
Effects of a ground-glass opacity component on the recurrence and survival of pathological stage IA3 lung adenocarcinoma: a multi-institutional retrospective study
Highlight box
Key findings
• We used multicenter data to demonstrate that pathological stage IA3 lung adenocarcinoma patients with GGO components had a better prognosis than those without GGO components. This important finding provides a reference value for the identification of patients with pathological stage IA3 lung adenocarcinoma and a high recurrence rate.
What is known and what is new?
• The prognostic value of GGO components in patients with pathological stage IA3 was previously investigated.
• This study analyzed the effects of GGO components on dynamic recurrence and death in patients with pathological stage IA3 lung adenocarcinoma.
What is the implication, and what should change now?
• Our study showed that active follow-up and postoperative adjuvant therapy should be adopted for pathological stage IA3 lung adenocarcinoma without GGO components in the future, which will change the current treatment method for patients with pathological stage IA3 lung adenocarcinoma.
Introduction
With the development of high-resolution computed tomography (HRCT) and multidetector computed tomography (MDCT), more cases of early-stage non-small cell lung cancer (NSCLC) are being detected (1). Based on radiological findings, early lung cancers exhibit pure ground-glass opacity (GGO), partial solids, or pure solids (2). A series of studies have shown that lung cancers without GGO components are more aggressive than those with GGO components (3,4). Therefore, a consolidation-to-tumor ratio (CTR) greater than 0.5 was defined as radiation-aggressive lung cancer by the Japanese Clinical Oncology Group (5). Moreover, the clinical T stage of the 8th Tumor Node Metastasis (TNM) staging system was determined primarily by the size of the solid component of the tumor, excluding the GGO component (6,7).
In recent years, an increasing amount of convincing evidence has shown that CTR and solid component size are associated with the prognosis of early NSCLC (8,9). The degree of malignancy of the tumors varies according to the different sizes of the GGO component, and tumors with more GGO components are less aggressive (10-13). However, previous results have shown that tumors with a small percentage of GGO on computed tomography (CT) scans of the thorax have a good prognosis compared to pure solid stage IA NSCLC (14), and this was confirmed by Kamigaichi et al. (15). In addition, some researchers have suggested that the presence of a GGO component itself is a favorable indicator (16), even for long-term survivors after radical lung adenocarcinoma surgery, while the absence of a GGO component still affects patients' recurrence and survival (17).
The effect of radiographically presented GGO components on the prognosis of patients with pathological stage IA3 lung adenocarcinoma (2–3 cm) is not well established. Therefore, we used multicenter data to compare the cumulative recurrence and mortality rates of patients with or without GGO components and to clarify the differences in the risk of recurrence and tumor-related death between these two groups. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-246/rc).
Methods
Study population
We conducted a retrospective analysis of 352 patients diagnosed with pathological stage IA3 lung adenocarcinoma at two medical organizations in China between July 2012 and July 2020. The participants included patients from the Fujian Medical University Union Hospital (n=235) and the First Hospital of Putian (n=117). The inclusion criteria were as follows: (I) pathological stage IA3 lung adenocarcinoma according to the 8th TNM staging system; (II) radical surgery; and (III) negative postoperative pathological margins. The exclusion criteria were as follows: (I) preoperative neoadjuvant chemoradiotherapy; (II) multiple primary lung adenocarcinomas; (III) palliative surgery; (IV) death within 30 days after surgery; and (V) incomplete clinicopathological information records and loss of follow-up (Figure S1).
This study was reviewed and approved by the Ethics Committee of the Fujian Medical University Union Hospital (No. 2018KY033) and the First Hospital of Putian (No. 2020KJT009), and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All of the included patients signed the informed consent.
Definition
The preoperative clinical staging and postoperative pathological staging were performed for all patients according to the 8th edition TNM staging. The patients underwent segmentectomy or lobectomy plus mediastinal lymphatic nodes dissection routinely; in case of poor lung function such as not to tolerate a lobectomy, the segmental resection was performed.
