
Assessing how lung cancer screening guidelines contribute to racial disparities in screening access
Highlight box
Key findings
• Non-White people who smoke have been systematically left out of lung cancer screening efforts, though they stand to benefit most and are at the highest risk for poor lung cancer outcomes.
What is known and what is new?
• Black people who smoke have a higher risk of developing late-stage lung cancer and a higher risk of lung cancer mortality than White people who smoke, though they smoke fewer cigarettes.
• Despite having lower average smoking exposure, most of non-White people who smoke have higher average levels of urinary cotinine, indicating a higher level of exposure to carcinogens found in tobacco.
What is the implication, and what should change now?
• Current lung cancer screening criteria should be re-examined, since it does not adequately capture the risk of non-White people who smoke. Biomarkers such as cotinine should be considered in the development of new risk-based models for screening eligibility.
Introduction
Lung cancer (LC) is currently the third most common cancer in the United States (U.S.) and the leading cause of cancer related deaths (1). In 2022, an estimated 236,740 people will be newly diagnosed with LC and 130,180 will die from this disease (2). Of those with LC in the U.S., only 19% are diagnosed at the localized stage while 55% are diagnosed at a later stage, when the cancer has already metastasized. The prognosis for such late diagnosed LC is poor, with a 5-year relative survival of only 7% (1). However, emphasis on screening and early detection in the past decade has resulted in a stage shift for LC diagnoses, with an increasing percentage of people being diagnosed with localized-stage LC—which has a 61.2% 5-year relative survival (1,2). Since LC outcomes are highly dependent on the stage of diagnosis, LC screening has been considered a powerful tool in decreasing LC mortality in the U.S. (2).
In 2021, The U.S. Preventive Services Task Force (USPSTF) released updated guidelines for annual LC screening. Currently, screening with low-dose computed tomography (LDCT) is recommended for adults between the ages 50 and 80 years old with at least a 20 pack-year smoking history who currently smoke or who have quit smoking in the past 15 years (3). These guidelines were modified from the original guidelines that the USPSTF released in 2013, which recommended screening for those between the ages 55 and 80 years old with at least a 30 pack-year smoking history who either currently smoke or who have quit smoking in the past 15 years (4).
The current USPSTF guidelines for LC screening were primarily informed by the results of three studies: The Early Lung Cancer Action Project (ELCAP), The National Lung Screening Trial (NLST), and Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) (3). ELCAP was the first study conducted in the U.S. which demonstrated that LDCT screening for LC increases the likelihood of earlier stage LC detection through increased detection of small non-calcified nodules (5). The results of ELCAP were echoed in NLST and NELSON, which were the only RCTs adequately powered to detect the impact of screening on LC mortality (3). In NLST, a 20% relative risk reduction for LC mortality was observed in those who were screened with LDCT compared to those screened with chest radiographs (6). Similarly, the incidence rate ratio for LC mortality in the NELSON trial was 0.75 in those screened using LDCT compared to no screening (7). Given these positive findings, the USPSTF recommended age and cigarette smoking exposure cutoffs for LC screening that were similar to the eligibility criteria in ELCAP, NLST, and NELSON (3).
It is important to note that the studies which informed the USPSTF LC screening guidelines include a homogeneous population in terms of the race and ethnicity of the participants. Of the 1,000 participants in the original ELCAP study, 91% of them were White, 5% were Black, 2% were Hispanic, and 2% were of other ethnic origin (5). Similar racial distributions were observed in NLST: of the 53,454 participants, 90.9% were White in the LDCT group and 90.8% were White in the chest radiography group, while only 4.5% were Black in the LDCT group and 4.4% were Black in the chest radiography group (6). The NELSON trial, conducted in the Netherlands and Belgium, did not report racial distributions (7). The lack of a conspicuous number of racial and ethnic minority participants in these studies begs the question whether the USPSTF guidelines for LC screening are truly generalizable to all racial groups.
Racial disparities in LC screening have become increasingly evident. For example, though Black men have the highest rates of LC incidence and mortality and though Black individuals are disproportionately diagnosed at later stages of LC, they are underrepresented in LC screening, primarily due to not meeting screening criteria (2). The USPSTF 2021 guidelines for LC screening acknowledge that the smoking exposure cutoff for screening was lowered from 30 pack-years in the 2013 guidelines to 20 pack-years in the current guidelines, in part to improve racial disparities in LC screening (8). We analyze here the National Health and Nutrition Examination Survey (NHANES) dataset to assess if racial disparities in screening eligibility still persist despite the changes in guidelines.
