
Real-world data on severe lung cancer: a multicenter retrospective study
Highlight box
Key findings
• The incidence rate of severe lung cancer among lung cancer patients in the cross-sectional study was 13.10% and treatment-related AEs are gradually accounting for more of the causes of severe lung cancer, surpassing cancer-related symptoms and comorbidities.
• We summarized the clinical characteristics of severe lung cancer patients and found that the prognosis of severe lung cancer due to adverse reactions was the worst.
What is known and what is new?
• Our team pioneered the concept of “advanced severe lung cancer” in 2017 and drafted the “International consensus on severe lung cancer-first edition” in 2021.
• We analyzed the epidemiological data of patients with severe lung cancer through a multicenter retrospective study. Additionally, we assessed clinicians’ acceptance of the severe lung cancer concept via a questionnaire.
What is the implication, and what should change now?
• We cannot ignore patients with severe lung cancer, especially due to treatment-related adverse effects.
Introduction
According to Global Cancer Incidence, Mortality and Prevalence (GLOBOCAN) 2020, lung cancer is currently the second most common cancer and the leading cause of death worldwide (1). Two-thirds of patients with lung cancer are diagnosed with stage IIIB, IIIC, and IV, and the prognosis of these patients is clinically poor (2). Eastern Cooperative Oncology Group (ECOG) performance status (PS) scores are evaluated on the basis of the patient’s ability of self-care, level of daily activity, and physical ability in terms of walking and working or percentage of time waking hours confined to a bed or chair (3). The assessment of PS scores in patients with cancer provides prognostic information and guides treatment intervention (4,5). As PS scores are highly heterogeneous and depend on subjective categorization, the evaluation results of different doctors for the same patient can be different. Besides, the results of the doctor’s evaluation and the patient’s self-evaluation will also differ (6). Nevertheless, the evaluation is still a reliable and relevant prognostic variable, being one of the most important independent prognostic factors in lung cancer (7,8). The recommendation against chemotherapy in patients with poor PS scores dates to the early 1980s, when poor PS score (PS 2–4) was a predictor of poor survival (9). For a long time, patients with poor PS score were typically ineligible for clinical trials, and existing evidence of limited benefits among patients with poor PS has been derived from only a handful of small trials (10,11).
The incidence of poor PS is high among patients in all stages of lung cancer. For example, in extensive epidemiological studies, Buccheri and Radzikowska found that 42–50% of patients with lung cancer had a PS score of 2–4 as assessed by their doctors at diagnosis (12-14). Furthermore, in recent years, the availability of targeted therapies, antiangiogenic agents, and immune checkpoint inhibitors (ICIs) has dramatically prolonged the survival of patients and made poor PS score in these patients less of a concern than that in patients treated with traditional chemotherapy (15,16). Moreover, an increasing number of clinical studies are enrolling patients with a PS score of 2, and some real-world studies have enrolled patients with PS scores of 3 and 4. In addition to advances in oncology, improved survival in patients with a poor PS score has also been attributed to advances in managing sepsis and associated organ failure (17,18). Therefore, we found it was necessary to distinguish a certain portion of patients with poor PS scores and end stage lung cancer who could benefit from modern treatment. Based on previous research, our team pioneered the concept of “advanced severe lung cancer” in 2017 (19,20). Moreover, we drafted the first edition of the international consensus on severe lung cancer in 2021 (21).
Severe lung cancer is a novel concept, and there is currently no relevant research or real-world data on those with severe lung cancer, such as incidence, cause, clinical features, and risk factors. Therefore, based on the first edition of the international consensus, we (I) conducted a real-world, multicenter study on patients with severe lung cancer and (II) used questionnaires to assess clinicians’ acceptance of the severe lung cancer concept. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-4/rc).
Methods
Patients
This multicenter, retrospective, observational study was conducted in 3 participating institutes through electronic medical records and consisted of 2 cohorts. One cohort was a cross-sectional study that collected data from patients with lung cancer who received antitumor treatment at different centers from January 1, 2022, to June 30, 2022. The other cohort included fatal cases from January 1, 2019, to June 2022. The participating institutes included the First Affiliated Hospital of Guangzhou Medical University (Hospital A), the First Affiliated Hospital of Anhui Medical University (Hospital B), and Shenzhen People’s Hospital (Hospital C). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the First Affiliated Hospital of Guangzhou Medical University (No. 2021-38). Each participating center was informed and agreed with this study. Individual consent for this retrospective analysis was waived.