All patients underwent chest CT scans and were analyzed by professional radiologist authors. In this study, 16-slice spiral CT was used as the main imaging method for the chest of patients, and the GGO component of all tumors was evaluated by the thickness of lung field imaging of 1.25–5 mm. “Lung window” is defined as window height of −500 to −700 H and window depth of 1,000–2,000 H. The CTR was calculated from the ratio of the solid component of the tumor to the maximum diameter on chest CT. The presence of a GGO component in this study was defined as 0≤ CTR <1 on CT (Figure 1A-1E), which were mainly divided into the following five groups: Group A: (CTR =0, Figure 1A), Group B: (0< CTR <0.25, Figure 1B), Group C: (0.25≤ CTR <0.5, Figure 1C), Group D: (0.5≤ CTR <0.75, Figure 1D) and Group E: (0.75≤ CTR <1, Figure 1E). The absence of GGO components was defined as CTR =1 on CT (Figure 1F).
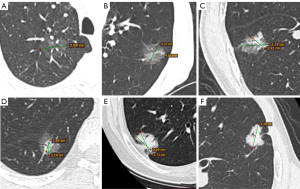
Histology was classified according to the most recent classifications (18) and micropapillary (MIP) component <5% was defined as the absence of an MIP component, while MIP component ≥5% was defined as the presence of an MIP component (19).
Local-regional recurrence (LRR) was defined as tumor recurrence at the surgical margins, anastomosis, ipsilateral lobe, or ipsilateral lymph nodes. Distant metastasis (DM) was defined as tumor metastasis to the contralateral lung lobe or lymph nodes, cervical lymph nodes, abdominal lymph nodes, brain, bone, liver, or other organs. Multiple metastases (LRR+DM) were defined as recurrence or metastasis at two or more sites.
Follow-up of patients
All patients with pathological stage IA3 lung adenocarcinoma who underwent radical resection were followed-up via outpatient visit or by telephone. Tumor markers, physical, and chest radiographs were performed every 3 months for 2 years postoperatively, and CT of the thorax evaluations were performed every 6 months. After 2 years, the evaluations were performed every 6 months, and chest CT examinations were performed annually. In case of recurrence, contrast enhancement CT scan or brain magnetic resonance imaging (MRI) or positron emission tomography-CT (PET-CT) scan were performed, as indicated.
Further tests were performed at follow-up based on the signs and symptoms of recurrence, including total abdominal ultrasound or CT, brain MRI, and 18F-FDG-PET/CT. Histological or radiological evidence of recurrence or metastasis was based on biopsy or surgical excision. Follow-up was available until death or July 2021.
Statistical analysis
The cumulative incidence of recurrence (CIR) was defined as the cumulative incidence of tumor recurrence due to lung cancer, and the cumulative mortality rate (CID) was defined as the cumulative incidence of patient death due to primary lung adenocarcinoma. Recurrence-free survival (RFS) was defined as the time interval between the date of surgery and tumor recurrence or the end of follow-up. Cancer-specific survival (CSS) was defined as the interval between the date of surgery and patient death due to the tumor or the end of follow-up.
The χ2 test or Fisher’s exact test was used to analyze the categorical variables between the two groups. Meanwhile, the continuous variables were shown as the mean ± standard deviation and tested by the t-test or Mann-Whitney U test. Differences in the CIR and CID between the two groups were assessed using the Kaplan-Meier methods and log-rank tests. We used the life table to analysis the annual risk of recurrence and death was calculated based on the number of patients with no recurrence or death at the beginning of each interval.
Cox univariate analysis was performed for all prognostic factors, and multivariate analysis was included if P<0.05. A new staging system was established according to the results of the Cox multivariate analysis. The Kaplan-Meier method was employed to analyze the RFS and CSS of the different groups. Through decision curve analysis (DCA), the net benefit of the prediction model was evaluated using the true and false positive rates of different risk thresholds to measure the clinical practicability of the prediction model.
All tests were bilateral and P<0.05 was considered statistically significant. SPSS (version 23.0, Chicago, IL, USA) and R language (version 3.6.3, Vienna, Austria) were used for statistical analysis.
Results
Clinicopathological information
A total of 352 patients with pathological stage IA3 lung adenocarcinoma were included in this study. There were 181 (51.4%) female patients and 171 (48.6%) male patients. Peripheral tumors were found in 343 (97.4%) cases, and central tumors were identified in nine (2.6%) cases. In addition, 82 (23.3%) patients had an MIP component and 186 (52.8%) patients did not have a GGO component (Table 1).