Methods
NHANES is a cross-sectional survey administered by the Centers for Disease Control and Prevention (CDC) that is intended to assess the health of civilians in the U.S. Non-institutionalized adults and children residing in the U.S. are included in the NHANES, which uses a complex, multi-stage probability sampling design to create a nationally representative sample of the U.S. population. Several thousand people residing in counties all over the U.S. are selected to participate in each 2-year data cycle of NHANES. NHANES consists of a health interview, physical examination, and laboratory testing. Data from NHANES are de-identified and publicly available.
Data for this study were derived from the NHANES 2013–2014 and NHANES 2015–2016 cycles, when 20,146 participants were interviewed, 12,105 of which were 18 years or older. This age cutoff was chosen since most of the variables regarding smoking status in NHANES are recorded only in participants ages 18 and older. The standard cutoff of at least 100 cigarettes smoked in a lifetime was used to designate which participants have ever smoked. Participants who did not know if they had smoked 100 cigarettes in their lifetimes, refused to answer this question, or who had never smoked (had smoked fewer than 100 cigarettes in their lifetimes) were excluded from the present analysis (nexcluded=7,104). The final cohort consisted of 5,001 participants, of which 2,669 were people who formerly smoked and 2,332 were people who currently smoke (Figure 1). Pack-years were estimated using age, lifetime duration of smoking cigarettes, and smoking intensity, defined as number of cigarettes smoked per day.

Eligibility for LC screening was defined as people who met the criteria set by the USPSTF and the Centers for Medicare and Medicaid Services: individuals aged 50 to 80 years old, who have at least a 20 pack-year smoking history, and either currently smoke or formerly smoked and quit within the past 15 years. Of the 5,001 participants who had ever smoked that were included in the cohort, 608 were eligible for LC screening and 4,393 were ineligible for LC screening. In this analysis, we used the age range of 50 to 79 years old for eligibility because the NHANES dataset codes participants ages 80+ as 80 years old.
Secondary analyses were conducted to assess racial differences in urinary cotinine levels within participants who had ever smoked. Cotinine is one of the primary metabolites of nicotine and can be used as a biomarker for tobacco and carcinogen exposure (9). Total urinary cotinine was measured in NHANES using isotope dilution high-performance liquid chromatography and tandem mass spectrometric methods. The lower limit of detection for urinary cotinine is 0.03 ng/mL. For those who had cotinine below the lower limit of detection, an imputed value was used, equal to the lower limit of detection divided by the square root of 2 (0.021 ng/mL). From the 5,001 participants who had ever smoked and who were aged 18 years and older in the NHANES 2013–2014 and NHANES 2015–2016 datasets, 1,543 participants had urinary cotinine data (nexcluded=3,458). The study was conducted in accordance with the ethical standards of the Declaration of Helsinki (as revised in 2013).
Statistical analysis
All statistical analyses were performed on Stata software, version 14.2. Since NHANES uses a multi-stage probability sampling design, weighted analyses were conducted using survey procedures to obtain national estimates. The data were declared as survey data using svyset. Summary statistics were obtained using svy linearized. Figures were generated using Stata 14.2.
All the continuous variables were compared using analysis of variance (ANOVA)/Kruskal-Wallis test, while all the categorical variables were compared using the Chi-square test of association.
Results
There were 608 participants who would have been eligible for screening according to national guidelines, 394 males and 214 females; of those 4,393 ineligible participants, 2,573 were males and 1,820 females. The mean age of the participants among the eligible group was significantly higher than in the ineligible group {59.8 years [standard error (SE): 0.4] versus 48.1 years (SE: 0.5); P≤0.001} and so was the mean pack-years [38.8 (SE: 1.0) versus 13.4 (SE: 0.6); P≤0.001]. The mean time since quitting smoking in years of the participants eligible for LC screening was 6.4 years (SE: 0.7), while in those who are ineligible it was 17.9 years (SE: 0.4) (P≤0.001).
Among screening eligible participants, there were no statistically significant differences in demographics or smoking variables across races. Most of the participants eligible for screening were people who currently smoked across all races (Table 1).
