
A protocol of a single arm, prospective, open-label, multicenter, phase II study of ramucirumab and erlotinib in treatment-naïve non-small cell lung cancer patients with EGFR mutation and brain metastases (SPIRAL-BRAIN study)
Introduction
Epidermal growth factor receptor (EGFR) gene mutation has been known to be closely involved in the onset, growth and metastases of non-small cell lung cancer (NSCLC). Discovery of EGFR mutation and development of tyrosine kinase inhibitors (TKIs) for EGFR marked the beginning of molecularly targeted treatment in patients with NSCLC (1), and a 3rd-generation EGFR-TKI (osimertinib) is now available on the market.
In the FLAURA study, a phase III study compared osimertinib with first-generation EGFR-TKIs in advanced, previously untreated NSCLC patients with EGFR mutation, exon 19 deletion (ex 19del) or exon 21 Leu858Arg mutation (L858R), both progression-free survival (PFS) and overall survival (OS) were significantly better for the osimertinib arm [18.9 vs. 10.2 months; hazard ratio (HR), 0.46; 95% confidence interval (CI): 0.37 to 0.57; P<0.001 and 38.6 vs. 31.8 months; HR, 0.80; 95% CI: 0.64 to 1.00; P=0.046, respectively] (2,3).
Combination treatment with EGFR-TKI and vascular endothelial growth factor (VEGF) or VEGF-receptor (VEGFR) inhibitor is another treatment option for NSCLC patients with EGFR mutation. Angiogenesis, the process leading to the formation of new blood vessels, is one of the hallmarks of cancer. The study established that VEGF, a key driver of sprouting angiogenesis, is overexpressed in NSCLC and inhibition of VEGF can suppress tumor growth (4).
Recently, in the RELAY study, combination with ramucirumab, a human monoclonal IgG1 antibody selectively targeting VEGFR2, and erlotinib achieved significantly better PFS compared with erlotinib alone (19.4 vs. 12.4 months; HR, 0.59; 95% CI: 0.46 to 0.76; P<0.0001) (5). This study indicates that dual blockade of the EGFR and VEGFR pathways in EGFR mutation-positive NSCLC has demonstrated improved tumor control compared with EGFR inhibition alone.
Osimertinib shows excellent efficacy for EGFR-mutated NSCLC; however, a subgroup analysis of the FLAURA study revealed that there was no significant OS difference in patients with L858R mutation, while those with ex 19del derived significant OS benefit from osimertinib (3). A following observational study also suggested that the efficacy of osimertinib for L858R mutation might be inferior to that for ex 19del (6). On the other hand, in RELAY study, the benefit was similar between patients with L858R and ex 19del, although OS data is not yet mature to date (5). Therefore, ramucirumab plus erlotinib is a promising treatment option regardless of ex 19del or L858R mutation status.
TP53 is common co-mutation with EGFR mutation in NSCLC (7) and the p53 family members regulates VEGF expression (8). Previous study demonstrates that the existence of TP53 co-mutation is negative prognostic factor for treatment of osimertinib in 2nd line or later line settings (9), whereas TP53 co-mutation leads to more additional efficacy of ramucirumab to erlotinib in RELAY study subgroup analysis (10,11).
Central nervous system (CNS) metastases are a common poor prognostic factor in patients with advanced NSCLC with EGFR mutations (occurring in approximately 30% of patients during treatment with an EGFR-TKI) (12). Osimertinib has shown favorable CNS penetration and FLAURA study also revealed the high CNS efficacy of osimertinib. Fewer patients in the osimertinib arm developed new brain lesions compared with the control arm (12% vs. 30%), supporting the protective role of osimertinib in the development of new CNS lesions (13). There also have been several reports that bevacizumab may improve the efficacy of EGFR-TKI in NSCLC patients with EGFR mutation and brain metastases (14,15). In a retrospective study, for example, combination of EGFR-TKI and bevacizumab demonstrated significantly better intracranial overall response rate (iORR) (66.1% vs. 41.6%, P=0.001), PFS (14.4 vs. 9.0 months; P<0.001), and OS (29.6 vs. 21.7 months; P<0.001) compared with EGFR-TKI alone (14). However, no data have been available for the combination with ramucirumab and erlotinib because the RELAY study excluded patients with brain metastases (5).
Thus, we aim to investigate prospectively the efficacy and the safety of erlotinib plus ramucirumab as the first-line therapy for advanced or recurrent EGFR-mutated NSCLC patients with brain metastasis. We present this article in accordance with the SPIRIT reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-109/rc).