Data collection
A total of 10 clinicians familiar with the international consensus on severe lung cancer collected the data from each center. Data entry is carried out after disputes are eliminated and a consensus is reached. First, we recorded the incidence, causes and baseline data of severe lung cancer in patients in the 2 study cohorts (3 primary causes are classified according to the consensus on severe lung cancer: cancer-related symptoms, treatment-related AEs, and comorbidities). We then collected the demographic and survival data for fatal cases (gender, age, preexisting comorbidities, pathological classification, neoplasm staging, genetic mutations, operation history, comorbidities, history of other tumors). The survival data recorded included the overall survival (OS) time from the first diagnosis of severe lung cancer to death.
The inclusion criteria for patients were the following: (I) age older than 18 years; (II) definite pathologic diagnosis of lung cancer and clinical lung cancer staging (IIIB, IIIC, and IV); (III) undergone at least 1 course of antitumor treatment; (IV) relatively complete baseline data; and (V) in fatal cases, recording of the cause of death in electronic medical records.
The exclusion criteria for patients were the following: (I) age younger than 18 years and (II) lack of antitumor therapy.
The inclusion criteria for severe lung cancer: We formulated the following three criteria based on the concept of severe lung cancer: (I) lung cancer patients with poor PS score [2–4] and thus led to discontinuation of current anticancer therapy; (II) the above patients have achieved improvement in the PS score [0–1] after supportive care and antitumor treatment based on dynamic and precise testing; (III) patients received at least two anti-tumor treatments after their PS scores improved.
Questionnaire for clinicians
We developed an electronic questionnaire regarding severe lung cancer and sent the electronic questionnaire to clinicians from mainland China who had treated patients with lung cancer (website address of online questionnaires: https://www.wjx.cn ID: 173715842; Appendix 1). The electronic questionnaire was implemented from August 1, 2022, to August 31, 2022. The answers from clinicians were collected and analyzed.
The inclusion criteria for clinicians were the following: (I) a specialist in treating lung cancer; (II) registration as a clinician; and (III) familiarity with the PS score criteria (Appendix 2).
Pathologic diagnosis and clinical TNM classification
The diagnostic criteria were those outlined in the “2015 Chinese guidelines for the diagnosis and treatment of primary lung cancer”. And the tumor classification of patients was performed according to the “World Health Organization Classification of Lung Tumor Tissue” (22,23). The staging criteria followed the eighth edition of the TNM staging method formulated by the International Union against Cancer in 2009 (24).
The concept of severe lung cancer
Severe lung cancer is a disease in which the patient has a poor PS score [2–4] in certain stages due to various acute or chronic comorbidities, cancer-related symptoms, and/or treatment-related adverse events (AEs) but also has a high probability of achieving survival benefit and/or improvement in the PS score after supportive care and antitumor treatment based on dynamic and precise testing (21).
Statistical analysis
An unpaired t test was used to compare continuous variables between the groups. The chi-squared or Fisher exact tests were used to compare categorical variables between the two groups. OS was calculated from the diagnosis of severe lung cancer until death. Cox proportional hazard regression models were used to estimate hazard ratios (HRs) with 95 % confidence intervals (CIs) for OS for multivariate models. Risk factors with P≤0.1 in the univariate analysis were included in the multivariate analysis. The hazard ratios (HR) the 95% confidence intervals (95% CIs) are reported. Kaplan-Meier survival curves were used to evaluate the OS of patients, and the log-rank test was used to assess differences between groups. A P value of <0.05 indicated statistical significance for all tests. The statistical significance levels were all 2-sided. SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used for analysis, and the graphs were generated using GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA)
Results
Cross-sectional study of patients with severe lung cancer
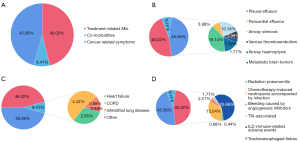
Clinical data of patients with lung cancer were collected and analyzed by 12 clinicians from 3 hospitals according to the inclusion criteria and the screening procedure for eligible cases (Figure S1). We collected data from 1,725 patients with advanced lung cancer who received antitumor therapy. Of them, 226 patients (13.10%) developed severe lung cancer (Figure S2). The demographic characteristics of the 1,725 advanced lung cancer patients were shown in Table 1. Severe lung cancer patients were mainly stage IV non-squamous NSCLC elderly male patients without gene mutation, and few patients had a history of lung cancer resection. We compared the differences in clinical characteristics between patients with severe lung cancer and non-severe lung cancer and found that the proportion of patients with smoking history (50.4%) in the severe lung cancer group was significantly more than that in the non-severe lung cancer group (40.8%; χ2=7.449; P=0.006; Table 1), and the proportion of patients with comorbidities in the severe lung cancer group (46.9%) was significantly more than that in the non-severe lung cancer group (36.4%; χ2=9.171; P=0.002; Table 1). We then analyzed the 3 primary causes of severe lung cancer in 226 patients (Figure 1A). Among them, treatment-related AEs were the most common primary cause (46.02%), followed by cancer-related symptoms (45.58%), and then pre-existing comorbidities (8.41%). Further analyzing the specific causes, we found that immunotherapy-related adverse events (irAEs) (25.66%) occurred the most frequently among the treatment-related AEs. For cancer-related symptoms, pleural effusion (18.18%) appeared the most often, and chronic obstructive pulmonary disease (COPD; 4.42%) was the most common comorbidity leading to severe lung cancer (Figure 1B-1D).
