
Immunotherapy for non-small cell lung cancer with EGFR or HER2 exon 20 insertion mutations: a real-world analysis
Highlight box
Key findings
• Immunotherapy combined with chemotherapy may play a role in first-line treatment for NSCLC patients with ex20ins mutations.
What is known and what is new?
• Chemotherapy with or without bevacizumab is the standard first-line treatment for NSCLC patients with ex20ins mutations.
• We analyzed NSCLC patients with ex20ins mutations who had received immunotherapy treatment and found it was efficacious and safe.
What is the implication, and what should change now?
• Immunotherapy may provide a new medication option for NSCLC patients with ex20ins mutations. A future RCT will be needed to further validate its advantages.
Introduction
Epidermal growth factor receptor (EGFR), also known as epidermal growth factor receptor-1 (ERBB-1) or human epidermal growth factor receptor-1 (HER-1), is the most common driver gene for non-small cell lung cancer (NSCLC). EGFR exon 20 insertion (ex20ins) is a relatively uncommon EGFR mutation, accounting for 2–10% of all EGFR-mutant NSCLC patients (1,2). Compared with patients with exon 19 deletion and exon 21 L858R, patients with ex20ins are reported to be resistant to classic EGFR tyrosine kinase inhibitors (TKIs), with a median PFS of less than three months (3,4). Human epidermal growth factor receptor 2 (HER2), as well as EGFR, belong to the ERBB tyrosine kinase receptor family. HER2-ex20ins is the most common mutation in HER2-mutated NSCLC patients, comprising about 2% of all NSCLC patients (5).
Amivantamab and mobocertinib have been approved by the U.S. Food and Drug Administration (FDA) for previously treated NSCLC patients with EGFR ex20ins (6,7). For patients with HER2 ex20ins, enhertu was also approved for later line setting (8). However, the accessibility and affordability of these novel target drugs are poor in most developing countries. And conventional therapy remains the preferred choice. Moreover, these novel TKIs were only approved for use in later-line treatment. As in the first-line setting, chemotherapy with or without bevacizumab is still the gold standard for patients with ex20ins mutations.
In the era of immunotherapy for NSCLC, the impact of driver genes on the efficacy of PD-1 inhibitors has been discrepant. The EGFR 19Del or L858R mutation was found to be negatively correlated with immunotherapy outcomes (9,10), whereas NSCLC patients with KRAS mutations showed benefit from immunotherapy alone or immunotherapy plus chemotherapy compared with KRAS wild-type (11,12). However, among patients with EGFR or HER2 ex20ins mutations, the use of PD-1 inhibitors in first-line treatment has been poorly reported, and their efficacy remains uncertain. Therefore, we conducted this retrospective study to assess the effectiveness of PD-1 inhibitors in the real world.
Although first-line chemotherapy with or without bevacizumab is still the gold standard treatment for NSCLC patients with ex20ins mutations, but whether these patients could benefit more from immunotherapy than chemotherapy remains uncertain. Therefore, we report the clinical characteristics and outcomes for NSCLC patients with EGFR ex20ins or HER2 ex20ins mutations who received ICI treatment or chemotherapy in our institution. A further assessment of the comparative efficacy between ICI treatment and chemotherapy was also performed. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-167/rc).
Methods
Study design and patients
This single-center analysis was conducted at the West China Hospital and included 72 patients admitted between January 2015 and December 2021. Informed consent of patients was exempt because of the retrospective nature of this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Biomedical Research Ethics Committee of the West China Hospital [No. 2020(637)]. Eligible patients were ≥18 years of age, had stage IV NSCLC (according to the staging criteria of the American Joint Committee on Cancer, 7th edition), and measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (13). Patients with known EGFR ex20ins or HER2 ex20ins treated with immunotherapy or chemotherapy as anti-tumor treatment in any line setting were included. Patients with ALK translocations or other gene mutation types were excluded. The major objective of this study was to determine the efficiency of ICI treatment in advanced NSCLC patients with ex20ins mutation.
