
Multiple bronchiolar adenomas/ciliated muconodular papillary tumors of the bilateral lung with tumor budding and potential malignant transformation into squamous cell carcinoma: a case report and literature review
Highlight box
Key findings
• Our case is the first to show that BA/CMPT can be more than 3 lesions and may involve multiple lesions in both lungs. The atypia of squamous metaplasia and tumor budding strongly supports the ability of malignant transformation.
What is known and what is new?
• The vast majority of BA/CMPT cases are solitary and inert. Related gene mutations such as EGFR, KRAS, and BRAF have been detected, and typical pathological features have been reported.
• We add that BA/CPMT can involve multiple (>3) lesions and have the possibility of more lesions in both lungs; our finding of tumor budding also strongly supports its ability of malignant transformation.
What is the implication, and what should change now?
• We believe that BA/CMPT has potential of malignant transformation. The accuracy of the intraoperative pathological report is key to determining the surgical method, and we need pathologists to strengthen their understanding of it.
Introduction
The ciliated muconodular papillary tumor (CMPT) is a rare peripheral lung tumor first reported and named by Ishikawa in 2002 (1). In 2018, Chang et al. (2) expanded its description and proposed diagnostic terminology for bronchial adenoma (BA). This tumor has now been added to the latest WHO classification of thoracic tumors (5th edition) (3). Its histological characteristic is bilayer bronchiolar-type proliferation with a continuous layer of basal cells. With the continuous supplementation of case reports, the biological characteristics of this tumor are gradually being recognized. In the published literature, BA/CMPT mostly grows in isolation. In addition, the potential of BA/CMPT for malignant transformation has been controversial. Herein, we report an extremely rare case of BA characterized by multiple bilateral lung lesions with potential malignant transformation to squamous cell carcinoma to raise awareness of the disease and review the relevant literature. We present this article in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-374/rc).
Case presentation
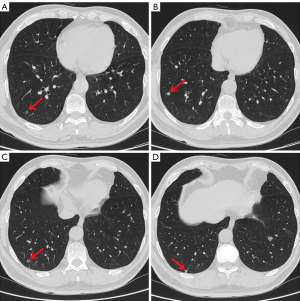
A 55-year-old Chinese man with a 30-year smoking history was admitted to the Ningbo No. 2 Hospital because of multiple small nodules in the lower lobes of both lungs detected during a physical examination seven years prior. Regular reexamination over the past three years showed no significant changes in the nodules. Two weeks before surgery, high-resolution chest computed tomography (HRCT) showed that the largest nodule (Figure 1A,1B) was located in the outer basal segment of the lower lobe of the left lung, approximately 10 mm in diameter, with irregular borders and small ridges on the edge, accompanied by a vacuole sign. There were multiple small nodules in the lower lobes of both lungs, approximately 3–7 mm in diameter with clear boundaries, most of which had solid components (Figure 1C-1E and Figure 2); some had ground-glass components (Figure 2A), and some had small cavities (Figure 2C). On admission, the patient had no symptoms, such as cough, expectoration, difficulty breathing or other uncomfortable symptoms. There was no family history of cancer. The physical examination revealed no obvious abnormalities. Serum levels of infection, inflammatory and tumor markers were all within the normal range, and only carbohydrate antigen 72-4 (CA 72-4) was measured at 16.37 U/mL (the reference value is less than 10.0 U/mL). No uptake of fluorodeoxyglucose (FDG) was observed on positron emission tomography computed tomography (PET-CT), and magnetic resonance imaging (MRI) of the head with contrast enhancement was unremarkable.
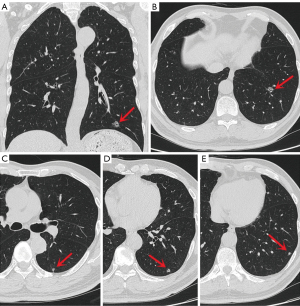
Lung cancer was highly suspected based on imaging findings. After completing the relevant examinations, the patient underwent video-assisted thoracoscopic S9+10 segmentectomy and S6 wedge resection of the left lung. On macrography, four lesions were located in S9+10, and the largest nodule was approximately 10 mm in diameter, medium in texture, and fish-like on the cut surface; one lesion was located in S6 with a diameter of approximately 6 mm, soft texture, and gray-white cut surface. Frozen pathology showed papillary hyperplasia of the bronchial epithelium of S9+10 lesions, three of which were rich in mucous cells, similar to a single-layered cell structure with atypical manifestations (Figure 3), and another lesion was bronchial epithelial hyperplasia with a double-layered cell structure. The S6 lesion showed bronchial epithelial hyperplasia with a double-layered cellular structure, visible mucous cells, and atypical focal epithelial cells. The initial diagnosis was mucinous adenocarcinoma; therefore, we proceeded to perform systematic lymph node dissection. The patient recovered well 3 days after surgery and was discharged.