Table 1
| Category and subcategory | Severe lung cancer (n=226) | Non-severe lung cancer (n=1,499) | Total | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 64.27±11.16 | 62.85±11.11 | 63.04±11.12 | 0.07 |
| Age, n (%) | 0.19 | |||
| ≥65 | 124 (54.9) | 752 (50.2) | 876 (50.8) | |
| <65 | 102 (45.1) | 747 (49.8) | 849 (49.2) | |
| Gender, n (%) | 0.50 | |||
| Male | 164 (72.6) | 1,055 (70.4) | 1,219 (70.7) | |
| Female | 62 (27.4) | 444 (29.6) | 506 (29.3) | |
| Smoking history, n (%) | 0.006 | |||
| No | 112 (49.6) | 887 (59.2) | 999 (57.9) | |
| Yes | 114 (50.4) | 612 (40.8) | 726 (42.1) | |
| Pathological classification, n (%) | 0.06 | |||
| Nonsquamous NSCLC | 146 (64.6) | 1,067 (71.2) | 1,213 (70.3) | |
| Squamous cell carcinomas | 57 (25.2) | 279 (18.6) | 336 (19.5) | |
| Small cell lung cancer | 23 (10.2) | 153 (10.2) | 176 (10.2) | |
| Location, n (%) | 0.08 | |||
| Central | 107 (47.3) | 618 (41.2) | 725 (42.0) | |
| Peripheral | 119 (52.7) | 881 (58.8) | 1,000 (58.0) | |
| Neoplasm staging, n (%) | 0.80 | |||
| IIIB and IIIC | 35 (15.5) | 242 (16.1) | 277 (16.1) | |
| IV | 191 (84.5) | 1,257 (83.9) | 1,448 (83.8) | |
| Genetic mutations, n (%) | 0.68 | |||
| No | 167 (73.9) | 1,127 (75.2) | 1,294 (75.0) | |
| Yes | 59 (26.1) | 372 (24.8) | 431 (25.0) | |
| Operation history, n (%) | 0.757 | |||
| No | 196 (86.7) | 1,311 (87.5) | 1,507 (87.4) | |
| Yes | 30 (13.3) | 188 (12.5) | 218 (12.6) | |
| Comorbidities, n (%) | 0.002 | |||
| No | 120 (53.1) | 953 (63.6) | 1,073 (62.2) | |
| Yes | 106 (46.9) | 546 (36.4) | 652 (37.8) | |
| History of other tumors, n (%) | 0.326 | |||
| No | 211 (93.4) | 1,423 (94.9) | 1,634 (94.7) | |
| Yes | 15 (6.6) | 76 (5.1) | 91 (5.3) | |
| Family history of cancer, n (%) | 0.973 | |||
| No | 219 (96.9) | 1,465 (97.7) | 1,684 (97.6) | |
| Yes | 7 (3.1) | 34 (2.3) | 41 (2.4) | |
SD, standard deviation; NSCLC, non-small cell lung cancer.
