
Risk factors for pulmonary embolism in lung cancer patients with lower limb deep venous thrombosis: a case-control study
Highlight box
Key findings
• Hypertension, long-term bedridden status, and elevated serum D-dimer could be independent risk factors of pulmonary embolism (PE) in lung cancer patients with limb extremity deep venous thrombosis.
What is known and what is new?
• Decreased physical activity and increased prevalence of obesity, as well as smoking and hypertension, contribute to the occurrence of PE.
• Hypertension, being bedridden for an extended period, and elevated serum D-dimer were identified as independent risk factors of PE in the present study.
What is the implication, and what should change now?
• Early diagnosis and urgent risk assessment of PE might potentially aid decision-making in the clinical setting or provide new strategies for the management of patients.
• Those variables associated to PE could help us to identify patients with lung cancer that may receive thromboprophylaxis to avoid venous thromboembolism.
Introduction
Pulmonary embolism (PE) is a common fatal emergency, manifested as a blockage in one of the pulmonary arteries. Due to changes in lifestyle, mainly characterized by decreased physical activity, a global increase in obesity, smoking and hypertension, PE has gradually become the third major cause of death from cardiovascular and cerebrovascular diseases (1). The mechanisms of PE in about 50% of cases are related to blood clots breaking off from vein walls in deep venous thrombosis (DVT) and traveling through the heart to the pulmonary arteries (2). Interestingly, patients with cancers are prone to venous thromboembolism (VTE) due to hypercoagulability, especially in patients with lung cancer (3).
Despite its associated frequency and fatality, the clinical symptoms of PE often lack specificity. Syncope and massive hemoptysis are rare, while symptoms such as palpitations, chest tightness, chest pain, dyspnea, cough or blood in sputum may be easily misdiagnosed as acute exacerbation of chronic obstructive pulmonary disease, coronary heart disease, or chronic pulmonary heart disease. Indeed, missed diagnosis and misdiagnosis contribute to the high mortality rate in PE (4,5). Therefore, exploring new methods and diagnostic algorithms is especially important.
In clinical practice, blood clots are the most significant risk factor of PE development, and VTE is a potentially life-threatening disease which leads to PE (6). Patients with cancer are 2–4 times more likely to have VTE compared to those without cancer, and several studies have shown that lung adenocarcinoma is the most common malignant tumor in patients with VTE (7), and approximately 3–13.9% of patients with lung cancers experience a complication with DVT, and 3.8% present with PE (8,9). With timely diagnosis and corresponding therapy, VTE is treatable, thus an effective method of risk assessment is urgently needed to provide early diagnosis and treatment for cancer patients. In view of this, the aim of this study was to analyze predictors of PE in lung cancer patients with lower limb DVT. We present this article in accordance with the STARD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-346/rc).
Methods
Study design and subjects
This retrospective case-control study enrolled lung cancer patients with DVT at the Shanghai Chest Hospital from January 2018 to December 2019. The inclusion criteria were as follows: (I) lung adenocarcinoma of stage I without metastasis confirmed by pathology biopsy evaluation and DVT diagnosed by ultrasound (US); (II) clinical data available; (III) computed tomographic pulmonary angiography (CTPA) was performed within 48 hours of admission; and (IV) DVT with or without PE was confirmed by CTPA. The exclusion criteria were: (I) incomplete US images or laboratory analysis; (II) recurrent DVT and vena cava filter implantation; and (III) any anticoagulant treatment prior to admission.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Shanghai Chest Hospital (No. KS1956) and individual consent for this retrospective analysis was waived.
Typical US examination
The US examination of deep veins in both lower limbs was performed with one of the following Color Doppler US devices: the S2000 (Siemens Medical Solutions, Mountain View, CA, USA) or the Logiq E9 (GE Medical Systems, Milwaukee, WI, USA). The examined deep veins included the femoral vein, popliteal vein and calf veins (tibial, peroneal, soleus and gastrocnemius veins). The DVT was considered as proximal (above or include popliteal vein), distal (limited to infra-popliteal veins) or mixed (both distal and proximal veins). All US images were analyzed by 2 experienced radiologists with more than 5 years of experience in deep vein imaging. When different opinions occurred, a third experienced radiologist was consulted for a final diagnosis. All image reviewers were adequately blinded to the clinical findings.
