
Efficacy of different therapies for brain metastases of non-small cell lung cancer: a systematic review and meta-analysis
Highlight box
Key findings
• Our study found that non-small cell lung cancer (NSCLC) patients with brain metastases (BM) who received immune checkpoint inhibitor (ICI)-based therapies achieved an impressive intracerebral objective response rate (icORR) and long-term survival benefit. In particular, patients who received first-line treatment or were programmed death-ligand 1 (PD-L1)-positive could benefit more from ICIs.
What is known and what is new?
• There is an ongoing concern and debate about the optimal therapies for BM patients with negative driver genes.
• Our study improves the understanding of different treatments in NSCLC patients with BM, suggesting that ICI-based therapies have potential clinical value for BM.
What are the implications, and what should change now?
• These results could help clinicians to select an optimal combination strategy for NSCLC patients with BM. Therapeutic strategies in these patients should be individualized based on their clinical characteristics. However, more prospective clinical trials are still needed to evaluate the efficacy and safety of different treatments in advanced patients with baseline BM.
• Report here about implications and actions needed.
Introduction
Despite the recent progress in therapeutic strategies for metastatic non-small cell lung cancer (NSCLC), the prognosis of NSCLC patients with brain metastases (BM), which is one of the most common metastatic sites and fatal factors, has failed to show substantial improvements. Without effective treatment, the overall survival (OS) of such patients ranges from several weeks to several months. However, the selectivity of the blood-brain barrier (BBB) limits the delivery of drugs to the brain parenchyma during systemic therapy, and the prognosis also relies on several essential characteristics of intracranial lesions, such as the number, size, locations, and central nervous system (CNS) symptoms (1). Generally, patients choose radiotherapy (RT) or surgery to rapidly alleviate their neurological symptoms. Among the systemic therapies, which include chemotherapy, angiogenesis inhibitors, immune checkpoint inhibitors (ICIs), and targeted agents, the latter are the best choice for patients with molecular drivers (2). However, there is an ongoing concern and debate about the optimal therapies for BM patients with negative driver genes or resistance to tyrosine kinase inhibitors (TKI) (3).
Unfortunately, few trials have evaluated the clinical benefits of systemic therapies for intracranial lesions in NSCLC patients who cannot benefit from targeted therapy. Traditionally, chemotherapy is reserved as a salvage therapy for BM because the BBB resists the passage of chemotherapeutic agents. Thus far, several chemotherapeutic agents, such as paclitaxel, vinorelbine or gemcitabine, cisplatin, and others, seem to be effective for CNS lesions (4). Currently, ICI-combined therapies are widely considered for patients with NSCLC. However, patients with BM are excluded from most clinical trials on programmed cell death-1 (PD-1) or programmed cell death-ligand 1 (PD-L1) inhibitors. KEYNOTE-189, which included the most extensive subgroup analysis of patients with BM, reported that the OS of patients treated with pembrolizumab combined with chemotherapy was significantly superior to those subjected to pure chemotherapy [hazard ratio (HR) =0.36; 95% confidence interval (CI): 0.20–0.62] (5,6).
A better understanding of the activity of different antitumor agents in the CNS is very important for making the optimal clinical choice. Therefore, in this study, we performed a meta-analysis to make reasonable suggestions for clinical treatment by comparing different therapies and assessing the most effective strategies for intracranial lesions in non-targeted therapy NSCLC patients. This meta-analysis was reported in accordance with the PRISMA reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-515/rc) (7).
Methods
From January 1, 2005, to October 1, 2021, the relevant information was systematically searched in the Embase, PubMed, and Cochrane Library electronic databases. Furthermore, we searched for abstracts from meetings of the European Society for Medical Oncology, the World Conference on Lung Cancer, and the American Society of Clinical Oncology. The following search terms were used: “(lung cancer or non-small cell lung cancer or NSCLC or lung adenocarcinoma or lung squamous)” and “(immunotherapy or immune checkpoint inhibitors or nivolumab or pembrolizumab or atezolizumab or durvalumab or nivolumab or ipilimumab or PD-1 or PD-L1) or (chemotherapy) or (angiogenesis inhibitors or bevacizumab) or (radiotherapy or radiation or radiosurgery)” and “(brain metastases or central nervous system)”.
Inclusion criteria
The inclusion criteria were as follows: (I) randomized controlled trials, non-randomized clinical trials, prospective or retrospective observational studies, or abstracts; (II) articles involving patients diagnosed with BM of NSCLC who received non-targeted therapy; (III) the study endpoints included the intracerebral objective response rate (icORR) or intracerebral progression-free survival (iPFS); and (IV) the proportion of patients with epidermal growth factor receptor (EGFR) gene or Kirsten rat sarcoma viral oncogene (KRAS) mutation did not exceed 25%.
The exclusion criteria were as follows: (I) case reports, reviews, editorials, meta-analyses, commentaries, and letters; and (II) studies that did not focus on any of the abovementioned endpoints.
Data extraction
Two researchers independently extracted information from the eligible studies on non-targeted therapy NSCLC patients with BM, including their clinical characteristics and outcomes. Specifically, sex, age, gene-mutation status, study type, Eastern Cooperative Oncology Group (ECOG) score, smoking, corticosteroid use, clinical treatments, histological type, line of treatment, percentage of asymptomatic nervous lesions, intracranial lesion status, and PD-L1 status were recorded. The main observational indicators were the intracranial outcomes, including icORR [defined as the proportion of patients reaching intracranial complete or partial response (PR) among the total number of NSCLC patients with BM] and iPFS. The OS was not regarded as an observational indicator because the influence of factors such as subsequent treatment and medical cost was complex. For each eligible study, the risk of bias was assessed by the Newcastle-Ottawa scale, and the score ranged from 0 to 9.
Statistical analysis
We used descriptive statistics to summarize the clinical characteristics obtained from the eligible studies (Table 1). We performed a meta-analysis using the random-effects method (I–V heterogeneity) following Freeman-Tukey double arcsine transformation with 95% CI. Statistical heterogeneity was assessed using the I2 test in the random-effects model. P<0.1 or I2>50% was considered to express significant heterogeneity. The significance of the difference in the pooled effect size was examined using the Z-test. All of the P values were two-sided, and P<0.05 was considered statistically significant. We also performed a sensitivity analysis to evaluate the stability of the results by sequentially excluding each study. Publication bias was evaluated with the funnel plot asymmetry test. The data analysis was performed by using R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria) and Stata software version 15.1 (Stata Corporation, College Station, TX, USA).
