
Activity of osimertinib in a patient with stage IV non-small cell lung cancer harboring HER2 exon 19, p.L755P mutation: case report
Highlight box
Key findings
• We present a case of a 68-year-old female with stage IV NSCLC harboring a ERBB2 exon 19 c.2262_2264delinsTCC, p.(L755P) mutation treated with osimertinib, resulting in intra- and extracranial response.
What is known and what is new?
• Osimertinib, a 3rd generation EGFR-TKI has been found in pre-clinical studies, both in vitro and in vivo, to have activity against various HER2 exon 19 aberrations, including HER2 exon 19, p.L755P mutations.
• Here we demonstrate for the first time in humans, that osimertinib was an effective and well tolerated treatment in a patient with stage IV NSCLC harboring HER2 exon 19, p.L755P mutation.
What is the implication, and what should change now?
• The efficacy of osimertinib against HER2 exon 19, p.L755P mutations and other HER2 exon 19 aberrations should be tested in clinical trials to determine its efficacy as a potential HER2 targeted treatment for patients harboring these mutations.
Introduction
Erb-B2 receptor tyrosine kinase 2 (ERBB2) is a member of the ErbB tyrosine receptor family, that also includes EGFR, ERBB3, and ERBB4. ERBB2 dimerizes either with itself or other ErbB members, causing activation of downstream signaling through two major pathways, the PI3K-AKT and MEK-ERK pathways, which induce cell proliferation and migration (1,2). HER2 dysregulation in non-small cell lung cancer (NSCLC) may arise as a result of protein overexpression, caused by gene amplification and/or aberrations in regulatory regions, or alternatively by the acquisition of gain of function coding mutations. ERBB2 gene amplification often emerges as a mechanism of acquired resistance to treatment, whereas somatic alterations usually represent oncogenic driver events (3-5).
Clinical trials combining anti-HER2 monoclonal antibodies with chemotherapy in NSCLC, aimed at targeting HER2 protein overexpression, have yielded disappointing results, which is in contrary to the success of these agents in breast and gastric cancer with HER2 protein overexpression (6).
The incidence of HER2 mutations in NSCLC is 2–4% (7), and have been associated with female sex, younger age, light or never smoking history, higher incidence of brain metastasis and worse prognosis (8,9). Therefore, HER2 targeting represents an urgent clinical need in NSCLC. Recurrent in-frame insertions within exon 20 causing duplication of amino acids YVMA represent 80% of all HER2 alterations in NSCLC. Tyrosine kinase inhibitors (TKIs) such as poziotinib, pyrotinib and antibody-drug conjugates (ADC) like trastuzumab deruxtecan (T-Dxd), have shown promising results in early phase clinical trials in NSCLC harboring HER2 aberrations (10-13). Currently there is no phase III clinical data available to guide treatment in this population and the activity of these agents in patients with ERBB2 exon 19 c.2262_2264delinsTCC, p.(L755P) mutation is limited. Osimertinib, a 3rd generation TKI that irreversibly and specifically targets both sensitizing and resistant T790M-mutated EGFRs, has been found to inhibit HER2 in pre-clinical studies (2,3). We present the following case in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-596/rc).
Case presentation
A 68-year-old Caucasian female with a past medical history of type 2 insulin dependent diabetes and a 3-pack-year smoking history (quit smoking in 1971) was diagnosed with adenocarcinoma of the lung (right lower lobe primary) in October 2016 via CT guided core needle biopsy. Immunohistochemical testing showed that the tumor was strongly positive for TTF-1 and CK7. At diagnosis her disease TNM staging was T3N3M1b according to AJCC 8th edition as she had metastatic disease to right hilar nodes, bilateral mediastinal nodes, and celiac axis nodes. Molecular testing showed that FISH for ALK and ROS was negative. PD-L1 tumor proportion score was <1% (22C3 assay). A 50 gene custom panel was performed using Ampliseq-based next generation sequencing on a NextSeq 550 (Illumina Inc., San Diego, CA, USA) and showed ERBB2 exon 19 mutation, c.2262_2264delGTTinsTCC, causing leucine to proline substitution at codon 755 (p.L755P). No other driver alterations were identified, including EGFR mutations. The timeline of this patient’s cancer treatment journey is portrayed in Figure 1.