Data collection
The demographic and clinical characteristics of the patients were collated, including age, gender, smoking, hypertension, surgical trauma, hyperlipidemia, long-term bedridden status, calf swelling, coronary heart disease, chronic pulmonary disease, DVT location, DVT type, prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen, and D-dimer. For bedridden status, in our study, “long-term” refers to more than one month of bedridden status persistently. The surgical trauma was performed within one month before the definite diagnose. In order to clarify PE or not, all the enrolled patients underwent CTPA.
Statistical analysis
SPSS version 22.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Normal distribution quantitative data (i.e., patients’ age, coagulation determinations) were expressed as mean ± standard deviation (SD) and Student’s t-test was used for continuous variables and the chi-squared or Fisher’s exact tests were used for comparison of categorical variables. The factors considered statistically significant by univariate analysis were further analyzed by multiple analysis to obtain risk factors. Multivariable logistic regression analysis was performed to determine the independent variables for predicting PE. We designed a scoring system to assess the diagnostic performance by calculating the number of positive risk factors. Linear regression and chi-squared tests were performed to confirm if PE can be predicted by the number of these risk factors. We made the receiver operating characteristic (ROC) to determine area under curve (AUC) to evaluate the diagnostic performance. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to evaluate the predictive value. Odds ratios (ORs) with 95% confidence intervals (CI) were also calculated. All tests were two-sided, and a P value <0.05 was considered statistically significant. The statistical analysis was performed using SPSS Software Version 25.0 for Windows (IBM, Armonk, NY, USA).
Results
A total of 128 lung cancer patients fulfilled the inclusion criteria. Among them, 8 patients were excluded due to recurrent DVT and vena cava filter implantation; 2 were excluded as they were previously under anticoagulation, thrombectomy and other treatments prior to admission, and another 28 patients were excluded due to incomplete clinical data. Finally, a total of 90 lung cancer patients with DVT were included in this analysis (Figures 1,2).
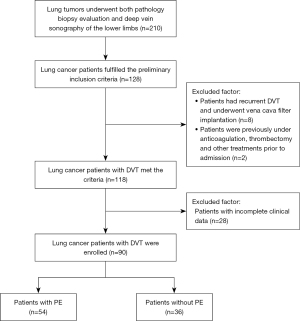

Among the 90 included patients, 54 (60%) presented with PE (Table 1). The mean age was 70.2±10.3 years for patients with DVT with PE and 65.8±8.4 years for patients without PE (P<0.005). Among patients with PE, 23 (42.6%) were men and 31 (57.4%) were women. We found statistically significant differences between two groups in age, comorbidities as hypertension, surgery, hyperlipidemia, risk factor as long-term bedridden status, signs as calf swelling, and proximal DVT (P<0.05 for all determinations) by univariate analysis. Among DVT types, the PE group had more mixed DVT cases than those in the non-PE group, but the difference was not significant after multivariate analysis. Among coagulations determinations, higher levels of D-dimer were more likely to be observed in DVT patients and PE compared to those without PE (6.8±5.2 vs. 2.8±1.9 mg/L; P=0.007; Table 2).
Table 1
| Characteristics | PE | P value | |
|---|---|---|---|
| Yes (n=54) | No (n=36) | ||
| Age (year), mean ± SD | 70.2±10.3 | 65.8±8.4 | 0.041* |
| Gender, n (%) | 0.085 | ||
| Male | 23 (42.6) | 22 (61.1) | |
| Female | 31 (57.4) | 14 (38.9) | |
| Smoking, n (%) | 0.140 | ||
| Yes | 20 (37.0) | 19 (52.8) | |
| No | 34 (63.0) | 17 (47.2) | |
| Hypertension, n (%) | 0.025* | ||
| Yes | 31 (57.4) | 12 (33.3) | |
| No | 23 (42.6) | 24 (66.7) | |
| Surgery, n (%) | 0.004* | ||
| Yes | 45 (83.3) | 20 (55.6) | |
| No | 9 (16.7) | 16 (44.4) | |
| Hyperlipidemia, n (%) | 0.025* | ||
| Yes | 34 (63.0) | 14 (38.9) | |
| No | 20 (37.0) | 22 (61.1) | |
| Long-term bedridden, n (%) | 0.002* | ||
| Yes | 39 (72.2) | 14 (38.9) | |
| No | 15 (27.8) | 22 (61.1) | |
| Calf swelling, n (%) | 0.001* | ||
| Yes | 36 (66.7) | 35 (97.2) | |
| No | 18 (33.3) | 1 (2.8) | |
| Coronary heart disease, n (%) | 0.102 | ||
| Yes | 32 (59.3) | 15 (41.7) | |
| No | 22 (40.7) | 21 (58.3) | |
| Chronic pulmonary disease, n (%) | 0.253 | ||
| Yes | 35 (64.8) | 19 (52.8) | |
| No | 19 (35.2) | 17 (47.2) | |
| DVT location, n (%) | 0.105 | ||
| Left | 11 (20.4) | 12 (33.3) | |
| Right | 19 (35.2) | 5 (13.9) | |
| Both lower extremities | 24 (44.4) | 19 (52.8) | |
| DVT type, n (%) | 0.001* | ||
| Distal | 29 (53.7) | 28 (77.8) | |
| Proximal | 6 (11.1) | 7 (19.4) | |
| Mixed | 19 (35.2) | 1 (2.8) | |
*, statistically significant difference. PE, pulmonary embolism; SD, standard deviation; DVT, deep vein thrombosis.