Table 1
| Author | Year | Type | Therapy regimen | Treatment line | Sex (F/M), % | Median age | Smoke % | Response Assessment method | ECOG 0–1, % | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| Sun L | 2021 | Retro | Pembro | ≥1 | 47.6/52.4 | 66 | 85.7 | – | 82.5 | (8) |
| Goldberg SB | 2020 | Pro | Pembro | ≥1 | 33/67 | 60 | 93 | mRECIST | 100 | (9) |
| Gauvain C | 2018 | Retro | Nivo | ≥1 | 24/76 | 59.5 | 91 | RECIST 1.1 | – | (10) |
| Dudnik E | 2016 | Retro | Nivo | – | 60/40 | 78 | 80 | mRECIST 1.1 | – | (11) |
| Hendriks L | 2019 | Retro | ICI | – | 62/28 | 61.5 | 93.4 | NS | 77.2 | (12) |
| Wakuda K | 2021 | Retro | Pembro | 1 | 74/26 | 70 | 91 | RECIST 1.1 | 39 | (13) |
| Zhang GW | 2020 | Retro | Nivo | ≥2 | 78/22 | 57.7 | – | RECIST 1.1 | 84 | (14) |
| Song P | 2019 | Retro | ICI | – | – | – | – | RECIST 1.1 | – | (15) |
| Skribek M | 2020 | Retro | ICI | ≥1 | 54.9/45.1 | 69 | 86.3 | mRECIST 1.1 | 75.8 | (16) |
| Kitai H | 2013 | Retro | Chem | – | 44.4/55.6 | 63 | 48 | – | – | (17) |
| Barlesi F | 2011 | Pro | PP | 1 | 67.4/32.6 | 60.4 | – | RECIST | 97.7 | (18) |
| Chouahnia K | 2010 | Pro | PP | – | – | – | – | – | 100 | (19) |
| Hu Q | 2011 | Retro | Chem | – | – | – | – | – | – | (20) |
| Chem + WBRT | – | – | – | – | – | – | ||||
| Bailon O | 2012 | Pro | PP | 1 | 70/30 | 58 | – | RECIST | 90 | (21) |
| Bearz A | 2010 | Retro | Pemetrex | – | – | – | – | RECIST | – | (22) |
| Monnet I | 2020 | Pro | PP + Bev | 1 | 78.3/22.7 | 60.5 | 89.2 | RECIST 1.1 | 100 | (23) |
| Tian Y | 2019 | Retro | PP | 1 | 40/60 | 54 | 24.4 | RECIST 1.1 | – | (24) |
| PP + Bev | 1 | 61.5/38.5 | 58 | 26.9 | NA | – | ||||
| Li X | 2019 | Retro | PP | 1 | – | – | – | RECIST 1.1 | – | (25) |
| PP + Bev | 1 | – | – | – | – | |||||
| Besse B | 2015 | Pro | TP + Bev | 1 | 69/31 | 61 | 79 | RECIST 1.0 | 100 | (26) |
| Ashinuma H | 2014 | Retro | Chem + Bev | – | 50/50 | 62.5 | – | RECIST 1.1 | – | (27) |
| Hirano S | 2006 | Retro | Chem | 1 | 52.6/47.4 | 61 | – | RECIST 1.1 | – | (28) |
| Metro G | 2021 | Retro | Pembro | 1 | 44.4/55.6 | 74 | 77.8 | mRECIST1.1 | 88.9 | (29) |
| Pembro + WBRT | 1 | 37.5/62.5 | 63 | 87.5 | mRECIST 1.1 | – | ||||
| Pembro + SRS | 1 | 76.9/22.1 | 69 | 92.3 | mRECIST 1.1 | – | ||||
| Shepard MJ | 2019 | Retro | ICI + SRS | – | 64.7/35.3 | 64.4 | – | RANO-BM | – | (30) |
| Afzal MZ | 2018 | Retro | PP + Pembro | ≥1 | – | 63.7 | 100 | RECIST 1.1 | 82.4 | (31) |
| He Q | 2017 | Pro | PP + WBRT | 1 | 34.4/65.6 | 58 | 31.3 | RANO-BM | – | (32) |
| Dinglin XX | 2013 | Pro | PP + WBRT | ≥1 | 64.3/35.7 | 55.4 | 54.8 | RECIST 1.0 | 100 | (33) |
| Quantin X | 2010 | Pro | Chem + WBRT | – | 75.7/24.3 | 59.1 | – | RECIST | – | (34) |
| – | 75.8/24.2 | 56 | – | – | – | |||||
| Chen L | 2009 | Pro | Chem + WBRT | ≥1 | 60.8/39.2 | 53 | – | RECIST | – | (35) |
| Lee DH | 2008 | Pro | Chem (earlier) + WBRT | 1 | 76/24 | 60 | – | WHO | 100 | (36) |
| Chem + WBRT (earlier) | 1 | 83/17 | 62 | – | WHO | 95.6 | ||||
| Carbone D | 2021 | Pro | Pembro + Ipili + Chem | 1 | 31.4/68.6 | 61 | 78 | mRECIST 1.1 | 98 | (37) |
| Chem | 1 | 48/52 | 64 | 92 | mRECIST 1.1 | 100 | ||||
| Lau SCM | 2021 | Retro | ICI + RT | – | 47/53 | 64 | 77 | RECIST 1.1 | 92 | (38) |
| Chem + RT | – | 51/49 | 62 | 67 | RANO—BM | 88 | ||||
| Lim SH | 2015 | Pro | Chem + SRS (earlier) | – | 71/29 | 58 | 58 | RECIST 1.1 | 100 | (39) |
| Chem (earlier) + SRS | – | 73/27 | 57 | 65 | 100 | |||||
| Azzam G | 2018 | Retro | ICI + SRS | – | – | – | – | – | – | (40) |
| Li J | 2020 | Pro | Pembro + ipili + SRS | – | – | – | – | – | – | (41) |
| Lee M | 2021 | Retro | ICI | – | 62.5/27.5 | 61 | – | – | 92.3 | (42) |
| ICI + RT (concurrent) | – | 80/20 | 62 | – | – | 100 | ||||
| ICI + RT (non-concurrent) | – | 70/30 | 59 | – | – | 100 | ||||
| Nadal E | 2019 | Pro | ICI + Chem | – | 29/71 | – | 75 | RANO—BM | 65 | (43) |
NSCLC, non-small cell lung cancer; BM, brain metastases; F, female; M, male; ECOG, Eastern Cooperative Oncology Group; Refs, references; Retro, retrospective; Pro, prospective; ICI, immune checkpoint inhibitor, Chem, chemotherapy; Pembro, pembrolizumab; Nivo, nivolumab; Ipili, ipilimumab; PP, pemetrexed plus cisplatin; TP, paclitaxel plus cisplatin; Bev, bevacizumab; No-sq, no-squamous; RT, radiotherapy; WBRT, whole brain radiotherapy; SRS, stereotactic radiosurgery.