Table 1
| Characteristics | Total (n=352) |
|---|---|
| Age (years), n (%) | |
| ≤65 | 224 (63.6) |
| >65 | 128 (36.4) |
| Sex, n (%) | |
| Female | 181 (51.4) |
| Male | 171 (48.6) |
| BMI (kg/m2), n (%) | |
| <18.5 | 50 (14.2) |
| 18.5–25 | 217 (61.6) |
| >25 | 85 (24.1) |
| Smoke history, n (%) | |
| No | 255 (72.4) |
| Yes | 97 (27.6) |
| Preoperative symptoms, n (%) | |
| No | 232 (65.9) |
| Yes | 120 (34.1) |
| Pathological tumor size (cm), mean ± SD | 2.47±0.26 |
| Primary tumor location, n (%) | |
| Central type | 9 (2.6) |
| Periphery | 343 (97.4) |
| CEA (ng/mL), n (%) | |
| <5 | 198 (56.3) |
| ≥5 | 154 (43.8) |
| Pulmonary lobectomy, n (%) | |
| No | 14 (4.0) |
| Yes | 338 (96.0) |
| Examined lymph node count, mean ± SD | 16.2±7.79 |
| MIP component, n (%) | |
| <5% | 270 (76.7) |
| ≥5% | 82 (23.3) |
| GGO component, n (%) | |
| Absence | 186 (52.8) |
| Presence | 166 (47.2) |
BMI, body mass index; SD, standard deviation; CEA, carcinoembryonic antigen; MIP, micropapillary; GGO, ground-glass opacity.
Recurrence pattern and survival analysis in the study population
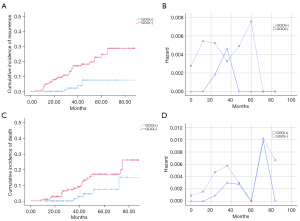
At the median follow-up of 40.5 months (10–107 months), the overall recurrence rate in the whole cohort was 10.5%; DM (5.1%) was the most frequent pattern of recurrence followed by LRR (3.1%) (Figure S2). At the end of follow-up, 37 patients had relapsed and 31 had died from relapse. In the overall patient population, the 5-year CIR and CID rates were 17.6% and 13.0%, respectively. During the study period, the time-dependent curve showed a double peak in patients’ risk of recurrence, respectively, at 3 and 5 years after surgery, after which the risk of recurrence showed a significant downward trend. Similarly, the risk of tumor-related death also exhibited a double peak at 4 and 6 years after surgery, respectively (Figure 2).

Clinicopathological features between the presence and absence of a GGO component
There were 166 (47.2%) patients in the presence-GGO group and 186 (52.8%) patients in the absence-GGO group. The proportion of centrally-located tumors in the absence-GGO group was significantly higher than that in the presence-GGO group (P=0.004). Patients with absence-GGO had a higher rate of lobectomy than those with presence-GGO (P=0.016). Postoperative pathology showed that the proportion of an MIP component ≥5% in the absence-GGO group was significantly higher than that in the presence-GGO (P=0.015). However, there were no significant differences in age, sex, body mass index (BMI), smoking history, preoperative symptoms, tumor size, preoperative carcinoembryonic antigen (CEA) concentration, and examined lymph node count among patients with presence-GGO and absence-GGO components (P>0.05) (Table 2).
Table 2
| Characteristics | GGO(+) (n=166) | GGO(−) (n=186) | P value |
|---|---|---|---|
| Age (years), n (%) | 0.762 | ||
| ≤65 | 107 (64.5) | 117 (62.9) | |
| >65 | 59 (35.5) | 69 (37.1) | |
| Sex, n (%) | 0.572 | ||
| Female | 88 (53.0) | 93 (50.0) | |
| Male | 78 (47.0) | 93 (50.0) | |
| BMI (kg/m2), n (%) | 0.236 | ||
| <18.5 | 29 (17.5) | 21 (11.3) | |
| 18.5–25 | 97 (58.4) | 120 (64.5) | |
| >25 | 40 (24.1) | 45 (24.2) | |
| Smoke history, n (%) | 0.371 | ||
| No | 124 (74.7) | 131 (70.4) | |
| Yes | 42 (25.3) | 55 (29.6) | |
| Preoperative symptoms, n (%) | 0.208 | ||
| No | 115 (69.3) | 117 (62.9) | |
| Yes | 51 (30.75) | 69 (37.1) | |
| Pathological tumor size (cm), mean ± SD | 2.42±0.25 | 2.51±0.26 | 0.964 |
| Primary tumor location, n (%) | 0.004 | ||
| Central type | 0 (0.00) | 9 (4.8) | |
| Periphery | 166 (100.0) | 177 (95.2) | |
| CEA (ng/mL), n (%) | 0.063 | ||
| <5 | 102 (61.4) | 96 (51.6) | |
| ≥5 | 64 (38.6) | 90 (48.4) | |
| Pulmonary lobectomy, n (%) | 0.016 | ||
| No | 11 (6.6) | 3 (1.6) | |
| Yes | 155 (93.4) | 183 (98.4) | |
| Examined lymph node count, mean ± SD | 15.8±7.87 | 16.5±7.71 | 0.843 |
| MIP component, n (%) | 0.015 | ||
| <5% | 137 (82.5) | 133 (71.5) | |
| ≥5% | 29 (17.5) | 53 (28.5) |
GGO, ground-glass opacity; BMI, body mass index; SD, standard deviation; CEA, carcinoembryonic antigen; MIP, micropapillary.