Clinical review
We performed a retrospective review of the clinical history of all eligible patients and designed a tabular questionnaire to collect the clinical data, which included basic patient information, therapeutic medications, presence of disease progression, reports of pathology and genetic testing, associated adverse events (AEs) or toxicity, and follow-up endpoints.
Molecular testing
Biopsy specimens were either from lung tumors or peripheral blood. The EGFR mutation was identified by the amplification refractory mutation system (ARMS) or next-generation sequencing (NGS). The HER2 mutation was identified by NGS. PD-L1 expression was evaluated by immunohistochemistry analysis.
Assessment of endpoint
Overall response rate (ORR) was defined as the proportion of patients with a complete or partial response (PR) at any point as assessed by the RECIST v1.1 criteria. Toxicities were assessed using the National Cancer Institute grading system.
Progression-free survival (PFS) was calculated from the date of first dose to first documented disease progression per RECIST v1.1 or death as a result of any cause, whichever occurred first. If the patient did not develop disease progression, censoring occurred on the date of the last follow-up.
Statistical analysis
All patients were included in the statistical calculations, and all cases were followed up and censored on 15 August 2022. The Kaplan-Meier method was used to calculate the curves for PFS. The unstratified Cox hazards model was used to calculate hazard ratios (HR) and confidence intervals for PFS in the subgroup analysis. Data were considered statistically significant from P<0.05. All statistical analyses were performed using SPSS and GraphPad Prism software.
To control for confounding factors between the ICI and chemotherapy groups, propensity score matching (PSM) was used to compare the efficacy of immunotherapy versus chemotherapy. The patients were matched 1:1 using the nearest-neighbor algorithm.
Results
Ex20ins incidence in our institution
Between January 2015 and December 2021, 5,235 patients showed positive EGFR results in the ARMS test. A total of 108 (2.1%) had ex20ins. In addition, 705 patients showed positive EGFR results in the NGS test, 37 (5.2%) with HER2 ex20ins and 28 (4.0%) with EGFR ex20ins. A total of 136 patients with EGFR ex20ins and 37 patients with HER2 ex20ins were identified in our center.
Characteristics of the population
Among all patients harboring ex20ins, we identified 72 patients with a HER2 ex20ins mutation or EGFR ex20ins mutation who received immunotherapy and/or chemotherapy. Most patients had EGFR ex20ins (59.7%, 43/72), were female (55.6%, 40/72), had lung adenocarcinoma (93.0%, 67/72), and were never-smokers (69.4%, 50/72), with a median age of 59 years (range, 35–77 years). In total, 38 (52.8%) patients had been treated with one line of single-agent immunotherapy or combined therapy, including immunotherapy, throughout the whole course of treatment. The ICIs included pembrolizumab, nivolumab, camrelizumab, and sintilimab. The remaining 34 (47.2%) patients had received chemotherapy with or without antiangiogenic therapy, but patients once treated with immunotherapy were excluded. All patients had advanced NSCLC. The clinical characteristics of the patients are shown in Table 1.
Table 1
| Characteristics | Number (%) |
|---|---|
| Gender | |
| Male | 32 (44.4) |
| Female | 40 (55.6) |
| Age (years), median [range] | 59 [35–77] |
| Smoking | |
| Former/ever smoker | 22 (30.6) |
| Never-smoker | 50 (69.4) |
| Histology | |
| Adenocarcinoma | 67 (93.0) |
| Adenosquamous | 1 (1.4) |
| Squamous cell carcinoma | 4 (5.6) |
| Mutation type | |
| EGFR ex20ins | 43 (59.7) |
| HER2 ex20ins | 29 (40.3) |
| PS score | |
| 0–1 | 69 (95.8) |
| ≥2 | 3 (4.2) |
| PD-L1 expression | |
| ≥50% | 10 (13.9) |
| 1–50% | 16 (22.2) |
| <1% | 32 (44.4) |
| Unknown | 14 (19.4) |
| Immunotherapy | |
| Yes | 38 (52.8) |
| Immunotherapy alone | 9 (23.7) |
| Immunotherapy combine chemotherapy | 29 (76.3) |
| No | 34 (47.2) |
| Brain metastasis | 24 (33.3) |
| Liver metastasis | 10 (13.9) |
EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; PS, Performance Status; PD-L1, programmed cell death-ligand 1.