Results
Characteristics of the included studies
A total of 3,713 records were obtained in the initial database search, and 864 duplicate studies were removed. After manually screening the abstracts and references, 36 studies were included after the final selection according to the eligibility criteria (Figure 1). Among these, 21 studies were retrospective and 15 were prospective trials. The therapeutic regimens included pure ICI (8-17), pure chemotherapy, chemotherapy plus bevacizumab, ICI plus RT, chemotherapy plus RT, ICI plus chemotherapy, nivolumab plus ipilimumab and RT, and nivolumab plus ipilimumab plus chemotherapy (18-36). Seven studies that only included iPFS data were also included (37-43).

These studies included a total of 1,774 patients who received eight different treatments, and all of the patients received whole-brain radiotherapy (WBRT) in the chemotherapy plus RT subgroup (41). As for the ICI plus RT subgroup, one study administered stereotactic radiosurgery (SRS), while another divided patients into two groups according to WBRT or SRS. We considered the two groups as a whole in the subgroup analysis based on the type of therapies. The details for each trial are shown in Tables 1,2. The proportion of the population with driver-gene mutation was required to be <25% in each study, except for one study where patients with EGFR or KRAS mutations reached 40.5% of the total population. That study reported the results of intracranial lesions in NSCLC patients with BM treated with pure ICI, which was the largest group (255 patients) examined in our study (12). Considering that the reported icORR was similar to the results of an important prospective study by Goldberg et al., we decided to include this study in the final analysis (9).
Table 2
| Author | Year | PD-L1 ≥1, % | Steroid, % | Number of BM, % | CNS symptom, % | Treated BM, % | Dose | Refs |
|---|---|---|---|---|---|---|---|---|
| Sun L | 2021 | – | – | – | – | 60.30 | – | (8) |
| Goldberg SB | 2020 | – | – | 100% 1–5 | – | 67 | – | (9) |
| Gauvain C | 2018 | – | – | 76% 1–3 | – | – | – | (10) |
| Dudnik E | 2016 | – | 0 | – | – | – | – | (11) |
| Hendriks L | 2019 | 61.5 | 27.4 | 47% 1–3 | 14.7 | 82.2 | – | (12) |
| Wakuda K | 2021 | – | – | – | – | 57 | – | (13) |
| Zhang GW | 2020 | – | – | – | – | 50 | – | (14) |
| Song P | 2019 | – | – | – | – | – | – | (15) |
| Skribek M | 2020 | 80 | 54.1 | 63.6% 1–3 | 54.5 | 78.8 | – | (16) |
| Kitai H | 2013 | – | – | – | – | – | – | (17) |
| Barlesi F | 2011 | – | – | – | 0 | – | – | (18) |
| Chouahnia K | 2010 | – | – | – | – | – | – | (19) |
| Hu Q | 2011 | – | – | – | – | – | – | (20) |
| Bailon O | 2012 | – | 63 | 60% 1–3 | 72 | 0 | – | (21) |
| Bearz A | 2010 | – | – | – | – | – | – | (22) |
| Monnet I | 2020 | – | – | 93.5% 1–5 | – | 0 | – | (23) |
| Tian Y | 2019 | – | – | – | 42.2 | – | – | (24) |
| – | – | – | 34.6 | – | – | |||
| Li X | 2019 | – | – | – | – | – | – | (25) |
| Besse B | 2015 | – | – | 100% 0–2 | – | – | – | (26) |
| Ashinuma H | 2014 | – | – | – | – | 80 | – | (27) |
| Hirano S | 2006 | – | – | 31.6% 1–2 | – | – | – | (28) |
| Metro G | 2021 | – | 11.1 | 66.7 % 1–3 | – | – | – | (29) |
| 100 | 37.5 | 12.5% 1–3 | – | 0 | – | |||
| 100 | 38.5 | 68.2% 1–3 | – | 0 | – | |||
| Shepard MJ | 2019 | 76.5 | 58.80 | – | – | 35.4 | Median 19 | (30) |
| Afzal MZ | 2018 | – | – | – | – | 50 | – | (31) |
| He Q | 2017 | – | – | 34% 1–2 | – | – | 30 | (32) |
| Dinglin XX | 2013 | – | 100 | 28.6% 1–2 | – | – | 30 | (33) |
| Quantin X | 2010 | – | – | 59.5 % 1–2 | – | – | 54 | (34) |
| – | – | 51.2 % 1–2 | – | – | – | |||
| Chen L | 2009 | – | – | 47.1% 1–3 | – | – | 30–60 | (35) |
| Lee DH | 2008 | – | – | – | 0 | – | – | (36) |
| – | – | – | 0 | – | 30 | |||
| Carbone D | 2021 | 64 | 6 | – | – | 36 | – | (37) |
| 67 | 16 | – | – | 45 | – | |||
| Lau SCM | 2021 | – | – | – | 44 | – | – | (38) |
| – | – | – | 39 | – | – | |||
| Lim SH | 2015 | – | – | Median 2 | 0 | 0 | – | (39) |
| – | – | Median 1.82 | 0 | 0 | – | |||
| Azzam G | 2018 | – | – | – | – | – | – | (40) |
| Li J | 2020 | – | – | – | – | – | – | (41) |
| Lee M | 2021 | 30.8 | – | Median 2 | – | 15.4 | – | (42) |
| 58.3 | – | Median 2 | – | 12.5 | Median 19 | |||
| 29.6 | – | Median 2 | – | 11.1 | – | |||
| Nadal E | 2019 | – | – | – | – | – | – | (43) |
NSCLC, non-small cell lung cancer; BM, brain metastases; Refs, references; PD-L1, programmed cell death ligand-1; CNS, central nervous system.