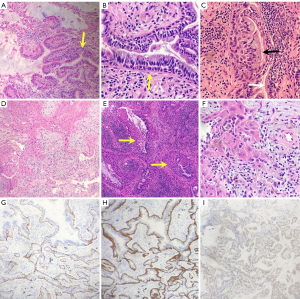
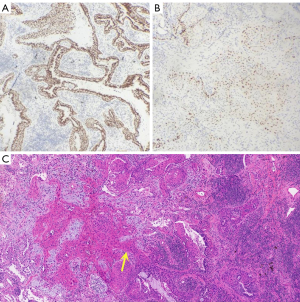
Hematoxylin and eosin staining of the paraffin sections showed that the mucinous cells in S9+10 were hyperplastic, ciliated structures were found in some areas, and the bronchial epithelium showed a double layer of cells (Figure 3A). The epithelium in the lesion area of S6 showed adenoid hyperplasia with a double-layer cell structure (Figure 3B), in which the luminal cells comprised mucinous cells and ciliated cells (Figure 3C). The basal cells were arranged in a bead-like manner; squamous metaplasia occurred in some areas of the basal cells; and irregular arrangement of the squamous epithelium in the focal area was observed, with increased nuclei, an irregular nuclear membrane and an increased nucleo-cytoplasmic ratio (Figure 3D). Continuation and transition between squamous metaplasia and ciliated columnar epithelium (Figure 3E). Immunohistochemical analysis showed that basal cells were positive for p40 (Figure 3G) and CK5/6 (Figure 3H), but basal cells were positive for p53 with varying degrees of intensity (Figure 3I). Cavity face cells were positive for TTF-1 (Figure 4A). In addition, tumor budding was observed in S6 (Figure 3F), which expressed p40 (Figure 4B) and CK5/6. Atypia appeared in normal squamous metaplastic cells (Figure 4C). The final diagnosis was proximal-type bronchiolar adenoma with basal cell squamous metaplasia, severe epithelial atypical hyperplasia and tumor budding. No tumor tissue metastases were found in the lymph nodes of either group. Next-generation sequencing (NGS) of all tumor tissues (including ALK, ROS-1, RET, EGFR-18/19/20/21, K-RAS-2, N-RAS-3, BRAF-15, PIK3CA-9/20 and HER2/neu-20) revealed no gene fusion or mutation in the target regions or mutation hotspots of all genes. The patient underwent chest CT scans every six months. Up to 37 months of follow-up, there was no recurrence or metastasis, and there were no significant changes in the multiple nodules in the right lower lung.
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The onset of BA/CMPT is insidious, and patients usually have no obvious clinical symptoms that are mainly detected during physical examination or the diagnosis and treatment of other diseases. We summarized some clinical features of BA/CMPT by comparing our case with 94 patients from the 40 included studies (Table 1).
Table 1
| Author | Age (yr)/sex | Smoking history | Location | CT findings | CT/growth size (mm) | Follow-up (moths) | Treatment | Molecular features | Frozen section diagnosis | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size [range] | Growth | Preoperative | Postoperative | |||||||||
| Present case | 55/M | + | LLL, RLL | Nodules | 3–10 | +0 | 84 | 28 NED | Seg, WR | – | Mucinous adenocarcinoma | |
| Ishikawa (1) | 50/F | + | RUL | Nodule | 15 | N/A | 120 NED | Lob | N/A | N/A | ||
| Harada (4) | 62/M | + | LLL | Nodule | N/A | N/A | 24 NED | PR | N/A | N/A | ||
| Sato (5) | 67/M | + | RUL | GGO | 5 to 9 | +4 | 28 | 10 NED | PR | N/A | Low-grade malignancy | |
| 59/F | N/A | RLL | GGO | 7 | N/A | 18 NED | PR | N/A | Low-grade malignancy | |||
| Hata (6) | 76/F | − | LUL | Nodule | 7 | +0 | 6 | 23 NED | Lob | – | Beyond recognition | |
| Chuang (7) | 68/M | + | RLL | GGN | 7 | +0 | 5 | 48 NED | WR | – | Adenocarcinoma | |
| Kamata (8) | Med: 62/M (n=7), F (n=3) | + (n=5) | RUL (n=1), RLL (n=5), LLL (n=4) | Nodule | Ave: 10 [6–15] | N/A | Ave: 43 NED (2–88) | WR (n=8), Seg (n=1), Lob (n=1) | N/A | CMPT (n=4), atypical glandular lesion (n=2), muconodular tumor (n=1), metaplastic lesion (n=1), mucinous carcinoma (n=1) | ||
| Lau (9) | 19/F | − | RLL | Nodule | 12 | +0 | 9 | N/A | WR | – | Mucinous neoplasm | |