Table 1
| Screening eligibility | Mexican American (n=637) | Other Hispanic (n=511) | Non-Hispanic White (n=2,267) | Non-Hispanic Black (n=1,045) | Non-Hispanic Asian (n=336) | Other, multi-racial (n=205) | Total (n=5,001) | P value |
|---|---|---|---|---|---|---|---|---|
| Eligible, n (%) | 61 (5.9) | 56 (7.5) | 314 (13.2) | 126 (9.9) | 19 (5.7) | 32 (16.9) | 608 | |
| Age, years, mean (SE) | 60.9 (0.9) | 58.8 (0.9) | 59.5 (0.4) | 60.5 (0.6) | 65.1 (2.5) | 61.1 (1.3) | 59.8 (0.4) | 0.2 |
| Sex, n (%) | 0.1 | |||||||
| Male | 43 (69.6) | 36 (66.6) | 189 (57.3) | 93 (69.9) | 16 (87.5) | 17 (47.5) | 394 | |
| Female | 18 (30.4) | 20 (33.4) | 125 (42.7) | 33 (30.1) | 3 (12.5) | 15 (52.5) | 214 | |
| Pack-years, mean (SE) | 32.8 (1.7) | 39.7 (3.0) | 39.7 (1.3) | 32.8 (1.8) | 35.7 (3.8) | 36.8 (2.6) | 38.8 (1.0) | 0.2 |
| Years since quitting smoking, mean (SE) | 4.2 (1.5) | 0.9 (0.5) | 6.9 (0.7) | 5.1 (1.3) | 6.2 (1.4) | 8.2 (4.6) | 6.4 (0.7) | 0.3 |
| Smoking status, n (%) | 0.1 | |||||||
| Former | 19 (34.3) | 15 (29.3) | 54 (19.6) | 25 (20.0) | 8 (39.2) | 8 (21.7) | 129 | |
| Current | 42 (65.7) | 41 (70.7) | 260 (80.4) | 101 (80.0) | 11 (60.8) | 24 (78.3) | 479 | |
| Ineligible, n (%) | 576 (94.1) | 455 (92.5) | 1,953 (86.8) | 919 (90.0) | 317 (94.3) | 173 (83.1) | 4,393 | |
| Age, years, mean (SE) | 43.0 (1.0) | 43.5 (1.0) | 49.6 (0.7) | 46.8 (0.7) | 46.4 (1.5) | 42.6 (2.0) | 48.1 (0.5) | <0.001 |
| Sex, n (%) | <0.001 | |||||||
| Male | 395 (71.7) | 270 (62.7) | 1,050 (52.4) | 510 (51.2) | 252 (76.6) | 96 (57.5) | 2,573 | |
| Female | 181 (28.2) | 185 (37.3) | 903 (47.6) | 409 (48.8) | 65 (23.4) | 77 (42.5) | 1,820 | |
| Pack-years, mean (SE) | 7.7 (0.5) | 8.5 (0.7) | 15.5 (0.7) | 8.7 (0.4) | 8.6 (0.8) | 10.1 (1.6) | 13.4 (0.6) | <0.001 |
| Years since quitting smoking, mean (SE) | 13.2 (0.5) | 13.6 (1.0) | 19.2 (0.6) | 16.5 (0.7) | 15.0 (1.1) | 12.7 (2.0) | 17.9 (0.4) | <0.001 |
| Smoking status, n (%) | <0.001 | |||||||
| Former | 362 (57.9) | 305 (61.3) | 1,193 (64.7) | 410 (39.0) | 197 (60.6) | 73 (45.4) | 2,540 | |
| Current | 214 (42.1) | 150 (38.7) | 760 (35.3) | 509 (61.0) | 120 (39.4) | 100 (54.6) | 1,853 |
*, all percentages and means are calculated using sampling weights. NHANES, National Health and Nutrition Examination Survey; SE, standard error.
Among screening ineligible participants, non-Hispanic White (NHW) participants were statistically significantly older and had a higher mean pack-years than the other racial and ethnic groups; the proportion of females was higher among NHW and non-Hispanic Black (NHB) participants than the other racial and ethnic groups.
The mean age was similar across races. Mean pack-years and years since quitting smoking were higher in NHW participants than in any other racial group. Two thirds of the ineligible NHB participants were people who currently smoked versus roughly a little more than one third of the other major racial groups.