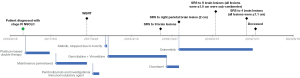
She was started on 1st line platinum-based doublet therapy with carboplatin and paclitaxel with an investigational agent through a clinical trial. After induction therapy she received pemetrexed switch-maintenance therapy. Her disease metastasized to the brain while receiving maintenance pemetrexed. She subsequently underwent whole-brain radiation with 30 Gy/10 fx and received 2nd line treatment with pembrolizumab and an investigational immunomodulatory agent as part of a clinical trial. However, she developed progression within two months.
She then started afatinib based on phase II data in patients with NSCLC and HER2 mutation, however, this was discontinued within a week due to grade 4 diarrhea.
After this, she received gemcitabine-vinorelbine combination resulting in stable intra- and extracranial disease for nine months. At which time there was disease progression in the primary lung lesion and brain. She underwent stereotactic radiosurgery (SRS) to brain lesions and started on docetaxel.
After three cycles, imaging showed multiple new brain lesions and she received SRS to the largest lesion only. At this time the patient was still fit for treatment. Therefore, clinical trials with anti-Her2 therapy were considered however she either did not qualify for available trials due to eligibility criteria or open slots were not available when enrollment was needed.
Nagano et al. had recently showed that among various TKIs, osimertinib had the best efficacy against the HER2 exon 19, p.L755P mutation (2). The authors demonstrated that the IC50 of osimertinib for the HER2 exon 19, p.L755P mutation was 23.8 nmol/L and low-dose osimertinib (8 mg/kg) could inhibit the growth of tumors with this mutation. Additionally, osimertinib was known to penetrate the blood brain barrier which was important since the main site of the patient’s disease progression was in the brain. Based on the findings from Nagano et al. osimertinib was commenced at a dose of 80 mg OD. Osimertinib was provided by Astrazeneca via patient assistance program at no cost to the patient. To maximize chances of intracranial disease control the dose of osimertinib was increased after two weeks of treatment to 160/80 mg alternating days and QTc monitored. The patient achieved a confirmed partial response (PR) to osimertinib intra- and extracranially according to RECIST criteria (Figures 2,3). Four months after starting osimertinib the patient was admitted with grade 3 fatigue and grade 2 transaminitis. It was thought that this could either have been related to osimertinib or other medications she was taking at that time (doxycycline). Osimertinib was withheld for two weeks with resolution of symptoms and dose was restarted at 160/80 mg alternating days. After six months on treatment with osimertinib, there was evidence of disease progression in the brain and nine small lesions were treated with SRS. Extracranial disease was stable at this time. Osimertinib dose was increased to 160 mg OD for another four months, after which the patient had further CNS progression with development of lethargy and disorientation, ultimately leading to death.


All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
To our knowledge, this is the first report to show osimertinib activity in a patient with NSCLC harboring HER2 exon 19, p.L755P mutation resulting in intra- and extracranial response. The patient lived for a total of 11-month after starting osimertinib. As well as being effective, osimertinib at alternating 160/80 mg daily dose was also well tolerated. This is an important observation considering toxicity concerns with some of the other HER2-targeting drugs. In the coming years, osimertinib could become a HER2 mutant targeted therapy for patients harboring exon 19 ERBB2 point mutations.
In a pre-clinical study using animal models, Liu et al. presented that osimertinib had antitumor efficacy against multiple HER2 aberrations in NSCLC, either as a single agent or in combination with JQ1, a BET inhibitor (3). Subsequently, Nagano et al. showed using both in vitro cell lines and in vivo animal models that HER2 variants at L755 were more sensitive to osimertinib compared to other TKIs, interestingly it was not effective against common exon 20 alterations (2). Because of the homology between HER2 and EGFR it has been speculated that the covalent binding site for osimertinib may be C805 (analogous Cys797 to EGFR) of human HER2 (3). No clinical trials have tested the efficacy of osimertinib in HER2 alterations.