Statistical analysis of fatal case data
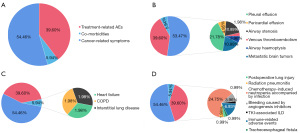
We included the data of 269 deceased patients with advanced lung cancer according to the inclusion criteria (Figure S3). Among them, 101 cases (37.55%) developed severe lung cancer from diagnosis to death (Figure S4). Furthermore, we analyzed the 3 primary causes of severe lung cancer development in these 101 patients, with the most common being cancer-related symptoms (54.46%), followed by treatment-related AEs (39.60%) (Figure 2A). Pre-existing comorbidities accounted more only a minor proportion of primary causes (5.94%). For the specific causes of severe lung cancer, pleural effusion (21.78%) was the most common cause of cancer-related symptoms. The most common cause of treatment-related AEs was chemotherapy-induced neutropenia accompanied by infection (24.75%). COPD, heart failure, and interstitial lung disease had equal proportions among the comorbidities (1.98%) (Figure 2B-2D).
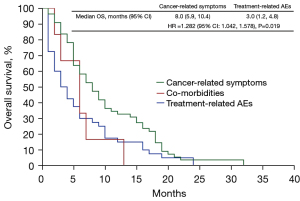
We further explored the risk factors for OS in deceased patients who developed severe lung cancer (n=101). The results showed that patients with genetic mutations (adjusted HR 0.619; 95% CI: 0.406–0.944) and severe lung cancer caused by treatment-related AEs (adjusted HR 1.282; 95% CI: 1.042–1.578) were significant risk factors associated with OS (Table 2, Figure 3). The OS of deceased patients with the genetic mutation was better than that of the control group (8 vs. 5 months; P=0.026; Figure S5). In addition, the OS of patients who developed severe lung cancer due to cancer-related symptoms was better than that of those who developed it from treatment-related AEs (8 vs. 3 months; P=0.019). The median OS for patients with comorbidities was 5 months, but due to the small number of patients in this subset (n=6), we did not statistically compare it with the other two causes (Figure 4). After excluding the 6 patients with severe lung cancer caused by comorbidities, we compared the baseline data of patients with severe lung cancer caused by cancer-related symptoms (n=55) with those from patients with treatment-related AE causes (n=40) (Table S1). After analyzing the data, we found that there are more patients aged 65 years and above in the treatment-related AE group (65.00%) than cancer-related symptoms group (35.50%), and the difference was statistically significant (χ2=8.615; P=0.003).
Table 2
| Category and subcategory | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≥65 vs. <65 years) | 0.90 (0.61, 1.33) | 0.60 | – | – | |
| Gender (male vs. female) | 0.91 (0.57, 1.46) | 0.70 | – | – | |
| Pathological classification | |||||
| Nonsquamous NSCLC | 1 (ref) | – | 1 (ref) | – | |
| Squamous cell carcinomas | 0.55 (0.28, 1.10) | 0.09 | 0.52 (0.26, 1.05) | 0.07 | |
| Small cell lung cancer | 0.62 (0.35, 1.10) | 0.10 | 0.89 (0.68, 1.66) | 0.72 | |
| Neoplasm staging (IIIB and IIIC vs. IV) | 1.10 (0.62, 1.94) | 0.74 | – | – | |
| Genetic mutations (no vs. yes) | 0.62 (0.41, 0.94) | 0.03 | 0.58 (0.36, 0.95) | 0.03 | |
| Operation history (no vs. yes) | 0.77 (0.44, 1.33) | 0.35 | – | – | |
| Comorbidities (no vs. yes) | 1.09 (0.68, 1.74) | 0.74 | – | – | |
| History of other tumors (no vs. yes) | 0.80 (0.25, 2.59) | 0.71 | – | – | |
| Primary causes | |||||
| Cancer-related symptoms | 1 (ref) | – | 1 (ref) | – | |
| Comorbidities | 2.03 (0.85, 4.83) | 0.11 | 0.64 (0.42, 0.98) | 0.78 | |
| Treatment-related AEs | 1.28 (1.04, 1.58) | 0.02 | 1.13 (0.47, 2.74) | 0.04 | |
HR, hazard ratio; CI, confidence interval; AEs, adverse events; NSCLC, non-small cell lung cancer.