Meta-analysis of the pooled icORR: all patients
We extracted the icORR and iPFS from the included 36 studies involving a total of 1,774 patients. Unfortunately, significant heterogeneity was observed in each subgroup, which may be attributable to the specificity of the single-arm study. Therefore, the random-effect model was adopted. We performed comparisons of the pooled icORR in different treatment subgroups, which ranged from 33% to 81% (Figure 2A). The most significant effect was observed in the ICI combined with RT subgroup, where the pooled icORR was 81% (95% CI: 16–100%, P=0.000), and the worst effect was found in the pure chemotherapy subgroup (33%, 95% CI: 24–42%, P=0.000). The pooled icORR was 56% (95% CI: 29–82%, P=0.000) in the ICI plus chemotherapy subgroup, 46% (95% CI: 34–57%, P=0.000) in the chemotherapy plus RT subgroup, 44% (95% CI: 23–66%, P=0.000) in the chemotherapy plus bevacizumab subgroup, and 34% (95% CI: 23–46%, P=0.000) for the ICI subgroup. Notably, although double ICI plus chemotherapy was only reported in one study, its icORR reached 51.3%, demonstrating the efficacy of double ICI combination therapy against BM. A more intuitive comparison of icORR is presented in Figure 2B.

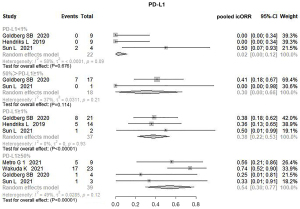
Meta-analysis of pooled icORR: PD-L1 status
Five of the 10 studies in the ICI subgroup divided patients based on PD-L1 status. Therefore, a subgroup analysis was performed to explore the possible correlation between PD-L1 status and the effect of immunotherapy, and the heterogeneity ranged from 0% to 58%. The pooled results revealed a significant difference between the groups based on the different PD-L1 expressions. The pooled icORR was 2.0% (95% CI: 0–12%, P=0.676) in the PD-L1 expression <1% subgroup, suggesting that pure ICI was ineffective for BM in this subgroup. The most significant icORR from the PD-L1 ≥50% subgroup was 54% (95% CI: 30–77%, P=0.000). The pooled icORRs of PD-L1 expression ≥1% and 1%≤ PD-L1 <50% were 38% (95% CI: 22–53%, P=0.000) and 30% (95% CI: 0–66%, P=0.114), respectively. Therefore, the beneficial effect of ICI on intracranial lesions may be positively correlated with PD-L1 status (Figure 3).
Meta-analysis of pooled icORR: first-line treatment
The icORR data for first-line treatment were provided in 12 studies. The pooled icORRs of the ICI, chemotherapy, chemotherapy combined with RT, and chemotherapy plus bevacizumab subgroups were 69% (95% CI: 51–85%, P=0.000), 33% (95% CI: 20–47%, P=0.000), 50% (95% CI: 32–68%, P=0.000), and 48% (95% CI: 22–75%, P=0.000), respectively (Figure 4A). As for the non-first-line subgroups, the icORR of these patients was 26.0% (95% CI: 21–30%, P=0.000) in the ICI subgroup, 31.0% (95% CI: 20–44%, P=0.000) in the chemotherapy subgroup, and 42.0% (95% CI: 28–57%, P=0.000) in the chemotherapy combined with RT subgroup (Figure 4B). Only one study included non-first-line treatment in the chemotherapy plus bevacizumab subgroup, so this group was ruled out in the subsequent analyses. The results demonstrated that the efficacy of ICI for BM showed the most significant improvement in the first-line treatment.

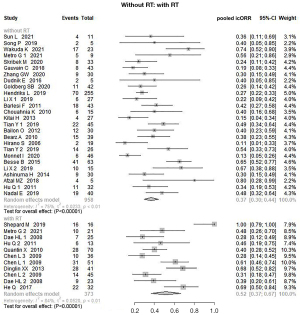
Meta-analysis of pooled icORR: with or without RT
Notably, subgroup analysis of antitumor agents plus RT compared with antitumor agents alone revealed a significant clinical benefit. The pooled icORR in the combination RT group was 52% (95% CI: 37–67%, P=0.000), as compared with 37% (95% CI: 30–44%, P=0.000) in patients without RT (Figure 5). We conducted further analysis on ICI plus RT according to the different types of RT (Figure 6). The pooled icORR was 75% (95% CI: 1–100%, P=0.054) for ICI plus SRS and 75% (95% CI: 35–97%, P=0.000) for ICI plus WBRT. As only one study was included, this was not sufficient to perform a subgroup analysis.

Meta-analysis of pooled icORR: prospective and retrospective
We performed further analyses of the pooled icORR according to the different types of studies (Figure 7). In the chemotherapy subgroup, the prospective arm showed a higher pooled icORR than the retrospective arm: 41% (95% CI: 31–52%, P=0.000) vs. 29% (95% CI: 17–41%, P=0.000). In contrast, a higher pooled icORR in the retrospective arm was observed in the ICI subgroup, 35% (95% CI: 23–49%, P=0.000) vs. 26% (95% CI: 14–42%, P=0.000). This may be explained by the inherent shortcomings of retrospective analyses, and further prospective trials are required to guide the selection of clinical therapeutic regimens. Other subgroups were not included in this analysis due to sample size limitations.

Discordance
Five studies offered data on the inconsistent response rate of primary and metastatic lesions, and the discordance response rate ranged from 12.7% to 60%.