Various other TKIs have been tested in NSCLC with HER2 alterations. Results from a multicenter, phase II trial looking at patients with HER2 exon 20 mutation treated with poziotinib after prior therapies (n=90) reported that the objective response rate (ORR) was 28% (10). We could not find any data regarding its activity in NSCLC with HER2 exon 19, p.L755P alteration. Pyrotinib has been found to produce an ORR of 30% in HER2-mutant NSCLC (n=60). This study included 4 patients with exon 19, p.L755P mutation of which 1 patient achieved a PR (11).
Ado-Trastuzumab emtansine, an ADC, demonstrated an ORR of 44% in 18 patients with HER2 mutant NSCLC (12). However, patients with exon 19 p.L755P did not respond (n=2). T-Dxd, another ADC, was evaluated in a phase II trial in patients with NSCLC harboring HER2 mutations (n=91). In this study, ORR was 55% and overall survival 17.8 months, 2 patients had an exon 19, p.L755P mutation. Their best responses were PR and stable disease, respectively (13). Toxicity concerns regarding fatal interstitial lung disease were noted in this trial.
Conclusions
Osimertinib, a 3rd generation EGFR-TKI, was effective and well tolerated in a patient with stage IV NSCLC harboring ERBB2 exon 19 c.2262_2264delinsTCC, p.(L755P) mutation, including response of intracranial metastases. Future research is required to illustrate the differential effects of targeted therapies in NSCLC harboring various types of HER2 alterations to select an optimal therapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-596/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-596/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-596/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tamaskovic R, Schwill M, Nagy-Davidescu G, et al. Intermolecular biparatopic trapping of ErbB2 prevents compensatory activation of PI3K/AKT via RAS-p110 crosstalk. Nat Commun 2016;7:11672. [Crossref] [PubMed]
- Nagano M, Kohsaka S, Ueno T, et al. High-Throughput Functional Evaluation of Variants of Unknown Significance in ERBB2. Clin Cancer Res 2018;24:5112-22. [Crossref] [PubMed]
- Liu S, Li S, Hai J, et al. Targeting HER2 Aberrations in Non-Small Cell Lung Cancer with Osimertinib. Clin Cancer Res 2018;24:2594-604. [Crossref] [PubMed]
- Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007;26:6469-87. [Crossref] [PubMed]
- Li BT, Ross DS, Aisner DL, et al. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J Thorac Oncol 2016;11:414-9. [Crossref] [PubMed]
- Lara PN Jr, Laptalo L, Longmate J, et al. Trastuzumab plus docetaxel in HER2/neu-positive non-small-cell lung cancer: a California Cancer Consortium screening and phase II trial. Clin Lung Cancer 2004;5:231-6. [Crossref] [PubMed]
- Stephens P, Hunter C, Bignell G, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 2004;431:525-6. [Crossref] [PubMed]
- Pillai RN, Behera M, Berry LD, et al. HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer 2017;123:4099-105. [Crossref] [PubMed]
- Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997-2003. [Crossref] [PubMed]
- Le X, Cornelissen R, Garassino M, et al. Poziotinib in Non-Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutations After Prior Therapies: ZENITH20-2 Trial. J Clin Oncol 2022;40:710-8. [Crossref] [PubMed]
- Zhou C, Li X, Wang Q, et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma After Platinum-Based Chemotherapy: A Multicenter, Open-Label, Single-Arm, Phase II Study. J Clin Oncol 2020;38:2753-61. [Crossref] [PubMed]
- Li BT, Shen R, Buonocore D, et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol 2018;36:2532-7. [Crossref] [PubMed]
- Li BT, Smit EF, Jänne PA. Trastuzumab Deruxtecan in Non-Small-Cell Lung Cancer. N Engl J Med 2022;386:1770-1. Reply. [Crossref] [PubMed]


