmonary nodule localization. With the minimization of insertion attempts, the pain and complications associated with the puncture will also reduce. Just as we showed in our study, the complication rate was 7.14% (2/28), which was lower than previously reported (22,25).
With the motion capture system to establish a virtual coordinate system in the real environment, and the locator pasted on the body surface as the marker, we realized the automatic matching between the real environment and virtual image to improve the accuracy of localization to a great extent, which are different from the previous research (26,27). Since the relative position of the locators and the experimental animals remained unchanged, and the relative position of the virtual puncture path and the locators remained the same, the absolute coincidence between the experimental animals and the virtual puncture path was ensured, which eliminated the possible errors caused by manual matching. In addition, we found it is inevitable that the direction of the hook-wire will be offset due to human factors during the operation, even if the localization is conducted by experienced operators who have suitably designed the puncture path before localization, which will reduce the accuracy in the usual process. Therefore, we tried to use a disposable guider to lead the puncture. We first matched the tip of the guider and the virtual puncture point on the skin. Then, the tail of the guider and the tail of the virtual puncture path were matched. We can well coincide the virtual puncture path with the path of the guider according to the principle that 2 points determine a straight line. Then, the hook-wire can follow the designed path to puncture completely, which further improves the accuracy.
As it is not necessary to perform a CT scan to determine the localization accuracy repeatedly with the MR-guidance, the time required for the localization process is significantly reduced (22,24). In addition, MR-guided localization generally requires only 1 insertion attempt, which also shortens the localization time to a certain extent. Meanwhile, due to the reduction of insertion attempts, the incidence of complications caused by localization decreased significantly. As MR-guided localization does not rely on CT assistance, patients will receive less CT scans than CT-guided localization, thus reducing radiation exposure. Chao et al. pointed out that preoperative localization outside the operating room significantly increases the risk time and the incidence of complications (25,28). Fortunately, with the gradual maturation of the technology, we will be able to adapt the MR-related equipment in the operating room to locate pulmonary nodules, which will circumvent the need for patients to go back and forth between the CT room and the operating room with the CT-guided localization. Better yet, MR-guided localization can be carried out after a stable anesthesia in the operating room, which avoids the stress and pain caused by the localization when the patient is awake. In addition, patients often need to wait for some time before surgery after the CT-guided localization, and pneumothorax or hemothorax caused by localization may be aggravated, or even become life-threatening in the waiting time. However, with the MR-guided localization, the patient can be directly treated in the operating room by thoracotomy, even if there are complications.
Our study also found that the accuracy of lower pulmonary nodule localization was lower than that of upper pulmonary nodule localization under MR guidance. We consider that it is related to the larger movement of the lower lung, whereas the coincidence between the experimental animal and the virtual image is significantly better than that of the lower lung due to the small movement of the upper lung. Similarly, with the continuous evolution of this technology, before puncture, we will be able to ask the anesthesiologist to monitor the tidal volume after anesthesia simulating the deep inspiratory volume of patients to offer the same lung volume as that of CT scan as best as possible, so as to avoid the localization error caused by respiratory movement. However, further study is required.
MR-guided localization requires the installation and debugging of the environment coordinate system by motion capture system and 3D reconstruction by DICOM, which needs surgeons to master the relevant knowledge; our surgeons have reported that it was easy to learn. In addition, it takes about 20 minutes for the motion capture system to install and debug the real environment coordinate system, about 15 minutes to do 3D reconstruction and import data into HoloLens. The automatic matching of real environment and virtual image takes about 5 minutes. Thus, the preparation time of MR-guided pulmonary nodule localization is a little long under the current conditions. However, with the establishment of an MR operating room, the preparation time will be shortened accordingly, to become more in line with the needs of clinical work.
There were still many limitations to this study. Although the simulated pulmonary nodule is hard and can be detected by touch, it is difficult to distinguish it from the lung tissue only by naked eye which according with the aim of the study is to help identify this type of nodule. But, for the experimenter, methylene blue and other dyes can be added to further increase the identification of nodules. In addition, the localization accuracy of deep nodules still needs to be further studied, as the simulated pulmonary nodules are located within 2 cm below the pleura. In addition, it is of vital importance to establish the MR operating room to further improve the localization method. In the end, the accuracy of the current MR is in the centimeter level, and it is hoped that the accuracy will be located in the millimeter level through further research.
Conclusions
This pilot animal study shows that the MR-guided localization of pulmonary nodules is feasible and safe, but further clinical randomized controlled trials are still needed to support the rationality of its clinical application (Register No. ChiCTR2100048313).