Table 2
| Coagulation determinations | PE | P value | |
|---|---|---|---|
| Yes (n=54) | No (n=36) | ||
| PT (s), mean ± SD | 13.6±8.5 | 13.2±3.4 | 0.252 |
| INR, mean ± SD | 1.1±0.7 | 1.1±0.2 | 0.175 |
| aPTT (s), mean ± SD | 37.9±57.6 | 31.5±6.6 | 0.171 |
| TT (s), mean ± SD | 19.1±0.6 | 18.4±1.6 | 0.091 |
| Fibrinogen (g/L), mean ± SD | 4.1±4.6 | 3.7±1.1 | 0.291 |
| D-dimer (mg/L), mean ± SD | 6.8±5.2 | 2.8±1.9 | 0.007* |
*, statistically significant difference. PE, pulmonary embolism; PT, prothrombin time; SD, standard deviation; INR, international normalized ratio; aPTT, activated partial thromboplastin time; TT, thrombin time; s, seconds.
As shown in Tables 1,2, older age, presence of hypertension and hyperlipidemia, history of surgical trauma or calf swelling, being bedridden for long periods, DVT type, and D-dimer levels differed significantly between lung cancer patients with PE and those without PE (all P values <0.05).
As depicted in Table 3, all variables with P<0.05 in univariate regression analyses were tested by multivariate logistic regression analysis. Hypertension (OR =7.883, 95% CI: 2.038–30.495, P=0.003), bedridden for long periods (OR =4.166, 95% CI: 1.236–14.044, P<0.01), and D-dimer levels (OR =2.123, 95% CI: 1.476–3.053, P=0.000) were independent risk factors for PE in lung cancer patients with DVT.
Table 3
| Parameter | B | S.E. | Odd ratio | 95% CI | P |
|---|---|---|---|---|---|
| Hypertension | 2.065 | 0.690 | 7.883 | 2.038–30.495 | 0.003* |
| Long-term bedridden | 1.427 | 0.620 | 4.166 | 1.236–14.044 | 0.021* |
| D-dimer (mg/L) | 0.753 | 0.185 | 2.123 | 1.476–3.053 | 0.000* |
*, statistically significant difference. PE, pulmonary embolism; S.E., standard error; CI, confidence interval.
Next, we proposed a scoring system (0–3) for each previously evaluated variables based on the cumulative number of suspicious clinical features (hypertension, bedridden for long periods, and high D-dimer levels) in lung cancer patients with DVT. Patients with PE were associated with more suspicious clinical features than patients without PE (P<0.001). When the number of suspicious clinical features was ≥1, the diagnosis for PE had the highest diagnostic accuracy, with a sensitivity of 98.1% and an NPV of 90.0%. When the number of suspicious clinical features was ≥3, the diagnosis for PE had the highest diagnostic accuracy, with a specificity of 100% and a PPV of 100% (Table 4). The ROC curve was plotted to achieve a good diagnostic performance of the number of suspicious US features for PE, and the AUC was 0.84 (P<0.001) (Figure 3). The cut-off value for predicting PE in lung cancers patients with VTE was 1.5.
Table 4
| Variables | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Odds ratios | P value |
|---|---|---|---|---|---|---|
| ≥1 characteristic | 98.1 | 25.0 | 66.3 | 90.0 | 17.667 | 0.008* |
| ≥2 characteristics | 81.5 | 75.0 | 83.0 | 73.0 | 13.200 | 0.000* |
| ≥3 characteristics | 31.5 | 100.0 | 100.0 | 49.3 | 34.067 | 0.002* |
*, statistically significant difference. PE, pulmonary embolism; DVT, deep vein thrombosis; PPV, positive predictive value; NPV, negative predictive value.