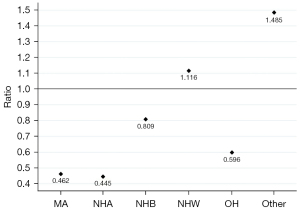
The ratios of the population proportion eligible for screening over the ineligible (Figure 2) shows that only for NHW and other including multi-racial participants is the number of eligible participants higher than the number of ineligible; for all other racial and ethnic groups, the eligibility ratio is below 1.
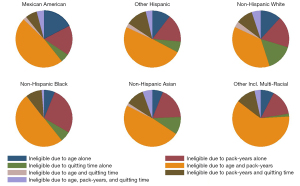
Reasons for ineligibility
Looking into the reasons of ineligibility for LC screening, we observed that age alone, pack-years alone and age along with pack-years were the most frequent reasons for ineligibility. Roughly half of the ineligible were excluded due to both age and pack-years, with significant differences across racial and ethnic groups; 51.4% of other Hispanic participants were ineligible because of age and pack-years, versus only 37% of NHW participants (Figure 3).
Within the subgroup of ineligible participants due to pack-years only, the mean pack-years in NHW participants [8.3 (SE: 0.3)] was significantly higher than in any other racial group (P≤0.001) (Table 2).
Table 2
| Reason for ineligibility* | Mexican American | Other Hispanic | Non-Hispanic White | Non-Hispanic Black | Non-Hispanic Asian | Other, multi-racial | P value (median) |
|---|---|---|---|---|---|---|---|
| Ineligible due to age alone | |||||||
| % within race | 13.9 | 10.7 | 12.0 | 6.9 | 7.9 | 13.7 | |
| Age, years | 36 | 39 | 40 | 36 | 36 | 34 | 0.02 |
| Ineligible due to pack-years alone | |||||||
| % within race | 15.6 | 15.0 | 17.8 | 27.6 | 18.0 | 19.3 | |
| Pack-years | 4.6 | 4.4 | 8.3 | 6.9 | 5.4 | 7.2 | <0.001 |
| Ineligible due to time since quitting smoking alone | |||||||
| % within race | 6.2 | 6.8 | 15.1 | 5.7 | 8.1 | 6.8 | |
| Quit time, years | 30 | 28.5 | 30 | 30 | 30 | 27.5 | 0.14 |
| Ineligible due to age and pack-years | |||||||
| % within race | 49.3 | 51.4 | 37.0 | 46.8 | 48.9 | 51.8 | |
| Age, years | 35 | 33 | 34 | 33 | 35 | 32 | 0.07 |
| Pack-years | 1.95 | 2 | 4 | 3.3 | 2.1 | 3 | <0.001 |
| Ineligible due to age and time since quitting smoking | |||||||
| % within race | 1.8 | 2.2 | 3.5 | 0.8 | 2.1 | 1.9 | |
| Age, years | 48.5 | 44.5 | 80 | 80 | 49 | 80 | <0.001 |
| Quit time, years | 22 | 20 | 40 | 35 | 28 | 33 | 0.001 |
| Ineligible due to pack-years and time since quitting smoking | |||||||
| % within race | 8.4 | 10.2 | 9.4 | 10.0 | 10.4 | 4.8 | |
| Pack-years | 4.6 | 6.2 | 7.5 | 8.2 | 9.5 | 12.1 | 0.002 |
| Quit time, years | 25 | 30 | 30 | 25 | 26 | 34 | 0.0118 |
| Ineligible due to age, pack-years, and time since quitting smoking | |||||||
| % within race | 4.7 | 3.7 | 5.2 | 2.4 | 4.7 | 1.7 | |
| Age, years | 45 | 48 | 80 | 80 | 48 | 41 | <0.001 |
| Pack-years | 7.4 | 5 | 6.8 | 6.1 | 5.3 | 2.6 | 0.4164 |
| Quit time, years | 20 | 22 | 27 | 30 | 20.5 | 22.5 | 0.003 |
*, all percentages are calculated using sampling weights.
Distribution of cotinine
The mean urinary cotinine from ineligible participants was very similar across racial groups, with the exception of Mexican American and other Hispanic participants, which both had significantly lower cotinine levels (Figure 4A).