| Ishikawa (10) | 66/M | + | RUL | Nodule | 13 | N/A | 58 NED | Lob | N/A | Mucinous cystic neoplasm | ||
| 82/F | − | LLL | Nodule | 10 | N/A | 55 NED | PR | N/A | No malignancy | |||
| 77/M | + | LLL | Nodule | 45 | N/A | 48 NED | Lob | N/A | Adenocarcinoma | |||
| 70/M | + | RLL | GGO | 35 | N/A | 19 NED | PR | N/A | CMPT | |||
| 67/F | − | RLL | Nodule | 5 | N/A | 28 NED | PR | N/A | No malignancy | |||
| Kon (11) | 80/M | N/A | LLL | Nodule | 7 | N/A | 29 NED | WR | N/A | N/A | ||
| 67/M | N/A | RLL | Nodule | 10 | N/A | 25 NED | WR | N/A | N/A | |||
| 66/M | N/A | RLL | Nodule | 13 | N/A | 14 NED | Lob | N/A | N/A | |||
| 73/F | N/A | LUL | Nodule | 9 | N/A | 5 NED | WR | N/A | N/A | |||
| 70/F | N/A | RLL | Nodule | 8 | N/A | 48 NED | WR | N/A | N/A | |||
| Liu (12) | 60/M | + | RLL | Nodule | 8 | +4 | 96 | 7 NED | WR | BRAF (n=1), AKT1 (n=1) | N/A | |
| 83/F | N/A | RML | Nodule | 4 | N/A | N/A | Lob | N/A | ||||
| 81/F | N/A | LL | Nodule | 4 | N/A | N/A | WR | N/A | ||||
| 71/F | N/A | LUL | Nodule | 12 | N/A | 120 NED | WR | N/A | ||||
| Taguchi (13) | 84/F | − | RLL | Nodule | 8 | N/A | 10 NED | PR | ALK | N/A | ||
| Jin (14) | 59/F | − | RLL | Nodule | N/A | N/A | 6 NED | Lob | ALK | Atypical glandular lesion | ||
| Chu (15) | 56/M | N/A | LUL | Nodule | 11 | N/A | 5 NED | Seg | N/A | Mucinous adenocarcinoma | ||
| Matsuoka (16) | 76/F | − | RLL | Nodule | 6 to 10 | +4 | 9 | 24 NED | WR | N/A | Mucinous adenocarcinoma | |
| Udo (17) | Med: 67/F (n=4) | − | N/A | N/A | Ave: 11 [8–25] | N/A | N/A | Seg (n=1), Lob (n=3) | BRAF (n=1), KRAS (n=1), ATK1 (n=1) | N/A | ||
| Kim (18) | 73/M | + | LLL | GGN | 9 | +0 | 6 | 36 NED | WR | BRAF | N/A | |
| Kataoka (19) | 58/F | − | N/A | N/A | 11 | N/A | 21 NED | Lob | BRAF | N/A | ||
| 69/F | + | N/A | N/A | 4 | N/A | 51 NED | PR | EGFR | N/A | |||
| 71/M | + | N/A | N/A | 5 | N/A | 17 NED | PR | – | N/A | |||
| 66/M | − | N/A | N/A | 6 | N/A | 36 NED | PR | KRAS EGFR | N/A | |||
| Chang (2) | Med: 72/M (n=11), F (n=10) | + (n=16) | N/A | Nodule | Ave: 6 [2–11] | +0 | 4–14 (n=4) | Ave :11 NED (1–108) (n=12) | WR | BRAF (n=6), KRAS (n=5), EGFR (n=5), HARS (n=1) | CMPT (n=1), IMA (n=1), mucous gland adenoma (n=1), adenocarcinoma (n=6) | |
| +1 | 5 (n=1) | |||||||||||
| +3 | 24 (n=1) | |||||||||||
| Miyai (20) | 67/F | + | RML | Nodule | 18 | N/A | 4 NED | PR | - | N/A | ||
| Kashima (21) | 71/M | N/A | RLL | N/A | 9 | N/A | 6 NED | N/A | - | N/A | ||
| 72/F | N/A | LUL | N/A | 9 | N/A | 12 NED | N/A | - | N/A | |||
| 73/F | N/A | LLL | N/A | 12 | N/A | 72 NED | N/A | BRAF | N/A | |||
| 64/M | N/A | LLL | N/A | 8 | N/A | 60 NED | N/A | BRAF | N/A | |||
| 56/F | N/A | RLL | N/A | 18 | N/A | 24 NED | N/A | BRAF | N/A | |||
| Yao (22) | 67/F | N/A | LUL | Nodule | 12 | +0 | 3 | 10 NED | Seg | N/A | No malignancy | |
| Mikubo (23) | 69/M | − | LLL | Nodule | 13 | N/A | 8 NED | WR | N/A | N/A | ||
| Shao (24) | 58/F | N/A | LLL, RLL | GGN, GGN | 15 | N/A | N/A | WR | KRAS | N/A | ||
| 66/F | N/A | RLL | Nodule | N/A | N/A | N/A | WR | – | N/A | |||
| Shen (25) | 58/M | − | RLL | Nodule | 11 | N/A | N/A | Lob | – | Papillary carcinoma | ||
| 64/F | − | LLL | Nodule | 5 to 8.5 | +3.5 | 144 | N/A | WR | – | Adenocarcinoma | ||
| Tachibana (26) | 77/F | − | RLL | GGN | 8 | +0 | 7 | 6 NED | WR | – | Adenocarcinoma | |
| Sun (27) | 68/F | − | LUL, LLL | Nodules | 65 | N/A | N/A | – | N/A | – | ||
| Liu (28) | 81/F | N/A | LLL | GGN | 6 to 8 | +2 | 14 | 17 NED | Seg | ALK BRAF |
Benign mucinous tumor | |
| Abe (29) | 76/M | + | RLL | Nodule | 14 | N/A | 14 NED | Seg | – | CMPT | ||
| Yang (30) | 30/F | − | LUL | GGO | 5 to 8 | +3 | 8 | 9 NED | Seg | EGFR | BA | |
| Matsushima (31) | 60/M | − | LLL | GGN | 4 to 10 | +6 | 144 | 20 NED | WR | N/A | N/A | |