Immunotherapy outcomes
Among the 38 patients, as listed in Table 2, 16 (42%) received ICIs in the first line, 11 (29%) in the second line, and 11 (29%) in the third line or later treatment. Except for 5 patients, all patients had evaluable PD-L1 expression, and 18 (47.4%) had a positive PD-L1 expression. To enhance the response rate, combined treatment included radiotherapy, chemotherapy, and anti-angiogenesis therapy. Nine (24%) patients received immunotherapy alone, and the remaining 29 (76%) patients received immunotherapy in combination with other treatments described above. Among all patients, PRs were seen in 9 patients harboring HER2 ex20ins and 5 harboring EGFR ex20ins, with an ORR of 36.8% (14/38).
Table 2
| Efficacy results | Immunotherapy (n=38) | ||
|---|---|---|---|
| First line (n=16, 42%) | Second line (n=11, 29%) | Third or later lines (n=11, 29%) | |
| Best response, n (%) | |||
| Partial response | 8 (50.0) | 5 (45.4) | 1 (9.1) |
| Stable disease | 8 (50.0) | 2 (18.2) | 4 (36.4) |
| Progression | 0 (0.0) | 4 (36.4) | 6 (54.5) |
| Overall response rate, % | 50.0% | 45.5% | 9.1% |
| PFS (months), median (95% CI) | 10.7 (8.2–13.2) | 3.4 (2.1–4.7) | 2.1 (1.0–3.2) |
| OS (months), median (95% CI) | Not reached | Not reached | Not reached |
PFS, progression-free survival; OS, overall survival; CI, confidence interval.
The median OS of all 38 patients was 41.4 months (95% CI: 24.2–58.7 months). In the first-line setting, most patients had HER2 ex20ins (11/16). Among them, 11 (68.8%) received ICI plus chemotherapy, 1 (6.3%) received ICI plus chemotherapy and radiotherapy, and 4 (25.0%) received a single agent. There were 5 patients harboring HER2 ex20ins and 3 harboring EGFR ex20ins who achieved PR in this line, with an ORR of 50% (8/16). The median PFS of first-line ICI treatment was 10.7 months (95% CI: 8.2–13.2 months). Among the 11 (68.8%) patients treated with second-line ICIs, 7 (63.6%) received ICI plus chemotherapy, 1 (9.1%) received ICI plus chemotherapy and radiotherapy, 1 (9.1%) had ICI plus radiotherapy, 1 (9.1%) had ICI plus chemotherapy and endostar, and the remaining patient (9.1%) had a single agent. The median PFS of second-line ICI treatment was 3.4 months (95% CI: 2.1–4.7 months), while the ORR was 45.5% (5/11). Of the 11 (29%) patients who received ICIs in third-line or later settings, 4 received a single agent, 4 had ICI plus chemotherapy, 1 received ICI plus anlotinib, 1 had ICI plus radiotherapy, and 1 received ICI plus chemotherapy and radiotherapy. The median PFS observed in this line was 2.1 months (95% CI: 1.0–3.2 months). Only 1 (9.1%) patient achieved PR in the third line or later setting. There was no significant difference in median PFS between 9 patients treated with immunotherapy alone and 29 patients treated with immunotherapy combined with other treatments (3.1 vs. 7.3, P=0.344).