Discussion
This study showed that hypertension, bedridden for long periods, and D-dimer levels were independent risk factors for PE in lung cancer patients with DVT. The probability of PE increases with the number of risk factors, which might further contribute to the decision-making of CTPA examination, as well as provide new strategies for the personalized management of patients.
Among the clinical characteristics, elevated systolic and diastolic blood pressure is a common feature observed in chronic cardiovascular diseases, and in our study, hypertension was one of the most prominent risk predictors of PE in DVT. Elevated blood pressure has been previously shown to influence thrombosis via hypercoagulability, low blood flow, and endothelial damage, which increases the risk of vascular wall damage, promoting or aggravating the formation of thrombus (10-12). Hypertension has been reported as a risk factor of VTE in patients with idiopathic pulmonary fibrosis (13), and is associated with a significantly reduced overall survival (OS) for patients with lung cancer (14). However, to the best of our knowledge, this current study is the first to investigate the relationship between chronic hypertension and PE in lung cancer. Our results suggested that lung cancer patients with a history of chronic hypertension, often aggravated by endovascular lesions, blood stasis, and other related conditions, are at higher risk of PE and should be approached with more caution. In addition, multivariate logistic regression analysis revealed that DVT type did not appear to be a statistically significant risk factor in our study, which contradicts some previous reports, where intramuscular vein thrombosis in VTE was reported to be critical (15). Our results suggest that the type and location of DVT may play a major role under conditions of hypertension, but might not be a major risk factor for PE in general.
Being bedridden for a long period is another risk factor of PE in the clinical setting. Reduced physical activity leads to stasis or slow blood flow, which in turn allows small clots to gradually fuse and form larger blood clots. Many studies have confirmed the relationship between long-term inactivity and DVT (16-19). In particular, Apenteng et al.’s prospective cohort study demonstrated that in the same population, the incidence of VTE was obviously higher in long-term bedridden people than in old people who were able to act on their own in care homes (17). Petterson et al. showed that nursing home residents had a substantially increased risk and the highest VTE incidence within 7 days after admission (18). Several similar reports have supported this view (20,21). In our study, 70% of all patients (63/90) were long-term bedridden due to their cancer condition, and this was shown to be the second strongest risk predictor of PE, with an OR of 4.166 (P<0.01), confirming previously reported risks in the study population of lung cancer patients (7-9).
The main component of thrombus is fibrin, which activates the fibrinolysis system and the production of plasma proteins. The dissolution of fibrin releases specific degradation products, such as D-dimer. Previous studies have reported high sensitivity and low specificity of D-dimer in PE, with less than 1% of patients with negative D-dimer results presenting with PE or PE and DVT within a three-month period (22,23). Other studies have also confirmed the predictive value of D-dimer in PE. Singer et al. demonstrated that D-dimer levels were higher in VTE patients with a proximal thrombus (24) and Choi et al. showed that PE can be excluded when D-dimer was below 500 mg/L and it had a good NPV for PE in pregnant and post-partum patients (25). However, in Fuchs et al.’s study, more than half of patients who had a low or intermediate risk for PE had higher D-dimer level (26). Kwon et al. found that D-dimer levels alone were not sufficient to negate the need for CTPA (27). Gawlitza et al. discovered that the predictive value of D-dimer for PE could be improved by machine-learning (28). In our study, all participants were elderly cancer patients with VTE and D-dimer levels were generally high. Nevertheless, D-dimer was confirmed to be one of the risk factors of PE and was the only one to be confirmed as a significant risk factor among all 6 coagulation indicators examined.
The commonly used clinical scoring systems for PE include the Wells score, the modified Wells score, the Simplified Wells score, the revised Geneva score, and the pulmonary embolism exclusion criteria (PERC) rules. However, clinical decision rules used alone might not exclude DVT or PE, and a structured evaluation system combined with D-dimer levels is needed (15,29). According to multivariate logistic regression analysis, we proposed a scoring system to assess the diagnostic performance by calculating the number of positive risk factors. When the number of risk factors was equal to or greater than one, the sensitivity was 98.1%, which was the highest of all. This indicated that we should be alert to the possibility of PE in lung cancer patients with DVT even if only 1 risk factor is present. Meanwhile, when all 3 risk factors were present, the specificity and PPV were 100%, suggesting that with the increase in risk factors, the rate of misdiagnosis might decrease. However, the sensitivity dropped to 31.5%. In our study, only 17 cases met all 3 risk factors criteria simultaneously and all were confirmed as PE by CTPA, posing the problem of high specificity, but low sensitivity. These results suggest that detailed and comprehensive analysis is still needed in each case to avoid unnecessary radiation exposure and excessive treatment.