| Han (32) | 70/M | + | RML | Nodule | 15 | N/A | N/A | Lob | KRAS | IMA | ||
| Chen (33) | 61/M | + | RLL | GGO | 14 | N/A | N/A | Lob | – | N/A | ||
| Patané (34) | 68/F | + | RLL | Nodule | N/A | N/A | N/A | Lob | N/A | N/A | ||
| Zhao (35) | 53/M | + | RLL | No solid mass | N/A | N/A | N/A | – | N/A | – | ||
| Li (36) | 56/F | N/A | LUL | GGN | 6 | +0 | 12 | N/A | WR | CCNE1 | N/A | |
| LLL | GGN | 7 to 8 | +1 | CCNE1 HER2 |
Adenoma | |||||||
| Wang (37) | 79/M | − | RUL | Nodule | 30 | N/A | 24 NED | WR | EGFR | Adenocarcinoma | ||
| Wang (38) | 64/F | N/A | RLL | Nodule | 12 | N/A | N/A | WR | – | N/A | ||
| Du (39) | 63/F | N/A | RLL | GGN | 6 | N/A | 11 NED | WR | – | N/A | ||
| 58/F | N/A | RUL | Nodule | 8 | N/A | 12 NED | WR | HER2 | N/A | |||
| Maroongroge (40) | 67/F | N/A | LLL | Nodule | 9 to 12 | +3 | 12 | 12 NED | SBRT | N/A | – | |
| Moon (41) | 39/M | N/A | RLL | Nodule | 5 to 10 | +5 | 132 | N/A | Lob | N/A | Mucinous adenocarcinoma | |
BA/CMPT, bronchial adenoma/ciliated muconodular papillary tumor; CT, computed tomography; F, female; M, male; +, positive result; −, negative result; N/A, not available; yr, year; Ave, average; Med, median; GGO, ground-glass opacity; GGN, ground grass nodule; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; Lob, lobectomy; Seg, segmentectomy; WR, wedge resection; PR, partial resection; NED, no evidence of disease; IMA, invasive mucinous adenocarcinoma; SBRT, stereotactic body radiation therapy.
There were 44 males (46.3%) and 51 females (53.7%). Excluding unknown information, the median age was 68 years (range, 19–84 years). Almost all patients were over 50 years old, and only three were younger than 40 years old, the youngest of whom was 19 years old (9). Thirty-nine patients had a history of smoking, and the correlation between BA/CMPT and smoking has not been confirmed (24).
Computed tomography (CT) showed that almost all lesions were of the peripheral type, mainly presenting as irregular nodules, which could be solid, partly solid or ground-glass shadows. Some lesions were accompanied by lobulation, central cavity or pleural depression. There is also a rare case of bilateral lung interstitial inflammation without a solid mass (35). Tumors ranged in diameter from 4 to 65 mm, with most measuring <15 mm. The vast majority are solitary growths, and a few can involve multiple lung segments (24,27,36). CT imaging does not usually provide much help in diagnosis and can easily be mistaken for a malignant tumor based on imaging findings alone. Twenty-three patients were followed up by preoperative imaging for 3–144 months, and half of them showed tumor growth, with growth rates ranging from 0.29 to 5.33 mm/y and a median growth rate of 1.61 mm/y. In addition to changes in tumor size, there were cases of new ground-glass opacities (GGOs) (5,30), central cavities (5,31,41) or ground-glass nodules transformed into solid nodules (31).
In our case, some nodules in the right lung showed radiographic features consistent with BAs in the left lung, such as irregular borders, small cavities and solid components, especially the nodule in Figure 2C. Combined with the tumor growth characteristics, we suspect that multiple nodules in the right lung are also BAs, which may be the first case of multiple BAs in both lungs. Considering the indolent growth of the tumor, we did not perform surgical resection of the multiple nodules in the right lower lung. We will continue to monitor changes in the right lung nodules and verify this speculation. Due to the small number of preoperative imaging follow-up cases, the growth characteristics and imaging changes of BA/CMPT need to be explored.