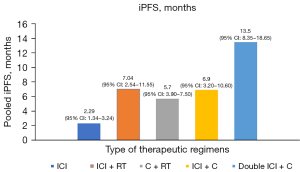
Meta-analysis of pooled iPFS
Due to the limitations of single-arm meta-analyses, only five subgroups provided sufficient data to perform an iPFS analysis (Figure 8). Since the difference of the pooled iPFS was statistically significant (P=0.000), we assessed the intracranial long-term survival benefit by comparing the pooled iPFS. The results showed that the highest median iPFS was 13.5 months (95% CI: 8.35–18.65 months) in patients who received nivolumab plus ipilimumab and chemotherapy. The median iPFS of ICI plus chemotherapy and ICI plus RT was 6.9 months (95% CI: 3.20–10.60 months) and 7.04 months (95% CI: 2.54–11.55 months), respectively. However, the iPFS of pure ICI in patients with BM was lower than the others, only 2.29 months (95% CI: 1.34–3.24 months). Finally, the median iPFS of chemotherapy plus RT was 5.7 months (95% CI: 3.90–7.50 months).
Publication bias
There was an apparent asymmetry in the funnel plots, which suggested the presence of publication bias (Figure 9). However, this finding can be explained by the high heterogeneity in each subgroup, which was an inevitable limitation of the single-arm study design. Therefore, we decided to include these studies in our analysis.
Discussion
Generally, most clinical trials for NSCLC exclude patients with BM. Although several clinical trials have reported on the long-term survival benefit of NSCLC patients with BM who received immune-based combination therapies, the intracranial efficacy against BM has not yet been elucidated. Alencar et al. conducted a meta-analysis to analyze the icORR of NSCLC patients who received pure ICI and revealed that ICI monotherapy was effective for intracranial lesions in NSCLC patients (44). However, considering the limited data in their study, it is difficult to evaluate differences among important clinical features, such as PD-L1 expression, treatment line, and the presence of driver-gene mutations. Most importantly, no studies have compared intracranial efficacy between different therapeutic regimens. Hence, we summarized the current literature and conducted a meta-analysis of intracranial efficacy to improve the understanding of different treatments in NSCLC patients with BM.
Our study suggests that ICI-based therapies have potential clinical value for patients with BM. ICI monotherapy did not show a strong advantage in the control of intracranial lesions, but the impact of the treatment line could explain this finding, considering that most patients who were enrolled in these studies had been treated with non-first-line therapy. Therefore, the analysis could underestimate the real effect of ICI, that is, patients who received ICI monotherapy as first-line treatment had a significant improvement in the pooled icORR, suggesting that early use of ICI is associated with superior BM control.
As the most common biomarker predicting the efficacy of ICI, the PD-L1 expression level in NSCLC patients with BM affected the intracranial efficacy of ICI, even though PD-L1 expression between primary tumors and BM exhibit temporal and spatial heterogeneity. Further analysis suggested limited intracranial efficacy in patients with negative PD-L1 expression. Conversely, the PD-L1 ≥50% subgroup showed the best control of intracranial lesions. The results suggested that PD-L1 status was positively associated with the clinical outcomes of BM, which also represents a significant biomarker for the intracranial prognosis of patients who receive ICI.
In our study, the discordance rate of extracranial and BM responses was between 12.7% and 60%, and the high discrepancy might be a result of the small sample size. As the sample size expanded, the responses of different sites tended to be congruent. Goldberg et al. reported a high consistency between BM and primary lesions’ PR, and BM PR was present in 8/9 (88%) patients diagnosed with systemic PR. The above-mentioned conclusions preliminarily suggested that ICI might have the same benefit for primary lung tumors and BM lesions in NSCLC (9,45).
Treatment line and PD-L1 expression played an important role in the efficacy of ICI against intracranial lesions, and patients who receive first-line ICI or those with PD-L1 ≥1 may benefit the most. Additionally, the key finding of this meta-analysis is that ICI-combined chemotherapy showed a synergistic effect and higher effectiveness compared to pure ICI. On the one hand, chemotherapy enhanced the efficacy of ICI in the intracranial immune microenvironment by increasing antigen presentation, inducing T-cell proliferation to activate the immune responses of T cells (46). On the other hand, ICI has a slow onset of efficacy, so chemotherapy could help prevent early disease progression before ICI takes effect. In KEYNOTE-407, the systemic objective response rate (ORR) of NSCLC patients with baseline BM who received platinum-doublet chemotherapy with pembrolizumab reached 92.7% (47). The combination of double ICI and chemotherapy provided the added advantage of iPFS and was ranked first in our study. The results consistently indicated that the combination of ICI and chemotherapy is a promising option for NSCLC patients with BM. Several ongoing clinical trials are testing this combined therapy, which will provide us with more evidence to support this conclusion. In the era of immunotherapy, it is worth exploring how to apply combination therapy to reasonably maximize efficacy.
RT still has irreplaceable advantages in the local treatment for BM, especially for severe neurological symptoms, in contrast to the poor results obtained by ICI treatment alone (48-50). In addition, active brain lesions are also a significant poor prognostic factor for NSCLC patients with BM (51). Our study obtained similar results, as BM patients who received ICI alone did not have a significant improvement. We found that ICI plus RT possessed a significant intracranial control, although the number of relevant articles was minimal. Theoretically, this effect could be explained by the following mechanisms: (I) ICI has a synergistic effect with RT (52); and (II) RT may increase the permeability of the BBB (48).
Notably, the synergistic effect between RT and ICI might be a double-edged sword (53), given that patients are at a higher risk of developing radiation necrosis following the combination of ICI with RT (53). In a retrospective study involving 480 patients with BM from various malignancies (including 294 patients with NSCLC) who were treated with RT with or without ICI, a higher incidence of radionecrosis was observed in patients who received ICI plus RT than in those who received RT alone, after adjustment for tumor histopathology (HR, 2.56; 95% CI: 1.35–4.86, P=0.004) (54). Another study involving 180 patients with BM who received SRS and various systemic therapies, including chemotherapy, ICI, and targeted therapy, showed that the incidence of radionecrosis reached 37.5% in patients who received RT plus ICI, which was significantly higher compared with patients who received targeted therapy or chemotherapy [odds ratio (OR), 2.40; 95% CI: 1.06–5.44, P=0.03] (55). Interestingly, there was a remarkable difference in iPFS between the concurrent- and non-concurrent-treated groups (36,39). Nevertheless, we did not collect sufficient data to assess whether the timing of ICI and RT was the source of these differences (39,42).