There were several limitations to this study. First, this retrospective study was undertaken in a single center and some degree of selection bias was unavoidable. Second, the sample size in our study was small and further studies should be performed to confirm the generalizability of these results. Third, the relationship between intramuscular vein diameter with thrombus and VTE was not explored. Fourth, the sign or symptoms of PE and the relationship between the stage of DVT and PE were not analyzed in our study. In the future, multi-centered studies should be conducted.
Conclusions
In conclusion, hypertension, being bedridden for long periods, and elevated serum D-dimer levels were identified as independent risk factors of PE in lung cancer patients with lower extremity DVT. The specificity of diagnosis increases with increasing number of risk factors, and presence of the two out of three of the above factors yields a sensitivity of 81.5% and specificity of 75.0% in predicting PE. This is high enough to merit clinical usability, especially since all factors are related to easily accessible clinical characteristics. These results contribute to the discussion on early diagnosis and the urgent risk assessment of PE patients, and may aid the decision-making process in the clinical setting, so as to improve patient management and prognosis.
Acknowledgments
Funding: This work was supported in part by the Cultivation Fund of Shanghai Chest Hospital (No. 2020YNJCQ12), the Municipality Scientific and Innovative Action Plan of Shanghai (CN) (No. 21142202200), and the Shanghai Key Laboratory Open Project (No. STCSM 22DZ2229005).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-346/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-346/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-346/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-346/coif). RHP received speaker fee from Medtronic, AMBU, AstraZeneca, Medela, and serves on the Advisory Board of AstraZeneca, BMS, Roche and MSD. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Shanghai Chest Hospital (No. KS1956) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lehnert P, Lange T, Møller CH, et al. Acute Pulmonary Embolism in a National Danish Cohort: Increasing Incidence and Decreasing Mortality. Thromb Haemost 2018;118:539-46. [Crossref] [PubMed]
- Meignan M, Rosso J, Gauthier H, et al. Systematic lung scans reveal a high frequency of silent pulmonary embolism in patients with proximal deep venous thrombosis. Arch Intern Med 2000;160:159-64. [Crossref] [PubMed]
- Cha SI, Shin KM, Lim JK, et al. Pulmonary embolism concurrent with lung cancer and central emboli predict mortality in patients with lung cancer and pulmonary embolism. J Thorac Dis 2018;10:262-72. [Crossref] [PubMed]
- Glise Sandblad K, Rosengren A, Sörbo J, et al. Pulmonary embolism and deep vein thrombosis-comorbidities and temporary provoking factors in a register-based study of 1.48 million people. Res Pract Thromb Haemost 2022;6:e12714. [Crossref] [PubMed]
- Xue X, Sista AK. Catheter-Directed Thrombolysis for Pulmonary Embolism: The State of Practice. Tech Vasc Interv Radiol 2018;21:78-84. [Crossref] [PubMed]
- Heit JA, Ashrani A, Crusan DJ, et al. Reasons for the persistent incidence of venous thromboembolism. Thromb Haemost 2017;117:390-400. [Crossref] [PubMed]
- Zhang Y, Shi Z, Yi J, et al. Correlation between clinicopathological characteristics of lung adenocarcinoma and the risk of venous thromboembolism. Thorac Cancer 2022;13:247-56. [Crossref] [PubMed]
- Tesselaar ME, Osanto S. Risk of venous thromboembolism in lung cancer. Curr Opin Pulm Med 2007;13:362-7. [Crossref] [PubMed]
- Connolly GC, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory patients with lung cancer. Lung Cancer 2012;78:253-8. [Crossref] [PubMed]
- Liang F, Chao M, Li JB, et al. Characteristics and risk factors of deep vein thrombosis in hemiplegic, healthy and bilateral limbs of hemiplegic patients: a 10-year retrospective study. J Thromb Thrombolysis 2021;51:798-804. [Crossref] [PubMed]