Pathologically, a continuous basal cell layer, mucinous cells and ciliary layer are critical for diagnosis. Immunohistochemical staining of p40 or p63 can highlight the presence of continuous layers of basal cells (42). In the literature reports we collected, 43 intraoperative pathological results were described, among which eight cases were diagnosed as BA/CMPT, a total of 15 cases including our patient were diagnosed with adenocarcinoma, nine cases were diagnosed with other malignant lesions, ten cases were diagnosed with benign lesions, and 1 case could not be identified. In addition, three cases (27,35,40) were diagnosed by needle biopsy without surgery because of the large lesion range or serious underlying diseases. BA/CMPT can exhibit cancer-like pathological features, which makes the diagnosis of BA/CMPT in intraoperative pathology challenging (15), mainly because some morphological characteristics of BA are difficult to identify in frozen sections. On the one hand, owing to the influence of freezing, some subtle structures of cells (such as cilia) are difficult to show in slices, and it should be noted that some nonclassic/distal types do not have typical cilia. On the other hand, sometimes the basal cells are pressed, or a certain section does not cut to the basal cells, which leads to the misdiagnosis of only a monolayer cell structure under the microscope. The classic/proximal type has significant mucinous characteristics and often needs to be distinguished from invasive mucinous adenocarcinoma (IMA) (2). One study found differences in mucin expression patterns between BA and IMA by elucidating the mucin core expression profile (43). HNF4a also is a useful marker for differentiation between BA and IMA; this marker is negative for BA but positive for IMA (44). In addition, mixed squamous cell and glandular papilloma is a rare benign tumor with similar morphology and cell composition to BA/CMPT, which can be distinguished by the anatomical site of the lesion (42), or the presence or absence of AKT1 mutations (45). Recently, Teng et al. (46) reported 5 cases of BA with squamous metaplasia. Interestingly, none of the cases had mucinous cells, and sheet-like spindle-oval cells with squamous metaplasia was more easily misdiagnosed as pulmonary sclerosing pulmonary (PSP), the expression level of TTF-1 is the key to distinguish between them.
The S6 nodules in our case showed obvious basal layer hyperplasia, while some basal cells underwent squamous metaplasia, accompanied by severe squamous epithelial hyperplasia and tumor budding. Tumor budding is a relatively newly recognized pathological pattern of tumor invasion defined as discrete clusters of tumor cells at the front of tumor invasion, and each cluster consists of 1–4 tumor cells (47), which is highly correlated with tumor invasiveness. Some studies have shown that it is an important prognostic factor for patients with solid tumors such as breast cancer (48), pancreatic cancer (49) and rectal cancer (50). In addition, tumor budding has also been identified as an unfavorable prognostic indicator for lung squamous cell carcinoma (51) and lung adenocarcinoma (52); however, an effective grading system has not been established for the clinical diagnosis of lung cancer. P53 immunohistochemistry is helpful in the diagnosis of squamous cell carcinoma, but our results did’t provide strong evidence. Due to the low malignant degree of the tumor, we strengthened follow-up as the main measure after surgery. Smoking appears to be a major risk factor for malignant transformation; thus, we believe that clinicians should focus on this group of patients with a history of smoking.
In terms of genetics, some common driver gene mutations in lung cancer, such as EGFR, KRAS, BRAF, ALK and AKT1 mutations, have been found in CMPT/BA, among which BRAF V600E is the most common. These genetic alterations suggest that CMPT is a neoplastic disease rather than a reactive or metaplastic lesion (8); however, its association with malignancy remains unclear (2). We examined multiple common loci but found no fusion or mutation in all lesions.
To date, no consensus has been reached on the benign and malignant characteristics of CMPT/BA, but an increasing number of cases of malignant transformation have been reported in recent years to reveal its characteristics. Miyai et al. (20) reported a case of CMPT with malignant transformation of basal tumor cells. Li et al. (36) reported a case of multiple BAs with adenocarcinoma cancerization in a local area. Zhao et al. (35) reported a case of a CMPT accompanied by adenocarcinoma in situ. Other researchers believe that CMPT is a precancerous lesion of mucinous adenocarcinoma. Lau et al. (9) proposed that CMPT is a precursor of mucinous adenocarcinoma based on the multistage pathogenesis of lung adenocarcinoma. Udo et al. (17) found two cases of CMPT with diffuse and strong staining for HNF-4α, which is similar to mucinous adenocarcinoma. Chen et al. (33) reported a case of mucinous adenocarcinoma caused by cancerization of the CMPT with discontinuous or absent basal cells in some areas. Han et al. (32) reported a case of malignant transformation from BA to invasive mucinous adenocarcinoma (IMA), both with the same KRAS mutation and the discontinuation of the basal cell layer in the junctional zone. Our case reports the potential capacity of BA/CMPT to malignant transformation into lung squamous cell carcinoma, which advances the understanding of the nature of BA/CMPT. Due to the limited reports on BA/CMPT, the potential relationship between BA/CMPT and malignant transformation requires further investigation.