Chemotherapy outcomes
Next, we analyzed the chemotherapy outcomes for 34 patients. The median OS of all 34 patients was 37.4 months (95% CI: 19.2–55.6 months). Patients who had received immunotherapy were excluded from the analysis. Most patients had first-line pemetrexed-based chemotherapy (n=23), and 7 received first-line paclitaxel-based chemotherapy. Six patients received additional antiangiogenic therapy, including bevacizumab (n=4), endostar (n=1), and anlotinib (n=1). The ORR and the median PFS for patients receiving first-line chemotherapy were 21.9% (7/32) and 4.6 months (95% CI: 3.6–5.6 months), respectively (n=32). In the second-line setting, of the 12 patients who received chemotherapy, 7 received second-line docetaxel-based chemotherapy, and 4 received pemetrexed-based chemotherapy. The ORR was 8.3% (1/12), and the median PFS was 5.0 months (95% CI: 2.6–7.4 months). For the 4 patients who received third-line chemotherapy, the median PFS was 3.0 months (95% CI: 0.0–6.3 months), and none of them achieved a partial or complete response in this line.
Immunotherapy versus chemotherapy outcomes
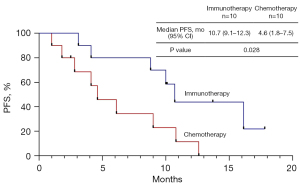
Due to the promising results derived from first-line ICI treatment, we directly compared the efficacy between ICI treatment and chemotherapy. There was no significant difference in median OS between immunotherapy and chemotherapy (41.4 vs. 37.4, P=0.957). Except for mutation type, no significant differences were found in baseline clinical characteristics between the immunotherapy and chemotherapy groups in the first-line setting before PSM. The 10.7 months (95% CI: 8.2–13.2 months) median PFS in the immunotherapy group was significantly longer than 4.6 months (95% CI: 3.6–5.6 months) in the chemotherapy group (P<0.001). There were only 4 patients who received single-agent immunotherapy in the first-line treatment. We found a trend for a better median PFS in single-agent immunotherapy compared with chemotherapy, but without significance (10.0 vs. 4.6, P=0.068). The median PFS with immunotherapy plus chemotherapy was significantly longer than with chemotherapy (10.7 vs. 4.6, P<0.001) (Figure 1). There was a trend for an increased ORR in patients who received ICIs compared with chemotherapy, but there was no statistical difference (50% vs. 21.9%, P=0.096). The PSM cohort included 20 patients for further analysis. After PSM, the clinicopathological features (age, gender, mutation type, PS score, PD-L1 expression, brain metastasis, and liver metastasis) of patients between the two groups were well matched (Table 3). The clinical characteristics of the patients before and after PSM are shown in Table 3. Consistent with previous results, the 10.7 months (95% CI: 9.1–12.3 months) median PFS with immunotherapy was significantly longer than 4.6 months (95% CI: 1.8–7.5 months) with chemotherapy in the PSM cohort (P=0.028) (Figure 2). The ORR of immunotherapy was close to that of chemotherapy (50% vs. 40%, P=1.000).
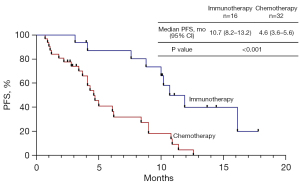
Table 3
| Feature | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| Immunotherapy (n=16) | Chemotherapy (n=32) | P | Immunotherapy (n=10) | Chemotherapy (n=10) | P | ||
| Gender, n (%) | 0.131 | 1.000 | |||||
| Male | 10 (62.5) | 12 (37.5) | 5 (50.0) | 4 (40.0) | |||
| Female | 6 (37.5) | 20 (62.5) | 5 (50.0) | 6 (60.0) | |||
| Median age, years [range] | 61 [35–77] | 60.5 [44–75] | 0.991 | 62 [35–77] | 57.5 [47–70] | 0.766 | |
| Smoking, n (%) | 0.110 | 0.650 | |||||
| Former/ever smoker | 8 (60.0) | 8 (22.6) | 5 (50.0) | 3 (30.0) | |||
| Mutation type, n (%) | 0.005 | 1.000 | |||||
| EGFR ex20ins | 5 (31.2) | 24 (75.0) | 3 (30.0) | 3 (30.0) | |||
| HER2 ex20ins | 11 (68.8) | 8 (25.0) | 7 (70.0) | 7 (70.0) | |||
| PS score, n (%) | 1.000 | 1.000 | |||||
| 0–1 | 15 (93.8) | 31 (96.9) | 10 (100.0) | 10 (100.0) | |||
| ≥2 | 1 (6.3) | 1 (3.1) | 0 (0.0) | 0 (0.0) | |||
| PD-L1 expression, n (%) | 0.310 | 0.656 | |||||
| ≥1% | 6 (37.5) | 7 (21.9) | 4 (40.0) | 6 (60.0) | |||
| <1% or unevaluable | 10 (62.5) | 25 (78.1) | 6 (60.0) | 4 (40.0) | |||
| Brain metastasis, n (%) | 6 (37.5) | 10 (31.3) | 0.750 | 3 (30.0) | 4 (40.0) | 1.000 | |
| Liver metastasis, n (%) | 2 (12.5) | 3 (9.4) | 1.000 | 2 (20.0) | 1 (10.0) | 1.000 | |
PSM, propensity score matching; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; PS, Performance Status; PD-L1, programmed cell death-ligand 1.