Furthermore, the type of RT was also a crucial factor when evaluating the efficacy of ICI combined with RT. Intracranial RT includes WBRT and SRS, and the choice of intracranial RT type depends on the characteristics of BM and the general status of patients. Most studies have suggested that WBRT is the standard option for patients with 5–20 BMs (56), while SRS is the standard option for patients with 1–4 BMs (57). Historically, WBRT has played an important role in the treatment of patients with BM. Compared to SRS, WBRT has significantly improved control of multiple or large intracranial lesions but leads to more neurocognitive function damage and a lower quality of life (58). A prospective trial evaluated the toxicity of WBRT plus SRS by comparing SRS to WBRT plus SRS in patients with BM; the results of this trial showed that patients who received WBRT plus SRS were significantly more likely (52%) to show a decline in neurocognitive function, such as memory and verbal learning, than patients who received SRS alone (24%) (58). There were also significant differences in local control and long-term survival between patients who received ICI plus WBRT and those who received ICI plus SRS. A retrospective analysis of patients with BM who received ipilimumab plus WBRT showed that the rate of intracranial lesion control reached 78% (59). Another retrospective study by Metro et al., which included eight patients who received ICI plus WBRT, 13 patients who received ICI plus SRS, and nine patients who received ICI alone (29), showed that the icORR was 31% in the SRS plus ICI group, 75% in the WBRT plus ICI group, and 55% in the ICI alone group. Moreover, the 12-month survival rates for SRS plus ICI, WBRT plus ICI, and ICI alone were 23.0%, 62.5% and 55.5%, respectively. Currently, clinical data is limited, and some prospective trials comparing ICI plus WBRT to ICI plus SRS have not yet been completed; the results from these ongoing trials may further increase the understanding of different types of ICI plus RT therapeutic regimens.
In our study, we did not observe a significant difference in icORR or iPFS between the WBRT and SRS subgroups, which might be related to data volume limitations. Similar results were obtained in a retrospective study that included 179 driver gene mutation-positive NSCLC patients with BM who received TKIs combined with RT; that is, WBRT did not offer better intracranial control than SRS, and this was probably related to the better systematic tumor control with TKI and ICI compared with pure chemotherapy (60). Another possible reason is the impact of the number of BMs. A study by Chen et al. classified 156 NSCLC patients with 1–4 BMs into three groups: those who received SRS, WBRT, and WBRT plus radiotherapy boost (RTB). The median OS and 2-year iPFS rates in the SRS group were not reached and 51.6%, those in the WBRT group were 33.3 months and 42.0%, and those in the WBRT plus RTB group were 27.9 months and 51.1%, respectively (61). There were no significant differences in OS and iPFS, which suggested that the number of BMs is an important factor in selecting the type of RT. Currently, several ongoing prospective trials are comparing SRS vs. WBRT for multiple BMs. For example, there is a phase III trial evaluating the OS in patients with 5–15 BM who received SRS compared with those who received hippocampal-avoidant WBRT plus memantine (NCT03550391). Other prospective trials are evaluating the differences in adverse effects of WBRT and SRS in patients with multiple BMs (NCT0192968; NCT 03075072). The results from these trials may help clinicians to select an optimal RT combination strategy.
We showed that pure chemotherapy has limited efficacy for BM. In contrast, a superior icORR and OS were observed in patients with BM treated with chemotherapy plus RT. Therefore, this therapy may be effective for non-targeted NSCLC patients with BM and a PD-L1-negative status. In addition, patients treated with chemotherapy plus bevacizumab had higher icORRs than patients treated with pure ICI, which may be explained by the fact that most patients in this subgroup received first-line treatment. The subgroup analysis confirmed this opinion.
Our study has several notable limitations. Most of the included studies were retrospective trials with small sample sizes, which could have resulted in selection bias. Furthermore, tumor response was not stratified according to certain inevitable influencing factors, such as the number and size of metastases. Further clinical trials are warranted to evaluate the efficacy of ICI-combined therapies and guide optimal clinical decisions.
Conclusions
For advanced non-targeted therapy NSCLC patients with BM, the current evidence suggests that the good clinical intracranial efficacy of ICI-based therapies, whether ICI plus chemotherapy or ICI plus RT, provides an impressive icORR and long-term survival benefits. In particular, patients who received first-line treatment or were PD-L1-positive could benefit more from ICI-based therapies, which leads to an improved icORR and prolonged survival. Moreover, chemotherapy plus RT had better efficacy for BM in patients with a PD-L1-negative status. The impacts of timing and technique on intracranial disease control need to be further validated in prospective trials. Currently, several ongoing trials are investigating the efficacy and safety of different treatments in advanced patients with baseline BM.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-515/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-515/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-515/coif). TL serves as an unpaid Associate Editor-in-Chief of Translational Lung Cancer Research from November 2017 to November 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim YH, Nagai H, Ozasa H, et al. Therapeutic strategy for non-small-cell lung cancer patients with brain metastases Biomed Rep 2013;1:691-6. (Review). [Crossref] [PubMed]