Given the low malignancy of BA/CMPT, which mostly occurs in the peripheral lung, wedge resection is generally considered sufficient, while excessive resection and systematic lymph node sampling are not necessary. The number and location of the nodules also affect the choice of surgical procedure, which is why we performed S9+10 segmentectomy. When BA/CMPT cannot be definitively diagnosed using frozen pathology, standard surgery for lung cancer is still required (6,8,10). For patients who cannot tolerate surgery, stereotactic body radiation therapy (SBRT) (40) has been reported to be chosen for treatment. However, as the study has shown, this treatment strategy cannot exclude the possibility of coexisting mucinous adenocarcinoma through core needle biopsy alone. Regardless of the surgical method, there has been no recurrence or metastasis after treatment (4–120 months) in any of the cases reported thus far. There is currently no recommended duration of follow-up, but 5 years appears to be safe enough for patients with complete resection. Furthermore, for cases of bilateral multiple nodules in which one side has been clearly diagnosed as BAs, clinicians should be aware of the possibility of multiple BAs in both lungs and choose an appropriate treatment method.
Conclusions
In conclusion, we report a rare case of multiple BAs/CMPTs with tumor budding and presumably the first case of multiple BAs/CMPTs in both lungs. Simultaneously, BA/CMPT was found to undergo potential malignant transformation into squamous cell carcinoma. Smoking appears to be the main risk factor, and further studies are needed to clarify the association between the two.
Questions to be further discussed and considered
- Mutations have been found in many cases of BA/CMPT. The discovery of a new mutation site seems to be a significant discovery. However, given the risk of the disease itself and the fact that testing the gene has not been helpful in treatment, is additional genetic testing generally necessary for BA/CMPT?
- For our case, can multiple nodules in the right lung be considered BAs/CMPTs, and what is the recommended follow-up interval?
- What are the current treatment options for BA/CMPT, and which is the most recommended?
Acknowledgments
Funding: This work was funded by the Ningbo Health Branding Subject Fund (grant No. PPXK2018-05); the Ningbo Natural Science Foundation (grant No. 2023J316); the Zhejiang Provincial Natural Science Foundation of China (grant No. LHDMD23H160001); the Science and Technology Innovation Guidance Fund Project of Hangzhou Medical College (grant No. CX2022006); the Medical Scientific Research Foundation of Zhejiang Province (grant No. 2021KY1009 and 2022KY1138); the Ningbo Top Medical and Health Research Program (No. 2022030208); the HwaMei Research Foundation of Ningbo No.2 Hospital (grant No. 2022HMKY37).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-374/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-374/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-374/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ishikawa Y. Ciliated muconodular papillary tumor of the peripheral lung: benign or malignant. Pathol Clin Med 2002;20:964-5.
- Chang JC, Montecalvo J, Borsu L, et al. Bronchiolar Adenoma: Expansion of the Concept of Ciliated Muconodular Papillary Tumors With Proposal for Revised Terminology Based on Morphologic, Immunophenotypic, and Genomic Analysis of 25 Cases. Am J Surg Pathol 2018;42:1010-26. [Crossref] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO classification of tumours Thoracic Tumours 5th ed Lyon: IARC Press; 2021.
- Harada T, Akiyama Y, Ogasawara H, et al. Ciliated muconodular papillary tumor of the peripheral lung: A newly defined rare tumor. Respiratory Medicine CME 2008;1:176-8. [Crossref]
- Sato S, Koike T, Homma K, et al. Ciliated muconodular papillary tumour of the lung: a newly defined low-grade malignant tumour. Interact Cardiovasc Thorac Surg 2010;11:685-7. [Crossref] [PubMed]
- Hata Y, Yuasa R, Sato F, et al. Ciliated muconodular papillary tumor of the lung: a newly defined low-grade malignant tumor with CT findings reminiscent of adenocarcinoma. Jpn J Clin Oncol 2013;43:205-7. [Crossref] [PubMed]
- Chuang HW, Liao JB, Chang HC, et al. Ciliated muconodular papillary tumor of the lung: a newly defined peripheral pulmonary tumor with conspicuous mucin pool mimicking colloid adenocarcinoma: a case report and review of literature. Pathol Int 2014;64:352-7. [Crossref] [PubMed]
- Kamata T, Yoshida A, Kosuge T, et al. Ciliated muconodular papillary tumors of the lung: a clinicopathologic analysis of 10 cases. Am J Surg Pathol 2015;39:753-60. [Crossref] [PubMed]
- Lau KW, Aubry MC, Tan GS, et al. Ciliated muconodular papillary tumor: a solitary peripheral lung nodule in a teenage girl. Hum Pathol 2016;49:22-6. [Crossref] [PubMed]
- Ishikawa M, Sumitomo S, Imamura N, et al. Ciliated muconodular papillary tumor of the lung: report of five cases. J Surg Case Rep 2016;2016:rjw144. [Crossref] [PubMed]
- Kon T, Baba Y, Fukai I, et al. Ciliated muconodular papillary tumor of the lung: A report of five cases. Pathol Int 2016;66:633-9. [Crossref] [PubMed]
- Liu L, Aesif SW, Kipp BR, et al. Ciliated Muconodular Papillary Tumors of the Lung Can Occur in Western Patients and Show Mutations in BRAF and AKT1. Am J Surg Pathol 2016;40:1631-6. [Crossref] [PubMed]
- Taguchi R, Higuchi K, Sudo M, et al. A case of anaplastic lymphoma kinase (ALK)-positive ciliated muconodular papillary tumor (CMPT) of the lung. Pathol Int 2017;67:99-104. [Crossref] [PubMed]
- Jin Y, Shen X, Shen L, et al. Ciliated muconodular papillary tumor of the lung harboring ALK gene rearrangement: Case report and review of the literature. Pathol Int 2017;67:171-5. [Crossref] [PubMed]
- Chu HH, Park SY, Cha EJ. Ciliated muconodular papillary tumor of the lung: The risk of false-positive diagnosis in frozen section. Human Pathology: Case Reports 2017;7:8-10.