Questionnaire for clinicians
In total, 617 clinicians from 27 provinces in mainland China completed the questionnaire, including 316 respiratory physicians, 232 oncologists, 22 thoracic surgeons, 19 radiotherapy physicians, and 5 interventional physicians (Figure S6). Furthermore, 97.01% of clinicians had experience diagnosing and treating newly diagnosed lung cancer patients with poor PS scores, and 41.03% of physicians frequently experienced the above. When asked to identify what caused a poor PS scores, 81.90% of clinicians indicated pre-existing comorbid conditions, and 89.37% indicated cancer-related symptoms (Figure S7A,S7B). When asked about treating using antitumor therapy in patients with lung with cancer, 99.26% of clinicians indicated they experienced treating patients with poor PS scores, and 40.49% of physicians indicated they frequently experienced the above. Moreover, 77.24% of clinicians believed that comorbidities can cause poor PS scores, 91.60% thought that cancer-related symptoms were one of the primary causes, and 78.73% believed that treatment-related AEs were one of the primary causes (Figure S7C,S7D). As for the severe lung cancer concept, 90.26% of clinicians agreed with this concept, 91.56% believed that the severe lung cancer concept has more therapeutic value than does that of end-stage lung cancer, and 98.21% thought that it is necessary to distinguish groups of patients with severe lung cancer (Figure S8A-S8C). For lung cancer patients with comorbidities, 92.54% of clinicians thought it necessary to treat both lung cancer and comorbidities (Figure S8D). In the questionnaire, we set up a 10-point scale assessment to allow clinicians to evaluate their ability to manage patients with severe lung cancer, with the majority of clinicians scoring between 5 and 8 points (Figure S9A). After comparing the scores of the two major groups of clinicians, we found that most respiratory clinicians scored 7 points (19.69%) while oncologists scored 6 points (28.95%) (Figure S9B). Concerning the treatment strategy recommended in the international consensus on severe lung cancer, each recommendation was supported by more than 75% of clinicians, and the highest rate of support was for multidisciplinary participation and individualized and comprehensive treatment (94.22%), while the lowest was for the escalation/de-escalation of chemotherapy drugs (75.75%) (Figure S10).
Discussion
Patients with lung cancer with poor PS [2–4] at diagnosis have poor survival rates and a high incidence in clinic (25,26). According to the questionnaire results, 97.01% of clinicians have treated patients with newly diagnosed lung cancer and poor PS scores, and 99.25% encountered poor PS scores in patients undergoing antitumor treatment. Based on this background, we defined a subset of patients with the potential for remission of severe lung cancer, as supported by 90.66% of clinicians. In the study, 12 clinicians from 3 hospitals conducted cross-sectional statistical analysis and found that 10.70–25.35% of patients with advanced lung cancer developed severe lung cancer. Moreover, further analyzing the data of fatal cases revealed that 18.29–56.30% had severe lung cancer at least once from tumor diagnosis to death. The results show that severe lung cancer is prevalent in patients with advanced lung cancer based on real-world evidence.
Distinguishing the causes of severe lung cancer is essential for the subsequent management of patients. Previous research points to cancer-related symptoms or pre-existing comorbid conditions that can affect PS scores of those with newly diagnosed lung cancer (12,27). According to the questionnaire, more than 81.89% of clinicians agreed that there are two main causes of the poor PS score of newly diagnosed patients. For patients with lung cancer and antitumor therapy, the occurrence of treatment-related AEs is also a fundamental cause of the deterioration of PS score. Targeted drugs and ICIs have dramatically benefited the survival of patients with lung cancer; however, even if ICIs are generally better tolerated than are chemotherapy, they are not free from toxicities (28-30). From analyzing the causes of severe lung cancer in 3 participating institutes, we found that the primary cause for severe lung cancer in the cross-sectional study was treatment-related AEs, with irAEs accounting for the majority. However, in the 101 fatal cases of severe lung cancer, pleural and pericardial effusion as cancer-related symptoms ranked first as causes of severe lung cancer, ahead of treatment-related AEs. We analyzed the factors underlying this contrast and found that the start date for enrollment in the cross-sectional study was January 1, 2022, and thus more patients had the opportunity to receive immunotherapy, resulting in more irAEs. On the other hand, the primary treatment for patients in fatal cases was chemotherapy, so the most common treatment-related adverse reaction was chemotherapy-induced neutropenia accompanied by infection (24.75%). Moreover, pleural and pericardial effusion are common symptoms of patients lung with cancer and can easily lead to poor PS scores and quickly be relieved by treatment in clinical practice. Hence, the survival time of the 101 fatal cases with advanced lung cancer who developed severe lung cancer caused by treatment-related AEs was shorter than that of those caused by cancer-related symptoms (8 and 3 months; P<0.05). Moreover, we believe that with the advances of antitumor treatment, treatment-related AEs will become the main contributor to severe lung cancer.