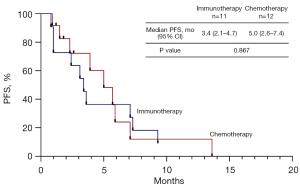
In the second line setting, there were no clinically significant abnormalities in baseline characteristics. A trend of increased ORR in patients who received ICIs was observed compared with chemotherapy, but there was no statistical difference (36.4% vs. 8.3%, P=0.155). The 3.4 months (95% CI: 2.1–4.7 months) median PFS observed in the immunotherapy group did not differ from the 5.0 months (95% CI: 2.6–7.4 months) median PFS observed in the chemotherapy group (P=0.867). We attempted to develop a PSM model to compare the efficacy of immunotherapy versus chemotherapy in the second-line setting. However, the number of cases was too small to obtain reliable results (Figure 3).
Safety of immunotherapy
All grades of AEs occurred in 52.6% (20/38) of patients. The AEs are summarized in Table 4. Fatigue, granulocytopenia, and hypo-/hyperthyroidism were the most common AEs. No deaths occurred. Grade 3–4 AEs were observed in 13.2% (5/38) of patients, with the majority being granulocytopenia (40%, 2/5). One patient (2.6%, 1/38) discontinued treatment due to a grade 3 rash after three cycles of ICI plus anlotinib treatment.
Table 4
| AEs | Grade 1–2, n (%) | Grade 3, n (%) | Grade 4 | Grade 5 | Total, n (%) |
|---|---|---|---|---|---|
| Anemia | 4 (10.5) | 0 | 0 | 0 | 4 (10.5) |
| Granulocytopenia | 3 (7.9) | 2 (5.3) | 0 | 0 | 5 (13.2) |
| Thrombocytopenia | 2 (5.3) | 1 (2.6) | 0 | 0 | 3 (7.9) |
| Fatigue | 5 (13.2) | 0 | 0 | 0 | 5 (13.2) |
| Rash | 1 (2.6) | 1 (2.6) | 0 | 0 | 2 (5.3) |
| Vomit | 2 (5.3) | 0 | 0 | 0 | 2 (5.3) |
| Diarrhea | 0 | 0 | 0 | 0 | 0 |
| Hypo-/hyperthyroidism | 4 (10.5) | 0 | 0 | 0 | 4 (10.5) |
| Increased AST/ALT | 2 (5.3) | 1 (2.6) | 0 | 0 | 3 (7.9) |
| Increased creatinine | 0 | 0 | 0 | 0 | 0 |
| Cough | 3 (7.9) | 0 | 0 | 0 | 3 (7.9) |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 |
AEs, adverse events; AST, aspartate transaminase; ALT, alanine aminotransferase.
Discussion
In this analysis conducted in a real-world setting, immunotherapy resulted in a clinically significant objective response rate (ORR) in NSCLC patients with ex20ins mutations, leading to a valuable PFS.