- Liu L, Bai H, Seery S, et al. Efficacy and safety of treatment modalities across EGFR selected/unselected populations with non-small cell lung cancer and brain metastases: A systematic review and Bayesian network meta-analysis. Lung Cancer 2021;158:74-84. [Crossref] [PubMed]
- Han X, Li H. Research Progress in the Treatment of Brain Metastases from Non-small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi 2020;23:1087-94. [PubMed]
- Weidle UH, Niewöhner J, Tiefenthaler G. The Blood-Brain Barrier Challenge for the Treatment of Brain Cancer, Secondary Brain Metastases, and Neurological Diseases. Cancer Genomics Proteomics 2015;12:167-77. [PubMed]
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. [Crossref] [PubMed]
- Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol 2019;20:1395-408. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Sun L, Davis CW, Hwang WT, et al. Outcomes in Patients With Non-small-cell Lung Cancer With Brain Metastases Treated With Pembrolizumab-based Therapy. Clin Lung Cancer 2021;22:58-66.e3. [Crossref] [PubMed]
- Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol 2020;21:655-63. [Crossref] [PubMed]
- Gauvain C, Vauléon E, Chouaid C, et al. Intracerebral efficacy and tolerance of nivolumab in non-small-cell lung cancer patients with brain metastases. Lung Cancer 2018;116:62-6. [Crossref] [PubMed]
- Dudnik E, Yust-Katz S, Nechushtan H, et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer 2016;98:114-7. [Crossref] [PubMed]
- Hendriks LEL, Henon C, Auclin E, et al. Outcome of Patients with Non-Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors. J Thorac Oncol 2019;14:1244-54. [Crossref] [PubMed]
- Wakuda K, Yabe M, Kodama H, et al. Efficacy of pembrolizumab in patients with brain metastasis caused by previously untreated non-small cell lung cancer with high tumor PD-L1 expression. Lung Cancer 2021;151:60-8. [Crossref] [PubMed]
- Zhang GW, Cheng RR, Wang HJ, et al. Therapeutic effect of nivolumab on non-small-cell lung cancer patients with brain metastases: a retrospective study. Zhonghua Zhong Liu Za Zhi 2020;42:961-5. [PubMed]
- Song P, Zhang J, Shang C, et al. Real-world evidenceand clinical observations of the treatment of advanced non-small cell lung cancer with PD-1/PD-L1 inhibitors. Sci Rep 2019;9:4278. [Crossref] [PubMed]
- Skribek M, Rounis K, Makrakis D, et al. Outcome of patients with NSCLC and brain metastases treated with immune checkpoint inhibitors in a ‘real-life’ setting. Cancers (Basel) 2020;12:3707. [Crossref] [PubMed]
- Kitai H, Asahina H, Takashina T, et al. Efficacy of pemetrexed in advanced non-small cell lung cancer with asymptomatic brain metastases. J Thorac Oncol 2013;8:S867.
- Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol 2011;22:2466-70. [Crossref] [PubMed]
- Chouahnia K, Bailon O, Bouillet T, et al. Pemetrexed (P) plus carboplatin (CB) as up-front treatment for patients with brain metastases of lung adenocarcinoma. Ann Oncol 2010;21:333.
- Hu Q, Ren S, Li A, et al. Effect of pemetrexed on advanced non small cell lung cancer patients with asymptomatic brain metastases. Respirology 2011;16:154.
- Bailon O, Chouahnia K, Augier A, et al. Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma. Neuro Oncol 2012;14:491-5. [Crossref] [PubMed]
- Bearz A, Garassino I, Tiseo M, et al. Activity of Pemetrexed on brain metastases from Non-Small Cell Lung Cancer. Lung Cancer 2010;68:264-8. [Crossref] [PubMed]
- Monnet I, Vergnenegre A, Robinet G, et al. Platin pemetrexed with or without bevacizumab with upfront versus “at progression” brain radiotherapy in advanced non squamous non-small cell lung cancer with asymptomatic brain metastasis: A randomized phase III trial (Metal2 trial). Ann Oncol 2020;31:S845. [Crossref]
- Tian Y, Zhai X, Tian H, et al. Bevacizumab in Combination with Pemetrexed and Platinum Significantly Improved the Clinical Outcome of Patients with Advanced Adenocarcinoma NSCLC and Brain Metastases. Cancer Manag Res 2019;11:10083-92. [Crossref] [PubMed]
- Li X, Abbas M, Li Y, et al. Comparative Effectiveness of Pemetrexed-platinum Doublet Chemotherapy With or Without Bevacizumab as First-line Therapy for Treatment-naive Patients With Advanced Nonsquamous Non-small-cell Lung Cancer in China. Clin Ther 2019;41:518-29. [Crossref] [PubMed]
- Besse B, Le Moulec S, Mazières J, et al. Bevacizumab in Patients with Nonsquamous Non-Small Cell Lung Cancer and Asymptomatic, Untreated Brain Metastases (BRAIN): A Nonrandomized, Phase II Study. Clin Cancer Res 2015;21:1896-903. [Crossref] [PubMed]
- Ashinuma H, Shingyoji M, Yoshida Y, et al. Safety and efficacy of bevacizumab-containing chemotherapy in NSCLC patients with brain metastases. Ann Oncol 2014;25:v96. [Crossref]
- Hirano S, Takeda Y, Izumi S, et al. Efficacy of chemotherapy in patients with asymptomatic brain metastases from non-small cell lung cancer. Japanese Journal of Lung Cancer 2006;46:111-6.