Recurrence pattern and survival analysis of patients with the presence and absence of GGO components
The Figure 3 showed that the total recurrence rate of patients with the presence of a GGO component was 3.0% and that of patients with the absence of a GGO component was 17.2%, with a significant difference between the two groups (P<0.001). Similarly, the incidence of LRR (5.4% vs. 0.6%, P=0.010), DM (8.1% vs. 1.8%, P=0.008), and LRR+DM (4.3% vs. 0.6%, P=0.028) were significantly higher in patients with the absence of a GGO component than in those with the presence of a GGO component.

The 5-year CIR in the GGO component group was significantly lower than that in the absence-GGO component group (7.5% vs. 24.5%, P<0.001) (Figure 4A). The Figure 4B shows a single peak of recurrence risk in the GGO component group at 3 years postoperatively. However, in the absence-GGO component group, there was a double-peak risk curve at 1 and 5 years after surgery. Moreover, the 5-year CID in the presence-GGO and absence-GGO component groups was 7.4% and 17.0%, respectively, and the difference between the two groups was statistically significant (P<0.001) (Figure 4C). The tumor-related death risk curve showed that the risk of death in both groups with and without GGO components presented respective double peaks at 3 and 6 years postoperatively, and the risk of death in both groups gradually decreased after reaching the peak (Figure 4D).
Further subgroup analysis showed significant differences in CIR in LRR, DM, and LRR+DM among patients in the presence-GGO and absence-GGO component group (all P<0.05), as well as significant differences in postoperative recurrence risk, as the survival time increased in both two groups (Figure S3A-S3F).
Prognostic value of presence-GGO in pathological IA3 stage lung adenocarcinoma
Cox proportional risk univariate regression analysis showed that sex, smoking history, MIP components, and GGO components were risk factors for RFS of pathological IA3 lung adenocarcinoma patients (all P<0.05). Multivariate analysis showed that smoking history [yes vs. no: hazard ratio (HR) 2.802; 95% confidence interval (CI): 1.201 to 6.540; P=0.017], MIP components (≥5% vs. <5%: HR 3.556; 95% CI: 1.792 to 7.057; P<0.001), and GGO components (absence vs. presence: HR 4.494; 95% CI: 1.742 to 11.591; P=0.002) were independent prognostic factors affecting RFS (Table 3).
Table 3
| Characteristics | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| ≤65 | |||||
| >65 | 1.331 (0.694–2.552) | 0.39 | |||
| Sex | |||||
| Female | |||||
| Male | 2.414 (1.211–4.813) | 0.012 | 1.486 (0.621–3.554) | 0.373 | |
| BMI (kg/m2) | |||||
| <18.5 | |||||
| 18.5–25 | 0.864 (0.327–2.285) | 0.769 | |||
| >25 | 0.982 (0.321–3.009) | 0.975 | |||
| Smoke history | |||||
| No | |||||
| Yes | 2.877 (1.503–5.505) | 0.001 | 2.802 (1.201–6.540) | 0.017 | |
| Preoperative symptoms | |||||
| No | |||||
| Yes | 1.049 (0.539–2.039) | 0.888 | |||
| Pathological tumor size (cm) | 3.107 (0.909–10.615) | 0.071 | |||
| Primary tumor location | |||||
| Central type | |||||
| Periphery | 0.606 (0.083–4.438) | 0.622 | |||
| CEA (ng/mL) | |||||
| <5 | |||||
| ≥5 | 1.411 (0.738–2.697) | 0.297 | |||
| Pulmonary lobectomy | |||||
| No | |||||
| Yes | 0.947 (0.129–6.938) | 0.957 | |||
| Examined lymph node count | 0.997 (0.956–1.039) | 0.877 | |||
| MIP component | |||||
| <5% | |||||
| ≥5% | 2.885 (1.511–5.510) | 0.001 | 3.556 (1.792–7.057) | <0.001 | |
| GGO component | |||||
| Presence | |||||
| Absence | 5.103 (1.985–13.119) | 0.001 | 4.494 (1.742–11.591) | 0.002 | |
RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index; CEA, carcinoembryonic antigen; MIP, micropapillary; GGO, ground-glass opacity.