In our data, the patients with severe lung cancer caused by comorbidities were 8.41% and 5.94% in the 2 study cohorts. We considered that there might be several reasons for the small proportion of patients with comorbidities being the cause of severe lung cancer: (I) as this was a retrospective observational study based on electronic medical records, clinicians might have been unable to distinguish the symptoms of comorbidities and tumors; (II) some clinicians could have ignored the diagnosis and treatment of comorbidities in the patients with lung cancer. For example, previous study has pointed out that oncologists may be neglectful in diagnosing and treating COPD in patients with lung cancer and COPD (31). However, comorbidity is not only a critical cause of severe lung cancer but also affects the patient’s prognosis. Previous studies by our own and Hyunji’s group found that standardized management of comorbidities can prolong the survival of those with lung cancer (32,33). Moreover, 92.54% of clinicians in the questionnaire thought that the treatment of both lung cancer and comorbidities was necessary.
The previous reports of poor outcomes of patients with cancer and poor PS scores in the literature might have led clinicians to forego early life-sustaining therapy; however, clinicians must be up to date with the literature to improve their clinical skills and willingness to care for patients (18,34). This was also the main impetus for developing our concept of severe lung cancer. The self-assessment results of clinician’ confidence in the questionnaire showed that most clinicians had the confidence and willingness to manage patients with severe lung cancer.
To our best knowledge, this is the first epidemiological study based on real-world data to examine a population of patients with severe lung cancer. Nonetheless, some limitations of the current study should be noted. First, the diagnostic criteria for severe lung cancer were based on the change in the PS score of the patient, and the PS score is a subjective standard. However, we did require at least two clinicians in each participating center to evaluate the PS score independently and negotiate a consensus on the score, but discrepancies were inevitable, especially between different centers. Second, we used a retrospective observational study design that relied on the electronic medical record system. Therefore, the reliability of the data concerning the statistical and analytical results of severe lung cancer causes should be scrutinized. Finally, the level of evidence of questionnaire studies is considered controversial, so the results of the clinician questionnaire should be verified further.
Conclusions
The incidence of severe lung cancer in advanced lung cancer patients is 13.10% in real-world clinical practice, and the concept of severe lung cancer is supported by the vast majority of clinicians in the field. Severe lung cancer patients were mainly stage IV non-squamous NSCLC elderly male patients without gene mutation, and few patients had a history of lung cancer resection. And compared with common lung cancer patients, the proportion of smoking in severe lung cancer patients is less but more comorbidities. With the development of antitumor treatment, treatment-related AEs have gradually become the most common cause of severe lung cancer, more so than cancer-related symptoms and co-morbidities. Furthermore, the prognosis of patients with advanced lung cancer who develop severe lung cancer due to treatment-related AEs is worse than that of patients who do so from cancer-related symptoms.
Acknowledgments
The authors appreciate the academic support from the China Respiratory Oncology Collaboration (CROC).