- Metro G, Gili A, Signorelli D, et al. Upfront pembrolizumab as an effective treatment start in patients with PD-L1 ≥ 50% non-oncogene addicted non-small cell lung cancer and asymptomatic brain metastases: an exploratory analysis. Clin Transl Oncol 2021;23:1818-26. [Crossref] [PubMed]
- Shepard MJ, Xu Z, Donahue J, et al. Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: a matched cohort study. J Neurosurg 2019; Epub ahead of print. [Crossref] [PubMed]
- Afzal MZ, Dragnev K, Shirai K. A tertiary care cancer center experience with carboplatin and pemetrexed in combination with pembrolizumab in comparison with carboplatin and pemetrexed alone in non-squamous non-small cell lung cancer. J Thorac Dis 2018;10:3575-84. [Crossref] [PubMed]
- He Q, Wang Y, Zou P, et al. Phase II Study of High-Dose Pemetrexed Plus Cisplatin as First-Line Chemotherapy In the Treatment of Patients with Brain Metastases from Lung Adenocarcinoma. World Neurosurg 2017;99:758-62. [Crossref] [PubMed]
- Dinglin XX, Huang Y, Liu H, et al. Pemetrexed and cisplatin combination with concurrent whole brain radiotherapy in patients with brain metastases of lung adenocarcinoma: a single-arm phase II clinical trial. J Neurooncol 2013;112:461-6. [Crossref] [PubMed]
- Quantin X, Bozonnat MC, Pujol JL. Recursive Partitioning Analysis Groups II-III brain metastases of non-small cell lung cancer: a phase II randomized study comparing two concurrent chemoradiotherapy regimens. J Thorac Oncol 2010;5:846-51. [Crossref] [PubMed]
- Chen L, Yang Q, Liang Y, et al. Brain radiotherapy combined with sequential chemotherapy in non-small-cell lung cancer patients with brain metastases. Chinese Journal of Lung Cancer 2009;12:896-900. [PubMed]
- Lee DH, Han JY, Kim HT, et al. Primary chemotherapy for newly diagnosed nonsmall cell lung cancer patients with synchronous brain metastases compared with whole-brain radiotherapy administered first: result of a randomized pilot study. Cancer 2008;113:143-9. [Crossref] [PubMed]
- Carbone D, Ciuleanu T, Cobo M, et al. First-line Nivolumab + Ipilimumab + Chemo in Patients With Advanced NSCLC and Brain Metastases: Results From CheckMate 9LA. J Thorac Oncol 2021;16:S862. [Crossref]
- Lau SCM, Poletes C, Le LW, et al. Durability of CNS disease control in NSCLC patients with brain metastases treated with immune checkpoint inhibitors plus cranial radiotherapy. Lung Cancer 2021;156:76-81. [Crossref] [PubMed]
- Lim SH, Lee JY, Lee MY, et al. A randomized phase III trial of stereotactic radiosurgery (SRS) versus observation for patients with asymptomatic cerebral oligo-metastases in non-small-cell lung cancer. Ann Oncol 2015;26:762-8. [Crossref] [PubMed]
- Azzam G, Park W, Mellon E, et al. Combined use of radiosurgery with concurrent PD-1/PD-L1 inhibition for metastatic brain lesions of NSCLC. Int J Radiat Oncol Biol Phys 2018;101:E24. [Crossref]
- Li J, Wang Y, Tang C, et al. Concurrent Nivolumab And Ipilimumab With Brain Stereotactic Radiosurgery For Brain Metastases From Non-Small Cell Lung Cancer: A Phase I Trial. Int J Radiat Oncol Biol Phys 2020;108:e744. [Crossref]
- Lee MH, Cho KR, Choi JW, et al. Immune Checkpoint Inhibitors for Non-Small-Cell Lung Cancer with Brain Metastasis: The Role of Gamma Knife Radiosurgery. J Korean Neurosurg Soc 2021;64:271-81. [Crossref] [PubMed]
- Nadal E, Castro RL, Juan O, et al. ATEZO-BRAIN, A Single-Arm Phase II Study of Atezolizumab Combined with Chemotherapy in Stage IV NSCLC Patients with Untreated Brain Metastases. J Thorac Oncol 2019;14:S405. [Crossref]
- Alencar V, Camandaroba M, Pirolli R, et al. Immunotherapy as Single Treatment for Brain Metastases of Non-small cell Lung Cancer: A Systematic Review and Meta-Analysis. J Thorac Oncol 2021;16:S359. [Crossref]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Durability of brain metastasis response and overall survival in patients with non-small cell lung cancer (NSCLC) treated with pembrolizumab. J Clin Oncol 2018;36:2009. [Crossref]
- Sun C, Zhou F, Li X, et al. PD-1/PD-L1 Inhibitor Combined with Chemotherapy Can Improve the Survival of Non-Small Cell Lung Cancer Patients with Brain Metastases. Onco Targets Ther 2020;13:12777-86. [Crossref] [PubMed]
- Powell SF, Rodríguez-Abreu D, Langer CJ, et al. Outcomes With Pembrolizumab Plus Platinum-Based Chemotherapy for Patients With NSCLC and Stable Brain Metastases: Pooled Analysis of KEYNOTE-021, -189, and -407. J Thorac Oncol 2021;16:1883-92. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. [Crossref] [PubMed]
- Geier M, Descourt R, Corre R, et al. Real-Life Intracerebral Efficacy of Nivolumab in Non-Small Cell Lung Cancer Patients with Brain Metastases. J Thorac Oncol 2018;13:S384-5. [Crossref]
- Tozuka T, Kitazono S, Sakamoto H, et al. Poor efficacy of anti-programmed cell death-1/ligand 1 monotherapy for non-small cell lung cancer patients with active brain metastases. Thorac Cancer 2020;11:2465-72. [Crossref] [PubMed]
- Kim PH, Suh CH, Kim HS, et al. Immune checkpoint inhibitor therapy may increase the incidence of treatment-related necrosis after stereotactic radiosurgery for brain metastases: a systematic review and meta-analysis. Eur Radiol 2021;31:4114-29. [Crossref] [PubMed]
- Weingarten N, Kruser TJ, Bloch O. Symptomatic radiation necrosis in brain metastasis patients treated with stereotactic radiosurgery and immunotherapy. Clin Neurol Neurosurg 2019;179:14-8. [Crossref] [PubMed]
- Martin AM, Cagney DN, Catalano PJ, et al. Immunotherapy and Symptomatic Radiation Necrosis in Patients With Brain Metastases Treated With Stereotactic Radiation. JAMA Oncol 2018;4:1123-4. [Crossref] [PubMed]
- Colaco RJ, Martin P, Kluger HM, et al. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg 2016;125:17-23. [Crossref] [PubMed]
- Shinde A, Akhavan D, Sedrak M, et al. Shifting paradigms: whole brain radiation therapy versus stereotactic radiosurgery for brain metastases. CNS Oncol 2019;8:CNS27. [Crossref] [PubMed]
- Schiff D, Messersmith H, Brastianos PK, et al. Radiation Therapy for Brain Metastases: ASCO Guideline Endorsement of ASTRO Guideline. J Clin Oncol 2022;40:2271-6. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Gerber NK, Young RJ, Barker CA, et al. Ipilimumab and whole brain radiation therapy for melanoma brain metastases. J Neurooncol 2015;121:159-65. [Crossref] [PubMed]
- El Shafie RA, Seidensaal K, Bozorgmehr F, et al. Effect of timing, technique and molecular features on brain control with local therapies in oncogene-driven lung cancer. ESMO Open 2021;6:100161. [Crossref] [PubMed]
- Chen Z, Zhou L, Zhao M, et al. Real-world analysis of different intracranial radiation therapies in non-small cell lung cancer patients with 1-4 brain metastases. BMC Cancer 2022;22:1010. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