As for CSS, age, sex, smoking history, and GGO component were risk factors for CSS (all P<0.05). Multivariate analysis showed that age (>65 vs. ≤65 years: HR 2.521; 95% CI: 1.200 to 5.297; P=0.015), smoking history (yes vs. no: HR 2.658; 95% CI: 1.094 to 6.459; P=0.031), and GGO components (absence vs. presence: HR 2.948; 95% CI: 1.115 to 7.793; P=0.029) were independent prognostic factors for patients’ CSS (Table 4).
Table 4
| Characteristics | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| ≤65 | |||||
| >65 | 2.464 (1.207–5.031) | 0.013 | 2.521 (1.200–5.297) | 0.015 | |
| Sex | |||||
| Female | |||||
| Male | 3.480 (1.486–8.150) | 0.004 | 1.650 (0.572–4.757) | 0.354 | |
| BMI (kg/m2) | |||||
| <18.5 | |||||
| 18.5–25 | 0.421 (0.162–1.094) | 0.076 | |||
| >25 | 0.470 (0.143–1.547) | 0.214 | |||
| Smoke history | |||||
| No | |||||
| Yes | 3.887 (1.905–7.929) | <0.001 | 2.658 (1.094–6.459) | 0.031 | |
| Preoperative symptoms | |||||
| No | |||||
| Yes | 0.887 (0.419–1.878) | 0.754 | |||
| Pathological tumor size (cm) | 2.348 (0.569–9.689) | 0.238 | |||
| Primary tumor location | |||||
| Central type | |||||
| Periphery | 0.584 (0.079–4.328) | 0.599 | |||
| CEA (ng/mL) | |||||
| <5 | |||||
| ≥5 | 1.195 (0.589–2.423) | 0.622 | |||
| Pulmonary lobectomy | |||||
| No | |||||
| Yes | 1.839 (0.246–13.755) | 0.553 | |||
| Examined lymph node count | 0.988 (0.943–1.036) | 0.615 | |||
| MIP component | |||||
| <5% | |||||
| ≥5% | 2.096 (0.989–4.441) | 0.054 | |||
| GGO component | |||||
| Presence | |||||
| Absence | 3.413 (1.304–8.931) | 0.012 | 2.948 (1.115–7.793) | 0.029 | |
CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index; CEA, carcinoembryonic antigen; MIP, micropapillary; GGO, ground-glass opacity.
Establishing a GGO component-based prognostic score staging method
Based on the results of Cox multivariate analysis, we established an individualized prognostic staging of RFS and CSS for patients with pathological stage IA3 lung adenocarcinoma. In terms of predicting RFS, we rated smoking history as 1 point, no smoking history as 0 point, the presence of an MIP component as 1 point, the absence of an MIP component as 0 point, the presence of a GGO component as 1 point, and the absence of a GGO component as 0 point (Figure S4A). Similarly, in terms of predicting CSS, we rated age >65 years old as 1 point, ≤65 years old as 0 point, smoking history as 1 point, no smoking history as 0 point, the absence of a GGO component as 1 point, and the presence of a GGO component as 0 point (Figure S4B).
Based on the GGO component, the predicted 5-year RFS of patients with scores of 0, 1, 2, and 3 was 97.4%, 88.1%, 64.6%, and 51.4%, respectively, with significant differences between these groups (P<0.001) (Figure 5A). Based on the GGO component, the predicted 5-year CSS of patients with scores of 0, 1, 2, and 3 was 100%, 88.3%, 83.1%, and 55.6%, respectively. Similarly, there were significant differences between the groups (P<0.001) (Figure 5B).