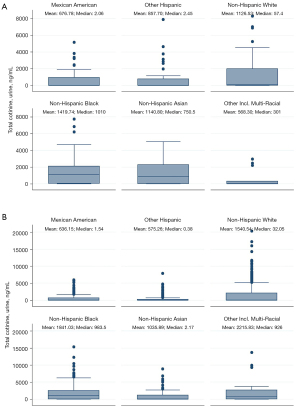
When the analysis was restricted to participants who were ineligible due to pack-years (Figure 4B), NHW, NHB and non-Hispanic Asian (NHA) participants had very similar mean values of urinary cotinine.
Discussion
Our analyses demonstrated that a larger percentage of racial and ethnic minority participants than White participants are still ineligible for LC screening, based on the 2021 USPSTF LC screening guidelines. It is evident that current, updated, LC screening guidelines systematically under-screen non-White people who smoke. However, this is the first attempt, to our knowledge, at using NHANES data to quantify the phenomenon and analyze the reasons for it, following the 2021 change in USPSTF LC screening guidelines. The observation of racial disparities in screening is not unique to LC. The development of new screening tests has historically increased racial disparities for cancer outcomes, at least initially (10). Though racial disparities in screening narrow as new screening tests become more widely implemented, they still persist, for example, for prostate, colorectal, and breast cancer, which are the cancers with the highest incidence and mortality aside from LC (2,11-15). People belonging to racial and ethnic minority groups are less likely to be screened for these cancers though they often have higher incidence and mortality rates than White people.
For LC in particular, stage at diagnosis is the strongest predictor for survival (16). LC has a relative 5-year survival rate of 22.9%, making it one of the most deadly cancers, more deadly than breast, colorectal, and prostate cancers (17). Racial and ethnic minorities are known to be diagnosed at a later stage (3,18,19). Since screening is one of the key tools for the early detection of cancer, it is important to understand why non-White populations, in particular, Black adults, are left out of screening efforts. In addition to classic barriers to screening for those who are eligible, Black people who smoke were found to be less likely to meet the 2013 USPSTF LC screening eligibility criteria in a previous study, primarily because they smoke fewer pack-years on average than White people who smoke (19). Furthermore, Black people who smoke are more likely to be diagnosed with LC at an earlier age than White people who smoke, suggesting that the screening age minimum cutoff of 50 years might not be equally appropriate for all races (16). We show here that non-White people who smoke who are ineligible for LC screening based on the 2021 USPSTF guidelines were still predominantly ineligible either due to not meeting both the minimum age and smoking exposure requirements or due to solely not meeting the minimum smoking exposure requirement. It is important to acknowledge that a smaller proportion of White than non-White people who smoke were ineligible due to these criteria.
From the data presented in this analysis, it is evident that the USPSTF LC screening guidelines fail to capture a significant portion of the at-risk population. By failing to base LC screening guidelines on information from more diverse study populations, non-White populations have been systematically left out of LC screening eligibility—even though results of previous studies have indicated, for example, that Black people may have the most to gain from LDCT screening in terms of reducing mortality rates (10).
In order to improve the risk profile estimate and expand screening to those who need it most, it is important to acknowledge that pack-years might not be the most appropriate measure of tobacco exposure, and that the addition of metabolic biomarkers could improve the individual assessment of LC risk. Cotinine is one of the main metabolites of nicotine, which can be used as an accurate measure of recent nicotine exposure, and consequently, tobacco exposure (20). It is easily measurable in saliva, serum, and urine and has a half-life of 16–18 hours (20,21). Previous studies have demonstrated a dose-dependent association between cotinine and LC risk (22,23). From the present analysis, we observed that though ineligible NHB participants had lower mean pack-years than ineligible NHW participants, they still had similar or higher mean urinary cotinine levels.
There are several reasons why non-White racial groups may have higher levels of exposure to carcinogens from tobacco at lower levels of cigarette smoking. Smoking behaviors differ across racial and ethnic groups. Black people who smoke are more likely to smoke menthol cigarettes than White people who smoke, which have been shown to promote deeper smoke inhalation through soothing respiratory irritation commonly associated with smoking (24-26). Some mentholated brands also have higher concentrations of tar and nicotine per cigarette (27). The chance of environmental tobacco exposure is not equally distributed across races. For example, Black children and adults have the highest risk of environmental tobacco smoke exposure, when compared to all other races (28). It has been reported that nicotine metabolism and clearance vary with race and ethnicity, even after adjusting for environmental exposures (27), although recent data have downplayed cotinine’s ability to adequately measure such racial differences in metabolism (29). Higher levels of cotinine are associated with increased risk of LC, thus screening guidelines that are primarily based on the amount of cigarettes smoked inadequately capture individual LC risk (30).