- Delis KT, Hunt N, Strachan RK, et al. Incidence, natural history and risk factors of deep vein thrombosis in elective knee arthroscopy. Thromb Haemost 2001;86:817-21. [Crossref] [PubMed]
- Kret MR, Liem TK, Mitchell EL, et al. Isolated calf muscular vein thrombosis is associated with pulmonary embolism and a high incidence of additional ipsilateral and contralateral deep venous thrombosis. J Vasc Surg Venous Lymphat Disord 2013;1:33-8. [Crossref] [PubMed]
- Sobiecka M, Szturmowicz M, Lewandowska K, et al. Chronic hypersensitivity pneumonitis is associated with an increased risk of venous thromboembolism: a retrospective cohort study. BMC Pulm Med 2021;21:416. [Crossref] [PubMed]
- Xiu W, Huang Y, Li Y, et al. Comorbidities and mortality risk among extensive-stage small-cell lung cancer patients in mainland China: impacts of hypertension, type 2 diabetes mellitus, and chronic hepatitis B virus infection. Anticancer Drugs 2022;33:80-90. [Crossref] [PubMed]
- Henry JC, Satiani B. Calf muscle venous thrombosis: a review of the clinical implications and therapy. Vasc Endovascular Surg 2014;48:396-401. [Crossref] [PubMed]
- Gong B, Xu Q, Pang Y, et al. Clinical features of patients with venous thromboembolism: 177 case analysis in 10 years. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019;31:453-7. [PubMed]
- Apenteng PN, Hobbs FR, Roalfe A, et al. Incidence of venous thromboembolism in care homes: a prospective cohort study. Br J Gen Pract 2017;67:e130-7. [Crossref] [PubMed]
- Petterson TM, Smith CY, Emerson JA, et al. Venous Thromboembolism (VTE) Incidence and VTE-Associated Survival among Olmsted County Residents of Local Nursing Homes. Thromb Haemost 2018;118:1316-28. [Crossref] [PubMed]
- Leibson CL, Petterson TM, Smith CY, et al. Rethinking guidelines for VTE risk among nursing home residents: a population-based study merging medical record detail with standardized nursing home assessments. Chest 2014;146:412-21. [Crossref] [PubMed]
- Heit JA, Crusan DJ, Ashrani AA, et al. Effect of a near-universal hospitalization-based prophylaxis regimen on annual number of venous thromboembolism events in the US. Blood 2017;130:109-14. [Crossref] [PubMed]
- Skeik N, Westergard E. Recommendations for VTE Prophylaxis in Medically Ill Patients. Ann Vasc Dis 2020;13:38-44. [Crossref] [PubMed]
- Lucassen W, Geersing GJ, Erkens PM, et al. Clinical decision rules for excluding pulmonary embolism: a meta-analysis. Ann Intern Med 2011;155:448-60. [Crossref] [PubMed]
- van Es N, van der Hulle T, van Es J, et al. Wells Rule and d-Dimer Testing to Rule Out Pulmonary Embolism: A Systematic Review and Individual-Patient Data Meta-analysis. Ann Intern Med 2016;165:253-61. [Crossref] [PubMed]
- Singer AJ, Zheng H, Francis S, et al. D-dimer levels in VTE patients with distal and proximal clots. Am J Emerg Med 2019;37:33-7. [Crossref] [PubMed]
- Choi H, Krishnamoorthy D. The diagnostic utility of D-dimer and other clinical variables in pregnant and post-partum patients with suspected acute pulmonary embolism. Int J Emerg Med 2018;11:10. [Crossref] [PubMed]
- Fuchs E, Asakly S, Karban A, et al. Age-Adjusted Cutoff D-Dimer Level to Rule Out Acute Pulmonary Embolism: A Validation Cohort Study. Am J Med 2016;129:872-8. [Crossref] [PubMed]
- Kwon H, Kim YJ, Her EJ, et al. Elevation of the D-dimer cut-off level might be applicable to rule out pulmonary embolism for active cancer patients in the emergency department. Intern Emerg Med 2022;17:495-502. [Crossref] [PubMed]
- Gawlitza J, Ziegelmayer S, Wilkens H, et al. Beyond the d-dimer - Machine-learning assisted pre-test probability evaluation in patients with suspected pulmonary embolism and elevated d-dimers. Thromb Res 2021;205:11-6. [Crossref] [PubMed]
- Wang KL, Kao YT, Chang WT, et al. Management of Venous Thromboembolisms: Part II. The Consensus for Pulmonary Embolism and Updates. Acta Cardiol Sin 2020;36:562-82. [PubMed]
(English Language Editor: J. Teoh)