- Matsuoka S, Kondo R, Ishii K. Differential diagnosis of a rare papillary tumor and mucinous adenocarcinoma. Asian Cardiovasc Thorac Ann 2017;25:391-4. [Crossref] [PubMed]
- Udo E, Furusato B, Sakai K, et al. Ciliated muconodular papillary tumors of the lung with KRAS/BRAF/AKT1 mutation. Diagn Pathol 2017;12:62. [Crossref] [PubMed]
- Kim L, Kim YS, Lee JS, et al. Ciliated muconodular papillary tumor of the lung harboring BRAF V600E mutation and p16(INK4a) overexpression without proliferative activity may represent an example of oncogene-induced senescence. J Thorac Dis 2017;9:E1039-44. [Crossref] [PubMed]
- Kataoka T, Okudela K, Matsumura M, et al. A molecular pathological study of four cases of ciliated muconodular papillary tumors of the lung. Pathol Int 2018;68:353-8. [Crossref] [PubMed]
- Miyai K, Takeo H, Nakayama T, et al. Invasive form of ciliated muconodular papillary tumor of the lung: A case report and review of the literature. Pathol Int 2018;68:530-5. [Crossref] [PubMed]
- Kashima J, Hishima T, Tonooka A, et al. Genetic and immunohistochemical analyses of ciliated muconodular papillary tumors of the lung: A report of five cases. SAGE Open Med Case Rep 2019;7:2050313X19830483.
- Yao X, Gong Y, Zhou J, et al. A surgical case of ciliated muconodular papillary tumor. Thorac Cancer 2019;10:1019-22. [Crossref] [PubMed]
- Mikubo M, Maruyama R, Kakinuma H, et al. Ciliated muconodular papillary tumors of the lung: Cytologic features and diagnostic pitfalls in intraoperative examinations. Diagn Cytopathol 2019;47:716-9. [Crossref] [PubMed]
- Shao K, Wang Y, Xue Q, et al. Clinicopathological features and prognosis of ciliated muconodular papillary tumor. J Cardiothorac Surg 2019;14:143. [Crossref] [PubMed]
- Shen L, Lin J, Ren Z, et al. Ciliated muconodular papillary tumor of the lung: report of two cases and review of the literature. J Surg Case Rep 2019;2019:rjz247. [Crossref] [PubMed]
- Tachibana M, Saito M, Kobayashi J, et al. Distal-type bronchiolar adenoma of the lung expressing p16(INK4a) - morphologic, immunohistochemical, ultrastructural and genomic analysis - report of a case and review of the literature. Pathol Int 2020;70:179-85. [Crossref] [PubMed]
- Sun Y, Liu M, Jiang Z, et al. Bronchiolar adenoma with diffuse pulmonary nodules: a extremely rare case report and review of literature. BMC Pulm Med 2020;20:192. [Crossref] [PubMed]
- Liu S, Liu N, Xiao M, et al. First case of bronchiolar adenoma lined purely by mucinous luminal cells with molecular analysis: A case report. Medicine (Baltimore) 2020;99:e22322. [Crossref] [PubMed]
- Abe M, Osoegawa A, Miyawaki M, et al. Ciliated muconodular papillary tumor of the lung: a case report and literature review. Gen Thorac Cardiovasc Surg 2020;68:1344-9. [Crossref] [PubMed]
- Yang C, Wang X, Da J, et al. Distal-type bronchiolar adenoma of the lung harboring an EGFR exon 21 p.L858R mutation: A case report. Thorac Cancer 2020;11:3596-8. [Crossref] [PubMed]
- Matsushima R, Mori T, Saeki S, et al. Long-term follow-up of ciliated muconodular papillary tumor of the lung by computed tomography: a case report. J Surg Case Rep 2020;2020:rjaa522. [Crossref] [PubMed]
- Han X, Hao J, Ding S, et al. Bronchiolar Adenoma Transforming to Invasive Mucinous Adenocarcinoma: A Case Report. Onco Targets Ther 2021;14:2241-6. [Crossref] [PubMed]
- Chen F, Ren F, Zhao H, et al. Mucinous adenocarcinoma caused by cancerization from a ciliated multinodular papilloma tumor: A case report. Thorac Cancer 2021;12:1629-33. [Crossref] [PubMed]