The majority of EGFR ex20ins occur after the αC-helix (residues 767–774), and most HER2 ex20ins are located in a similar position (775–783 in HER2) (14,15). The binding ability of targeted drugs is limited due to the confined size of the drug-binding pocket of these mutations (16). Based on the similarity of EGFR ex20ins and HER2 ex20ins, we assessed the efficiency of ICI treatment and chemotherapy in patients with both of these mutations. We observed that the clinical efficacy of first-line immunotherapy was not inferior to standard chemotherapy in patients presenting with ex20ins-mutated NSCLC.
Patients with EGFR or ALK mutations were excluded from a few clinical trials because of the low sensitivity to ICI treatment. Because of the low proportion of patients harboring EGFR ex20ins or HER2 ex20ins, the clinical data for ICI use in these patients are lacking. It remains uncertain whether these patients could benefit from immunotherapy. Among EGFR ex20ins patients who received first-line chemotherapy, the ORR and median PFS were 19.2% and 6.4 months, respectively, in a previous study (17). The outcome for HER2-mutant patients who received chemotherapy was similar to that of patients with EGFR mutations (18,19). The ORR of 21.9% and median PFS of 6.1 months achieved in our study were similar to the results from a previous study of first-line chemotherapy use (17). Furthermore, the ORR and median PFS with first-line ICIs in this study were better than those with chemotherapy. Another retrospective multicenter study reported the result of 10 EGFR ex20ins patients treated with ICIs; the ORR of first-line ICI treatment was 60% (3/5), while the first-line median PFS was 1.5–6.7 months (17). The data for first-line ICI treatment in HER2 ex20ins patients had been lacking until the current study. The 10.7-month median PFS of first-line ICI treatment achieved in this study is similar to the 10.2-month PFS achieved in EGFR-mutant patients that received atezolizumab plus bevacizumab plus chemotherapy in the Impower150 trial (20). Meanwhile, the 10.7-month median PFS in this study is longer than the PFS achieved in patients without sensitizing EGFR or ALK mutations treated with pembrolizumab plus chemotherapy in the keynote189 trial (21).
In the second-line setting, although 45.5% (5/11) of patients achieved PR, the median PFS did not show survival benefit from ICI treatment in patients with ex20ins mutations. The phase III IMPRESS trial showed a median PFS of 5.4 months (95% CI: 4.6–5.5 months) with second-line chemotherapy in EGFR-positive NSCLC, which was comparable to the 5.0-month median PFS of second-line chemotherapy observed in this study (22). Amivantamab and mobocertinib were approved for treatment of NSCLC patients with EGFR ex20ins mutations whose disease had progressed after platinum chemotherapy. In the phase I study CHRYSALIS, amivantamab showed an ORR of 40% and a median PFS of 8.3 months in treated NSCLC patients (6). Meanwhile, mobocertinib achieved an ORR of 35% and a median PFS of 7.3 months in the reported data (7). The median PFS with second-line ICI treatment observed in this study seemed shorter than the PFS with second-line chemotherapy and treatment with amivantamab or mobocertinib. Since ICI treatment had a shorter median PFS and poor response in the third-line or later settings, ICIs seem to bring limited clinical benefit to this subgroup of populations in second-line or later settings.