Risk prediction models have been recommended as an alternative method of identifying high-risk individuals who should be eligible for LC screening. One risk-based prediction model that has been validated by researchers in several countries is the PLCOm2012 model (31), which utilizes variables such as age, race or ethnicity, education, body mass index, personal history of cancer, family history of LC, smoking status, intensity, duration, and quitting time to assess an individual’s 6-year risk of developing LC (32). Though the PLCOm2012 model has been shown to decrease screening disparities between White and Black people who smoke when compared to the USPSTF 2021 LC screening guidelines, racial disparities in screening still persisted (32). Therefore, individual biomarkers might serve as a powerful tool to improve prediction models and inform screening guidelines.
The results of this study should be interpreted by taking into account several limitations. We were unable to utilize the most recent data cycles from NHANES because they were lacking certain smoking questionnaire variables necessary to calculate pack-years.
Our selection included participants aged from 50 to 79 because NHANES codes participants ages 80 and older as 80 years old. Hence we lost the potentially eligible participants who were exactly 80 years old.
Smoking history is derived from a questionnaire, and, as such, is subject to self-reporting bias. Additionally, cotinine is not the measure of life-long exposure, given its relatively short half-life.
However, this review has several strengths. NHANES provides us a nationally representative dataset, hence the conclusions can be generalized for the whole U.S. population. Furthermore, to our knowledge, this the first study to use urinary cotinine data from NHANES in relation to screening eligibility and report its distribution across racial and ethnic groups.
Conclusions
Current LC screening guidelines do not adequately capture at-risk individuals who are non-White. Future analyses should focus on determining appropriate individualized guidelines and consider including biomarkers. This is essential to ensure insurance coverage for LDCT screening in high-risk populations that are currently underscreened (10). Urinary cotinine is an example of a good, rapid, non-invasive measure to determine recent exposure to nicotine and, by proxy, exposure to tobacco carcinogens. Risk-based models that include several indicators of tobacco exposure, not just pack-years, should be utilized to improve LC outcomes in non-White populations and to decrease the racial disparity in LC mortality.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-816/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the ethical standards of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program, Cancer Stat Facts: Lung and Bronchus Cancer [cited 2022 Nov 10]. Available online: https://seer.cancer.gov/statfacts/html/lungb.html
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- U.S. Preventive Services Task Force. Final Recommendation Statement, Lung Cancer: Screening. [cited 2022 Nov 11]. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening#citation1
- U.S. Preventive Services Task Force. Archived: Lung Cancer: Screening [cited 2022 Nov 11]. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer 2007;120:868-74. [Crossref] [PubMed]
- Jonas D, Reuland DS, Reddy SM, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: An Evidence Review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 198. Agency for Healthcare Research and Quality. 2021. AHRQ publication 20-05266-EF-1.