- Patané AK, Poleri C, Martín C. Ciliated muconodular papillary tumor and thymoma: unusual presentation for two types of rare tumors: a case report. Mediastinum 2021;5:18. [Crossref] [PubMed]
- Zhao L, Willson CM, Givens NT, et al. A rare case of ciliated muconodular papillary tumor accompanied with adenocarcinoma in situ. BMC Pulm Med 2021;21:223. [Crossref] [PubMed]
- Li X, Wu Y, Hui D, et al. Multiple bronchiolar adenomas with malignant transformation and CCNE1 mutation: a case report and literature review. J Cardiothorac Surg 2021;16:307. [Crossref] [PubMed]
- Wang F, Shen MH, Cao D, et al. Malignant Ciliated Muconodular Papillary Tumors of the Lung: A Case Report. Int J Surg Pathol 2021;29:520-3. [Crossref] [PubMed]
- Wang Y, Wang D, Wang J, et al. Primary ciliated muconodular papillary tumor: A rare pulmonary disease and literature review of 65 cases. Thorac Cancer 2021;12:1917-22. [Crossref] [PubMed]
- Du Y, Wang ZY, Zheng Z, et al. Bronchiolar adenoma with unusual presentation: Two case reports. World J Clin Cases 2022;10:4541-9. [Crossref] [PubMed]
- Maroongroge S, Weissferdt A, Buszek S, et al. Management of Bronchial Adenoma/Ciliated Muconodular Papillary Tumor with Definitive Stereotactic Body Radiation Therapy (SBRT): A Case Report. Clin Lung Cancer 2022;23:e335-8. [Crossref] [PubMed]
- Moon J, You S, Sun JS, et al. Ciliated muconodular papillary tumor of the lung with cavitary change: A case report with 11-year preoperative follow-up. Thorac Cancer 2022;13:1866-9. [Crossref] [PubMed]
- Shirsat H, Zhou F, Chang JC, et al. Bronchiolar Adenoma/Pulmonary Ciliated Muconodular Papillary Tumor. Am J Clin Pathol 2021;155:832-44. [Crossref] [PubMed]
- Kishikawa S, Hayashi T, Takamochi K, et al. Comprehensive clinicopathological characteristics and mucin core protein expression profiles of bronchiolar adenoma. Histopathology 2023;82:264-75. [Crossref] [PubMed]
- Sasaki E, Masago K, Kogure Y, et al. Mucous Gland Adenoma of the Lung: A Neoplastic Counterpart of Mucinous Bronchial Glands. Mod Pathol 2023; Epub ahead of print. [Crossref] [PubMed]
- Sasaki E, Masago K, Fujita S, et al. AKT1 Mutations in Peripheral Bronchiolar Papilloma: Glandular Papilloma and Mixed Squamous Cell and Glandular Papilloma Is Distinct From Bronchiolar Adenoma. Am J Surg Pathol 2021;45:119-26. [Crossref] [PubMed]
- Teng X, Chen Z, Zhang L, et al. Bronchiolar adenoma with squamous metaplasia: a distinct phenotype. Histopathology 2023; Epub ahead of print. [Crossref] [PubMed]
- Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 2017;30:1299-311. [Crossref] [PubMed]
- Salhia B, Trippel M, Pfaltz K, et al. High tumor budding stratifies breast cancer with metastatic properties. Breast Cancer Res Treat 2015;150:363-71. [Crossref] [PubMed]
- Karamitopoulou E, Zlobec I, Born D, et al. Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur J Cancer 2013;49:1032-9. [Crossref] [PubMed]
- Ueno H, Murphy J, Jass JR, et al. Tumour 'budding' as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002;40:127-32. [Crossref] [PubMed]
- Masuda R, Kijima H, Imamura N, et al. Tumor budding is a significant indicator of a poor prognosis in lung squamous cell carcinoma patients. Mol Med Rep 2012;6:937-43. [Crossref] [PubMed]
- Kadota K, Yeh YC, Villena-Vargas J, et al. Tumor Budding Correlates With the Protumor Immune Microenvironment and Is an Independent Prognostic Factor for Recurrence of Stage I Lung Adenocarcinoma. Chest 2015;148:711-21. [Crossref] [PubMed]