We further evaluated the clinical benefit rate of RFS by GGO component-based prognostic score staging using DCA. The results showed that when comparing model A (smoking history), model B (MIP component), and model C (smoking history + MIP component) with model D (GGO component + smoking history + MIP component), the latter exhibited a better clinical value in predicting patients’ RFS over a considerable range (Figure 6A). Similarly, model A (age), model B (smoking history), model C (age + smoking history), and model D (GGO component + age + smoking history) were compared to predict the clinical value of CSS; the model D showed a higher clinical benefit rate (Figure 6B). The results using DCA showed that over a wide threshold probability range (RFS: 5–65%, CSS: 5–75%), the net clinical benefit was greater in models that included GGO components than in models that did not.

Discussion
In this study, multi-center data was utilized to compare the effects of a GGO component on the recurrence and survival of patients with pathological stage IA3 lung adenocarcinoma. Our results showed that the CIR and CID of patients with the presence of a GGO component were lower than those with the absence of a GGO component. The recurrence risk curve of patients with the presence of a GGO component showed a single peak, while those of patients with the absence of a GGO component exhibited a double peak.
Some large sample size data has shown that there are significant differences in the clinicopathological features and tumor outcomes between the presence and absence of GGO components, as well as in the types of epidermal growth factor receptor (EGFR) mutations between the two groups (4,10,20,21). Furthermore, a nationwide study in Japan confirmed the effect of GGO components on early-stage NSCLC and its value as a good prognostic factor (5). Similarly, we observed a significant difference between the CIR in pathological stage IA3 lung adenocarcinoma patients between the two groups (7.5% vs. 24.5%). In terms of tumor-related mortality, patients without GGO components had a significantly higher CID than those with GGO components (17.0% vs. 7.4%). A previous study demonstrated that pathological stage IA patients with GGO components have significantly higher OS than those without GGO components (97% vs. 84%) (22). Lung adenocarcinomas showing GGO components on CT exhibit lepidic growth pattern, whereas solid lung adenocarcinomas exhibit aggressive tumor histology (23). More importantly, radiologically solid masses are associated with lymph node metastasis, vascular invasion, and pleural invasion, all of which are associated with poor prognosis (24).
Similar to previous studies, our study found that the presence of MIP in pathological stage IA3 lung adenocarcinoma with GGO components was significantly lower than that without GGO components. Kamiya et al. reported that the presence of an MIP component promoted the proliferative ability of cells in the vascular and lymphatic systems, leading to the occurrence of micrometastases, which were a key cause of tumor recurrence after radical surgery (25). Watanabe et al. showed that patients with an MIP component had a high risk of postoperative recurrence, and this risk persisted for a long time (19). Therefore, pathological stage IA3 lung adenocarcinoma with or without GGO components may be two heterogeneous lesions with different tumor aggressiveness. At present, different countries hold varying views on postoperative adjuvant therapy for early lung adenocarcinoma. National Comprehensive Cancer Network (NCCN) guidelines recommend that patients with pathological stage IA3 lung adenocarcinoma do not require any adjuvant therapy after radical surgery (26). Meanwhile, adjuvant chemotherapy has become the standard treatment in Japan for patients with pathological stage IA and IB tumors larger than 2.0 cm in total size (27,28). Our findings suggest that patients with pathologic stage IA3 lung adenocarcinoma with solid component could be considered for closer follow-up or post-operative adjuvant chemotherapy if at high risk or recurrence, and prospective clinical trials are needed to confirm this conclusion.
To date, the same follow-up strategy has been used for the postoperative review of early NSCLC, including pathological stage IA3. However, with the emergence of molecularly targeted therapies, which have significantly improved the survival and quality of life of patients with advanced relapsed NSCLC (29,30), early detection and treatment are critical for patients with relapsed NSCLC. The relapse risk curve provides more useful postoperative follow-up information to help identify more patients at a high risk of early recurrence. Our investigation showed that patients without GGO components had a higher risk of recurrence in the first 3 years after surgery than those with GGO components. In addition, the risk curve of patients without GGO components showed a double peak at 1 and 5 years after surgery, while patients with GGO components showed a single peak at 3 years postoperatively. So, the GGO component should be considered in postoperative follow-up strategies for patients with pathological IA3 lung adenocarcinoma and an individualized and precise follow-up plan should be developed.