- Jung S, Lee IS, Kim SB, et al. Urine Cotinine for Assessing Tobacco Smoke Exposure in Korean: Analysis of the Korea National Health and Nutrition Examination Survey (KNHANES). Tuberc Respir Dis (Seoul) 2012;73:210-8. [Crossref] [PubMed]
- Carter-Harris L, Slaven JE Jr, Monahan PO, et al. Understanding lung cancer screening behavior: Racial, gender, and geographic differences among Indiana long-term smokers. Prev Med Rep 2018;10:49-54. [Crossref] [PubMed]
- Hansen M, Hamieh NM, Markt SC, et al. Racial Disparities in Prostate Cancer: Evaluation of Diet, Lifestyle, Family History, and Screening Patterns. Cancer Epidemiol Biomarkers Prev 2022;31:982-90. [Crossref] [PubMed]
- Lu CD, Adeyemi O, Anderson WE, et al. Racial Disparities in Prostate Specific Antigen Screening and Referral to Urology in a Large, Integrated Health Care System: A Retrospective Cohort Study. J Urol 2021;206:270-8. [Crossref] [PubMed]
- Liss DT, Baker DW. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med 2014;46:228-36. [Crossref] [PubMed]
- Chowdhury R, David N, Bogale A, et al. Assessing the Key Attributes of Low Utilization of Mammography Screening and Breast-self Exam among African-American Women. J Cancer 2016;7:532-7. [Crossref] [PubMed]
- Miranda PY, Tarraf W, González HM. Breast cancer screening and ethnicity in the United States: implications for health disparities research. Breast Cancer Res Treat 2011;128:535-42. [Crossref] [PubMed]
- Haddad DN, Sandler KL, Henderson LM, et al. Disparities in Lung Cancer Screening: A Review. Ann Am Thorac Soc 2020;17:399-405. [Crossref] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program, Cancer Statistics Explorer Network: Lung and Bronchus SEER 5-Year Relative Survival Rates, 2012-2018 [cited 2022 Nov 11]. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=47&data_type=4&graph_type=5&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&series=9&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2#graphArea
- Jones CC, Mercaldo SF, Blume JD, et al. Racial Disparities in Lung Cancer Survival: The Contribution of Stage, Treatment, and Ancestry. J Thorac Oncol 2018;13:1464-73. [Crossref] [PubMed]
- Japuntich SJ, Krieger NH, Salvas AL, et al. Racial Disparities in Lung Cancer Screening: An Exploratory Investigation. J Natl Med Assoc 2018;110:424-7. [Crossref] [PubMed]
- Chang CM, Edwards SH, Arab A, et al. Biomarkers of Tobacco Exposure: Summary of an FDA-Sponsored Public Workshop. Cancer Epidemiol Biomarkers Prev 2017;26:291-302. [Crossref] [PubMed]
- Raja M, Garg A, Yadav P, et al. Diagnostic Methods for Detection of Cotinine Level in Tobacco Users: A Review. J Clin Diagn Res 2016;10:ZE04-6. [Crossref] [PubMed]
- Yuan JM, Gao YT, Murphy SE, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res 2011;71:6749-57. [Crossref] [PubMed]
- Larose TL, Guida F, Fanidi A, et al. Circulating cotinine concentrations and lung cancer risk in the Lung Cancer Cohort Consortium (LC3). Int J Epidemiol 2018;47:1760-71. [Crossref] [PubMed]
- St Helen G, Dempsey D, Wilson M, et al. Racial differences in the relationship between tobacco dependence and nicotine and carcinogen exposure. Addiction 2013;108:607-17. [Crossref] [PubMed]
- Willis DN, Liu B, Ha MA, et al. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J 2011;25:4434-44. [Crossref] [PubMed]
- Weinberger AH, Giovenco DP, Zhu J, et al. Racial/ethnic differences in daily, nondaily, and menthol cigarette use and smoking quit ratios in the United States: 2002 to 2016. Prev Med 2019;125:32-9. [Crossref] [PubMed]
- Shastri SS, Talluri R, Shete S. Disparities in Secondhand Smoke Exposure in the United States: National Health and Nutrition Examination Survey 2011-2018. JAMA Intern Med 2021;181:134-7. [Crossref] [PubMed]
- Tsai J, Homa DM, Gentzke AS, et al. Exposure to Secondhand Smoke Among Nonsmokers - United States, 1988-2014. MMWR Morb Mortal Wkly Rep 2018;67:1342-6. [Crossref] [PubMed]
- Sipe CJ, Koopmeiners JS, Donny EC, et al. UGT2B10 Genotype Influences Serum Cotinine Levels and Is a Primary Determinant of Higher Cotinine in African American Smokers. Cancer Epidemiol Biomarkers Prev 2020;29:1673-8. [Crossref] [PubMed]
- Flores RM, Liu B, Taioli E. Association of serum cotinine levels and lung cancer mortality in non-smokers. Carcinogenesis 2016;37:1062-9. [Crossref] [PubMed]
- Tammemägi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med 2014;11:e1001764. [Crossref] [PubMed]
- Aredo JV, Choi E, Ding VY, et al. Racial and Ethnic Disparities in Lung Cancer Screening by the 2021 USPSTF Guidelines Versus Risk-Based Criteria: The Multiethnic Cohort Study. JNCI Cancer Spectr 2022;6:pkac033. [Crossref] [PubMed]