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-884/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-884/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-884/coif). GD reports personal fees from ASTRA ZENECA, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2021SL036) granted by the Ethics Committee of Shanghai Changzheng Hospital, in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Walter JE, Heuvelmans MA, Bock GH, et al. Characteristics of new solid nodules detected in incidence screening rounds of low-dose CT lung cancer screening: the NELSON study. Thorax 2018;73:741-7. [Crossref] [PubMed]
- Henschke CI. Early lung cancer action project: overall design and findings from baseline screening. Cancer 2000;89:2474-82. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-544.e2. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Hsu PK, Chuang LC, Wu YC. Electromagnetic Navigation-Guided Preoperative Localization of Small Malignant Pulmonary Tumors. Ann Thorac Surg 2020;109:1566-73. [Crossref] [PubMed]
- Seo JM, Lee HY, Kim HK, et al. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg 2012;143:809-14. [Crossref] [PubMed]
- Finley RJ, Mayo JR, Grant K, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 2015;149:26-31. [Crossref] [PubMed]
- Templeton PA, Krasna M. Needle/wire lung nodule localization for thoracoscopic resection. Chest 1993;104:953-4. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016;8:S749-55. [Crossref] [PubMed]
- Vandoni RE, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg 1998;14:265-70. [Crossref] [PubMed]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Ding N, Wang K, Cao J, et al. Targeted Near-Infrared Fluorescence Imaging With Iodized Indocyanine Green in Preoperative Pulmonary Localization: Comparative Efficacy, Safety, Patient Perception With Hook-Wire Localization. Front Oncol 2021;11:707425. [Crossref] [PubMed]
- Rostambeigi N, Scanlon P, Flanagan S, et al. CT Fluoroscopic-Guided Coil Localization of Lung Nodules prior to Video-Assisted Thoracoscopic Surgical Resection Reduces Complications Compared to Hook Wire Localization. J Vasc Interv Radiol 2019;30:453-9. [Crossref] [PubMed]
- Park JB, Lee SA, Lee WS, et al. Computed tomography-guided percutaneous hook wire localization of pulmonary nodular lesions before video-assisted thoracoscopic surgery: Highlighting technical aspects. Ann Thorac Med 2019;14:205-12. [Crossref] [PubMed]
- Li C, Liu B, Jia H, et al. Computed tomography-guided hook wire localization facilitates video-assisted thoracoscopic surgery of pulmonary ground-glass nodules. Thorac Cancer 2018;9:1145-50. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Morimoto T, Kobayashi T, Hirata H, et al. XR (Extended Reality: Virtual Reality, Augmented Reality, Mixed Reality) Technology in Spine Medicine: Status Quo and Quo Vadis. J Clin Med 2022;11:470. [Crossref] [PubMed]
- Tepper OM, Rudy HL, Lefkowitz A, et al. Mixed Reality with HoloLens: Where Virtual Reality Meets Augmented Reality in the Operating Room. Plast Reconstr Surg 2017;140:1066-70. [Crossref] [PubMed]
- Chen Z, Zhang Y, Yan Z, et al. Artificial intelligence assisted display in thoracic surgery: development and possibilities. J Thorac Dis 2021;13:6994-7005. [Crossref] [PubMed]
- Lin CY, Chang CC, Huang LT, et al. Computed Tomography-Guided Methylene Blue Localization: Single vs. Multiple Lung Nodules. Front Med (Lausanne) 2021;8:661956. [Crossref] [PubMed]
- Peng M, Yu L, Zhou Y, et al. Augmented reality-assisted localization of solitary pulmonary nodules for precise sublobar lung resection: a preliminary study using an animal model. Transl Lung Cancer Res 2021;10:4174-84. [Crossref] [PubMed]
- Ong CW, Tan MCJ, Lam M, et al. Applications of Extended Reality in Ophthalmology: Systematic Review. J Med Internet Res 2021;23:e24152. [Crossref] [PubMed]
- Wish-Baratz S, Crofton AR, Gutierrez J, et al. Assessment of Mixed-Reality Technology Use in Remote Online Anatomy Education. JAMA Netw Open 2020;3:e2016271. [Crossref] [PubMed]
- Li C, Zheng Y, Yuan Y, et al. Augmented reality navigation-guided pulmonary nodule localization in a canine model. Transl Lung Cancer Res 2021;10:4152-60. [Crossref] [PubMed]
- Zhang JT, Zhang T, Qiu ZW, et al. Metal-marked mixed reality technology for pulmonary nodule localization. Interact Cardiovasc Thorac Surg 2019;29:494. [Crossref] [PubMed]
- Wacker FK, Vogt S, Khamene A, et al. An augmented reality system for MR image-guided needle biopsy: initial results in a swine model. Radiology 2006;238:497-504. [Crossref] [PubMed]
- Chao YK, Pan KT, Wen CT, et al. A comparison of efficacy and safety of preoperative versus intraoperative computed tomography-guided thoracoscopic lung resection. J Thorac Cardiovasc Surg 2018;156:1974-1983.e1. [Crossref] [PubMed]
(English Language Editor: J. Jones)