The multivariate Cox analysis showed that smoking history, MIP components, and GGO components were independent prognostic factors affecting patients’ RFS; however, when the GGO component was included in the analysis, the MIP component exhibited no significant correlation with patients’ CSS. EGFR mutations are reported to be the most common mutation in Asian populations, accounting for more than 70% of lung adenocarcinomas containing MIP components (31,32). In our study population, a total of 37 patients developed local recurrence and distant metastasis after surgery, and about 27 patients (75.7%) had EGFR mutations. Since EGFR mutation-targeted inhibitors are effective in the treatment of recurrent lung adenocarcinoma, the presence of an MIP component is not a prognostic indicator of long-term survival in pathologic stage IA3 lung adenocarcinoma. Furthermore, according to the multivariate analysis results, we innovatively established a scoring staging system based on the GGO component. By calculating the net benefits of screening for various risk thresholds, we found that the model incorporating the GGO component had greater clinical value than other models.
Although this study was a retrospective analysis of the prospective data collected by two institutions, there were still some limitations that should be noted. Firstly, among the 352 patients with pathological stage IA3 lung adenocarcinoma included, only 37 patients had relapse or metastasis at the end of the follow-up period, indicating that the sample size was still small. And, patients with lung adenocarcinoma 2 or 3 different GGOs were excluded, so it is unclear whether the amount of GGO affects survival. In addition, the univariate and multivariate analyses we performed did not include all prognostic factors, such as the type of gene mutation, because genetic testing for pathological stage IA3 lung adenocarcinoma is not routinely performed in our two centers. Finally, the presence of GGO components was empirically determined by individual observers at each center, and future prospective data validation requires independent monitoring committees to evaluate and clearly define the “presence of a GGO component”.
Conclusions
Overall, the CIR and CID of pathological stage IA3 lung adenocarcinoma in the presence-GGO component group were significantly lower than those in the absence-GGO component group, and the risk of recurrence was significantly different between the two groups. The presence of a GGO component is an independent factor for good RFS and CSS, and the novel staging based on GGO components has a higher clinical benefit rate. Therefore, pathological stage IA3 lung adenocarcinomas with or without a GGO component are two lesions with different invasive abilities, and postoperative adjuvant therapy and more frequent follow-up strategies should be considered for patients without GGO components.
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: This study was supported by grants from the Key Laboratory of Cardio-Thoracic Surgery (Fujian Medical University), Fujian Province University (No. 0713304); the Natural Science Foundation in Fujian Province (No. 2020J011004); the Fujian provincial health technology project (No. 2020CXA028); the cohort study of the School of Public Health, Fujian Medical University (No. 2021HX003); the Joint Funds for the innovation of science and Technology, Fujian province (No. 2020Y9076); and the National Nature Science Foundation of China (No. 82273415).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-246/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-246/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-246/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-246/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the Fujian Medical University Union Hospital (No. 2018KY033) and the First Hospital of Putian (No. 2020KJT009). Informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Maeyashiki T, Suzuki K, Hattori A, et al. The size of consolidation on thin-section computed tomography is a better predictor of survival than the maximum tumour dimension in resectable lung cancer. Eur J Cardiothorac Surg 2013;43:915-8. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Neither Maximum Tumor Size nor Solid Component Size Is Prognostic in Part-Solid Lung Cancer: Impact of Tumor Size Should Be Applied Exclusively to Solid Lung Cancer. Ann Thorac Surg 2016;102:407-15. [Crossref] [PubMed]