ICI-combined treatments were correlated with superior efficiency compared with ICI monotherapy in previous trials. Among patients who achieved PR in this study, 78.6% (11/14) received combined ICI treatment. A meta-analysis including five phase III studies suggested comparable clinical benefits for patients with EGFR mutations receiving second-line ICI monotherapy versus chemotherapy (23). Mazieres et al. retrospectively investigated the effectiveness of ICI monotherapy in patients with advanced NSCLC, where mPFS was 2.1 months in patients with EGFR mutations and 2.5 months in HER2 mutations (24). The result suggested that single ICIs failed to improve the survival of patients harboring EGFR mutations. Meanwhile, increasing evidence has demonstrated that chemotherapy, radiotherapy, and anti-angiogenesis therapy have a synergistic effect with ICIs, and such combined strategies could bring survival benefits for NSCLC patients. A retrospective study analyzing median survival with chemotherapy with or without immunotherapy, osimertinib, and novel targeted agents in patients with EGFR 20 mutations showed that mPFS with chemotherapy plus immunotherapy appeared to be longer (25). Another first-line phase III Impower150 trial conducted in non-squamous NSCLC patients showed prolonged PFS and OS in EGFR or ALK mutant patients treated with atezolizumab plus bevacizumab and chemotherapy (20). The results indicated that among oncogene-driven NSCLC patients, a combined ICI strategy might be a promising choice in first-line treatment, and the outcomes of our study validated this finding in ex20ins patients. However, a subsequent phase III Impower130 study exhibited similar results between atezolizumab plus chemotherapy versus chemotherapy in patients with EGFR or ALK mutations (26). Compared with the results of the Impower150 trial, bevacizumab might play a role in ICI treatment in this population. Although the role of bevacizumab was not observed in our study, further investigations are required to determine how to add other treatments to ICIs to boost the clinical response. Among ex20ins-mutant patients that might be less sensitive to ICI treatment, PD-L1 expression as well as ICI-combined treatment should be taken into consideration when using ICIs in this subgroup of populations. A recent study reported that cyclo-Arg-Gly-Asp-D-Phe-Lys (cRGD) peptide-modified PEG-PLA (cRGD-PEG-PLA) copolymer can deliver curcumin (CUR) and doxorubicin (DOX), while (CUR-DOX)/cRGD-M combination therapy promotes apoptosis in lung cancer cells, which may have potential clinical value for ICI combination therapy for lung cancer (27).
PD-L1 expression is the most significant biomarker for ICI use in NSCLC patients (28). The role of PD-L1 expression remains uncertain when ICIs are used for oncogene-driven NSCLC patients. Meanwhile, 60.0% (6/10) of responders had a positive PD-L1 expression in this study. The phase III Keynote-010 trial compared pembrolizumab with docetaxel in previously treated NSCLC patients with positive PD-L1 expression, and subgroup analysis revealed that the ORR was correlated with PD-L1 expression levels for EGFR-mutant patients (29). PD-L1 expression has been demonstrated to correlate with the efficacy of ICIs in EGFR-mutant patients. However, due to the small sample size of this study, whether PD-L1 is a predictive biomarker and its predictive ability in ex20ins patients deserves further investigation and verification. A previous study found that higher ORR and median PFS were observed in patients with EGFR ex20ins mutations than in patients with HER2 ex20ins or classic EGFR mutations (30). Another study of a Hispanic sample showed that the mean PD-L1 expression in EGFR exon20ins seems higher than for patients with common EGFRm (31). These findings implied that patients with EGFR ex20ins are more likely to benefit from ICI treatment owing to their higher PD-L1 expression.
This study found that most of the AEs of ICI or ICI combined treatment among ex20ins patients were mild and tolerable. A study investigating the relationship between AEs and treatment sequence among EGFR-mutant patients found that severe immune-related AEs (irAEs) could occur when immunotherapy was followed by osimertinib, but no irAEs occurred when reversing the treatment order of immunotherapy and osimertinib (32). It is worth noting that severe irAEs might arise when choosing osimertinib for a patient who has recently received immunotherapy.
Like all real-world evidence (RWE) studies, our study had some limitations. First, the study was a retrospective, single-center study with a small amount of data to draw more comprehensive and definitive conclusions. Second, all response assessments are conducted at any point in time at the discretion of the treating physician, rather than at predetermined intervals like prospective tests. Therefore, PFS is a treatment time. At the same time, some comparisons were made between unbalanced random subgroups, so there was subjective bias. Finally, the study is also limited by missing data for some patients. Therefore, the results of mPFS should be interpreted with caution before the results of prospective clinical trials are available.
Conclusions
In conclusion, this analysis is a single-center retrospective cohort study, and its result showed that first-line ICI treatment combined with chemotherapy might be beneficial for NSCLC patients with ex20ins mutations. The clinical response requires further exploration in first-line treatment.