Funding: This study was supported by grants from State Key Laboratory of Respiratory Disease-The Open Project (No. SKLRD-OP-202011), the Beijing Xisike Clinical Oncology Research Foundation (No. Y-HS202102-0118), and the Wu Jieping Medical Foundation (No. 320.6750.2021-17-7).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-4/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-4/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-4/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the First Affiliated Hospital of Guangzhou Medical University (No. 2021-38). Each participating center was informed and agreed with this study. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Hong QY, Wu GM, Qian GS, et al. Prevention and management of lung cancer in China. Cancer 2015;121:3080-8. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Blazeby JM, Brookes ST, Alderson D. Prognostic value of quality of life scores in patients with oesophageal cancer. Br J Surg 2000;87:362-73. [PubMed]
- Llobera J, Esteva M, Rifà J, et al. Terminal cancer. duration and prediction of survival time. Eur J Cancer 2000;36:2036-43. [Crossref] [PubMed]
- Dall'Olio FG, Maggio I, Massucci M, et al. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer 2020;145:95-104. [Crossref] [PubMed]
- Albain KS, Crowley JJ, LeBlanc M, et al. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 1991;9:1618-26. [Crossref] [PubMed]
- Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest 2002;122:1037-57. [Crossref] [PubMed]
- Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst 1980;65:25-32. [PubMed]
- Liu S, Wang D, Chen B, et al. The safety and efficacy of EGFR TKIs monotherapy versus single-agent chemotherapy using third-generation cytotoxics as the first-line treatment for patients with advanced non-small cell lung cancer and poor performance status. Lung Cancer 2011;73:203-10. [Crossref] [PubMed]
- Baka S, Ashcroft L, Anderson H, et al. Randomized phase II study of two gemcitabine schedules for patients with impaired performance status (Karnofsky performance status J Clin Oncol 2005;23:2136-44. [Crossref] [PubMed]
- Lilenbaum RC, Cashy J, Hensing TA, et al. Prevalence of poor performance status in lung cancer patients: implications for research. J Thorac Oncol 2008;3:125-9. [Crossref] [PubMed]
- Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer 1996;32A:1135-41. [Crossref] [PubMed]
- Radzikowska E, Głaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol 2002;13:1087-93. [Crossref] [PubMed]
- Gonsalves W, Ganti AK. Targeted anti-cancer therapy in the elderly. Crit Rev Oncol Hematol 2011;78:227-42. [Crossref] [PubMed]
- Bersanelli M, Brighenti M, Buti S, et al. Patient performance status and cancer immunotherapy efficacy: a meta-analysis. Med Oncol 2018;35:132. [Crossref] [PubMed]
- Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015;372:1629-38. [Crossref] [PubMed]
- Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013;188:220-30. [Crossref] [PubMed]
- Qin YY, Zhang DH, Lin XQ, et al. Clinical analysis of 36 cases of advanced non-small cell lung cancer (NSCLC) with performance status (PS) scores between 2 and 4. Zhonghua Zhong Liu Za Zhi 2017;39:855-61. [PubMed]
- Xie ZH, Zhou CZ, Qin YY, et al. Diagnosis and treatment strategy for advanced severe lung cancer. Chinese Journal of Practical Internal Medicine 2019;416-9.
- Zhou C, Li S, Liu J, et al. International consensus on severe lung cancer-the first edition. Transl Lung Cancer Res 2021;10:2633-66.
- Zhi X, Shi Y, Yu J. Standards for the diagnosis and treatment of primary lung cancer (2015 version) in China. Zhonghua Zhong Liu Za Zhi 2015;37:67-78. [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Sweeney CJ, Zhu J, Sandler AB, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a Phase II trial in patients with metastatic nonsmall cell lung carcinoma. Cancer 2001;92:2639-47. [Crossref] [PubMed]
- Ruckdeschel JC, Finkelstein DM, Ettinger DS, et al. A randomized trial of the four most active regimens for metastatic non-small-cell lung cancer. J Clin Oncol 1986;4:14-22. [Crossref] [PubMed]
- Chang VT, Hwang SS, Feuerman M, et al. Symptom and quality of life survey of medical oncology patients at a veterans affairs medical center: a role for symptom assessment. Cancer 2000;88:1175-83. [Crossref] [PubMed]
- Weber JS, Hodi FS, Wolchok JD, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 2017;35:785-92. [Crossref] [PubMed]
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119-42. [Crossref] [PubMed]
- Siefker-Radtke AO, Necchi A, Park SH, et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a phase 2 study. Lancet Oncol 2022;23:248-58. [Crossref] [PubMed]
- Zhang J, Zhou JB, Lin XF, et al. Prevalence of undiagnosed and undertreated chronic obstructive pulmonary disease in lung cancer population. Respirology 2013;18:297-302. [Crossref] [PubMed]
- Jo H, Park S, Kim NE, et al. Impact of COPD Treatment on Survival in Patients with Advanced Non-Small Cell Lung Cancer. J Clin Med 2022;11:2391. [Crossref] [PubMed]
- Wang F, Xie XH, Lin XQ, et al. Exploration of the treatment model for patients with advanced non-small cell lung cancer complicated with chronic obstructive pulmonary disease based on real-world data. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:450-4. [PubMed]
- Darmon M, Bourmaud A, Georges Q, et al. Changes in critically ill cancer patients' short-term outcome over the last decades: results of systematic review with meta-analysis on individual data. Intensive Care Med 2019;45:977-87. [Crossref] [PubMed]
(English Language Editor: J. Gray)