- Hattori A, Suzuki K, Takamochi K, et al. Prognostic impact of a ground-glass opacity component in clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;161:1469-80. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Deng J, Zhao M, Wang T, et al. A modified T categorization for part-solid lesions in Chinese patients with clinical stage I Non-small cell lung cancer. Lung Cancer 2020;145:33-9. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Tsutani Y, Miyata Y, Yamanaka T, et al. Solid tumors versus mixed tumors with a ground-glass opacity component in patients with clinical stage IA lung adenocarcinoma: prognostic comparison using high-resolution computed tomography findings. J Thorac Cardiovasc Surg 2013;146:17-23. [Crossref] [PubMed]
- Stiles BM, Nasar A, Mirza F, et al. Ratio of positron emission tomography uptake to tumor size in surgically resected non-small cell lung cancer. Ann Thorac Surg 2013;95:397-403; discussion 404. [Crossref] [PubMed]
- Sun K, You A, Wang B, et al. Clinical T1aN0M0 lung cancer: differences in clinicopathological patterns and oncological outcomes based on the findings on high-resolution computed tomography. Eur Radiol 2021;31:7353-62. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Hayashi T, et al. Prognostic Impact of the Findings on Thin-Section Computed Tomography in Patients with Subcentimeter Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:954-62. [Crossref] [PubMed]
- Ye T, Deng L, Wang S, et al. Lung Adenocarcinomas Manifesting as Radiological Part-Solid Nodules Define a Special Clinical Subtype. J Thorac Oncol 2019;14:617-27. [Crossref] [PubMed]
- Nelson DB, Godoy MCB, Benveniste MF, et al. Clinicoradiographic Predictors of Aggressive Biology in Lung Cancer With Ground Glass Components. Ann Thorac Surg 2018;106:235-41. [Crossref] [PubMed]
- Watanabe Y, Hattori A, Nojiri S, et al. Clinical impact of a small component of ground-glass opacity in solid-dominant clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2022;163:791-801.e4. [Crossref] [PubMed]
- Kamigaichi A, Tsutani Y, Mimae T, et al. The prognostic impact of the ground-glass opacity component in nearly pure-solid stage IA non-small-cell lung cancer. Eur J Cardiothorac Surg 2022;62:ezac166. [Crossref] [PubMed]
- Fu F, Zhang Y, Wen Z, et al. Distinct Prognostic Factors in Patients with Stage I Non-Small Cell Lung Cancer with Radiologic Part-Solid or Solid Lesions. J Thorac Oncol 2019;14:2133-42. [Crossref] [PubMed]
- Shigefuku S, Shimada Y, Hagiwara M, et al. Prognostic Significance of Ground-Glass Opacity Components in 5-Year Survivors With Resected Lung Adenocarcinoma. Ann Surg Oncol 2021;28:148-56. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Watanabe K, Sakamaki K, Ito H, et al. Impact of the micropapillary component on the timing of recurrence in patients with resected lung adenocarcinoma. Eur J Cardiothorac Surg 2020;58:1010-8. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Radiological classification of multiple lung cancers and the prognostic impact based on the presence of a ground glass opacity component on thin-section computed tomography. Lung Cancer 2017;113:7-13. [Crossref] [PubMed]
- Hong SJ, Kim TJ, Choi YW, et al. Radiogenomic correlation in lung adenocarcinoma with epidermal growth factor receptor mutations: Imaging features and histological subtypes. Eur Radiol 2016;26:3660-8. [Crossref] [PubMed]
- Miyoshi T, Aokage K, Katsumata S, et al. Ground-Glass Opacity Is a Strong Prognosticator for Pathologic Stage IA Lung Adenocarcinoma. Ann Thorac Surg 2019;108:249-55. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Yokouchi H, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer 2001;33:17-25. [Crossref] [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol 2008;21:992-1001. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19:254-66. [Crossref] [PubMed]
- Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 2004;350:1713-21. [Crossref] [PubMed]
- Hamada C, Tsuboi M, Ohta M, et al. Effect of postoperative adjuvant chemotherapy with tegafur-uracil on survival in patients with stage IA non-small cell lung cancer: an exploratory analysis from a meta-analysis of six randomized controlled trials. J Thorac Oncol 2009;4:1511-6. [Crossref] [PubMed]
- Meador CB, Sequist LV, Piotrowska Z. Targeting EGFR Exon 20 Insertions in Non-Small Cell Lung Cancer: Recent Advances and Clinical Updates. Cancer Discov 2021;11:2145-57. [Crossref] [PubMed]
- Oizumi S, Kobayashi K, Inoue A, et al. Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of North East Japan Study Group 002 Trial. Oncologist 2012;17:863-70. [Crossref] [PubMed]
- Huo Y, Sun L, Yuan J, et al. Comprehensive analyses unveil novel genomic and immunological characteristics of micropapillary pattern in lung adenocarcinoma. Front Oncol 2022;12:931209. [Crossref] [PubMed]
- Chao L, Yi-Sheng H, Yu C, et al. Relevance of EGFR mutation with micropapillary pattern according to the novel IASLC/ATS/ERS lung adenocarcinoma classification and correlation with prognosis in Chinese patients. Lung Cancer 2014;86:164-9. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