Acknowledgments
Funding: The study was supported by the Dazhou-Sichuan University project of Dazhou Bureau Science and Technology (No. 2021CDDZ-26).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-167/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-167/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-167/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-167/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Biomedical Research Ethics Committee of the West China Hospital [No. 2020(637)]. Informed consent of patients was exempt because of the retrospective nature of this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. [Crossref] [PubMed]
- Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 2013;8:179-84. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Chen D, Song Z, Cheng G. Clinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutations. Onco Targets Ther 2016;9:4181-6. [Crossref] [PubMed]
- Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997-2003. [Crossref] [PubMed]
- Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J Clin Oncol 2021;39:3391-402. [Crossref] [PubMed]
- Zhou C, Ramalingam SS, Kim TM, et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial. JAMA Oncol 2021;7:e214761. [Crossref] [PubMed]
- Tsurutani J, Iwata H, Krop I, et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov 2020;10:688-701. [Crossref] [PubMed]
- Jin R, Zhao J, Xia L, et al. Application of immune checkpoint inhibitors in EGFR-mutant non-small-cell lung cancer: from bed to bench. Ther Adv Med Oncol 2020;12:1758835920930333. [Crossref] [PubMed]
- Yamada T, Hirai S, Katayama Y, et al. Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer. Cancer Med 2019;8:1521-9. [Crossref] [PubMed]
- Sun L, Hsu M, Cohen RB, et al. Association Between KRAS Variant Status and Outcomes With First-line Immune Checkpoint Inhibitor-Based Therapy in Patients With Advanced Non-Small-Cell Lung Cancer. JAMA Oncol 2021;7:937-9. [Crossref] [PubMed]
- Nakajima EC. Outcomes of first-line immune checkpoint inhibitors with or without chemotherapy according to KRAS mutational status and PD-L1 expression in patients with advanced NSCLC: FDA pooled analysis. J Clin Oncol 2022;40:abstr 9001.
- Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 2018;88:38-47. [Crossref] [PubMed]
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. [Crossref] [PubMed]
- Takeda M, Sakai K, Hayashi H, et al. Clinical characteristics of non-small cell lung cancer harboring mutations in exon 20 of EGFR or HER2. Oncotarget 2018;9:21132-40. [Crossref] [PubMed]
- Heymach J, Negrao M, Robichaux J, et al. OA02.06 A Phase II Trial of Poziotinib in EGFR and HER2 exon 20 Mutant Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol 2018;13:S323-4. [Crossref]
- Yang G, Li J, Xu H, et al. EGFR exon 20 insertion mutations in Chinese advanced non-small cell lung cancer patients: Molecular heterogeneity and treatment outcome from nationwide real-world study. Lung Cancer 2020;145:186-94. [Crossref] [PubMed]
- Mazières J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol 2016;27:281-6. [Crossref] [PubMed]
- Zhou J, Ding N, Xu X, et al. Clinical outcomes of patients with HER2-mutant advanced lung cancer: chemotherapies versus HER2-directed therapies. Ther Adv Med Oncol 2020;12:1758835920936090. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015;16:990-8. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:210-6. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Mountzios G, Planchard D, Metro G, et al. Molecular Epidemiology and Treatment Patterns of Patients With EGFR Exon 20-Mutant NSCLC in the Precision Oncology Era: The European EXOTIC Registry. JTO Clin Res Rep 2022;4:100433. [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Zhang Y, Li T, Hu Y, et al. Co-delivery of doxorubicin and curcumin via cRGD-peptide modified PEG-PLA self-assembly nanomicelles for lung cancer therapy. Chinese Chemical Letters 2022;33:2507-11. [Crossref]
- Aguiar PN Jr, De Mello RA, Hall P, et al. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy 2017;9:499-506. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Negrao MV, Reuben A, Robichaux JP, et al. Association of EGFR and HER-2 exon 20 mutations with distinct patterns of response to immune checkpoint blockade in non-small cell lung cancer. J Clin Oncol 2018;36:abstr 9052.
- Cardona AF, Rojas L, Zatarain-Barrón ZL, et al. EGFR exon 20 insertion in lung adenocarcinomas among Hispanics (geno1.2-CLICaP). Lung Cancer 2018;125:265-72. [Crossref] [PubMed]
- Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol 2019;30:839-44. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