Methods
Study design
This study is a single arm, prospective, open-label, multicenter, phase II study. The present study has begun in July 2022 and is currently in progress. The schema of this study is shown in Figure 1. Participating institutions are following: Kyoto Prefectural University of Medicine (Kyoto, Japan), Uji-Tokushukai Medical Center (Kyoto, Japan), Japanese Red Cross Kyoto Daini Hospital (Kyoto, Japan), North Medical Center Kyoto Prefectural University of Medicine (Kyoto, Japan), Izumi City General Hospital (Osaka, Japan), Saiseikai Suita Hospital (Osaka, Japan), Japan Community Health care Organization Kobe Central Hospital (Hyogo, Japan), Iizuka Hospital (Fukuoka, Japan), Asahi General Hospital (Chiba, Japan), Nagasaki University Hospital (Nagasaki, Japan), Omi Medical Center (Shiga, Japan), University of Fukui Hospital (Fukui, Japan), Hyogo Medical University Hospital (Hyogo, Japan), Teikyo University Hospital (Tokyo, Japan), Fujita Health University Hospital (Aichi, Japan), Okinawa National Hospital (Okinawa, Japan), Kobe Minimally Invasive Cancer Center (Hyogo, Japan), Kyoto Yamashiro General Medical Center (Kyoto, Japan), Shonan-Fujisawa Tokushukai Hospital (Kanagawa, Japan), Kanazawa Medical Center (Ishikawa, Japan), Fukuchiyama City Hospital (Kyoto, Japan), Rakuwakai Otowa Hospital (Kyoto, Japan), Kanazawa University Hospital (Ishikawa, Japan), Kitakyushu Municipal Medical Center (Fukuoka, Japan), Himeji Medical Center (Hyogo, Japan), Otsu City Hospital (Shiga, Japan), Osaka Metropolitan University Hospital (Osaka, Japan), and Japanese Red Cross Kyoto Daiichi Hospital (Kyoto, Japan). The ethical committees of the Clinical Research Review Board of Kyoto Prefectural University of Medicine (No. 2022001-3) approved the study protocol and informed consent documents. The other participating hospitals were informed about the study and provided their agreement. All patients will be required to provide informed consent. The study will be conducted in compliance with the provisions of the Declaration of Helsinki (as revised in 2013).
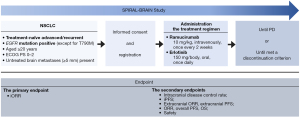
Eligibility criteria
The inclusion and exclusion criteria of this study are shown in Table S1.
Dose and treatment regimen
Erlotinib at a dose of 150 mg will be administered orally once daily and ramucirumab at a dose of 10 mg/kg intravenously once every 2weeks. The treatment regimen will be continued until disease progression or until a discontinuation criterion (Table S2) is met. We also set a discontinuation criterion for ramucirumab. Even after discontinuation of ramucirumab, treatment with erlotinib may be continued at the discretion of the clinical investigator. If treatment with erlotinib has been discontinued, the protocol treatment will be discontinued.
Statistical analysis
This trial is based on the following clinical hypothesis: the combined ramucirumab plus erlotinib therapy is safe and effective for treatment-naïve advanced/recurrent NSCLC patients with EGFR mutation and brain metastases. Therefore, the primary endpoint of this study is iORR. Secondary endpoints are intracranial disease control rate, intracranial progression-free survival (iPFS), extracranial ORR, extracranial PFS, ORR, overall PFS, OS, and safety. The efficacy of this therapy is judged as positive if the lower bound of the 95% confidence interval for the iORR exceeds 40%. The threshold level of 40% was set based on the data from the FLAURA study, in which iORR for the first-generation EGFR-TKI arm was 40% [95% confidence interval (CI): 41–81%] in all patients with brain metastases (n=67) and 63% (95% CI: 41–81%) in the patients having a 10-mm or larger brain metastases (n=19). The expected iORR was set to be 65% because iORR for the osimertinib arm was 57% (95% CI: 45–69%) in all patients with brain metastases (n=61) and 77% (95% CI: 57–90%) in the patients having a 10-mm or larger brain metastases (n=22). The number of subjects needed for a valid study with the use of one-sample binomial test (normal approximation) is 30 under the assumption of the threshold level 40%, the expected level 65%, the significance level 5% (two-tailed) and the detective power 80%. With possible dropout before the start of treatment taken into consideration, the planned number of patients to be registered was thus set as 32.
Discussion
Ramucirumab plus erlotinib is one of the treatment options for previously untreated NSCLC patients with EGFR mutation, and the efficacy of which is independent with EGFR subtypes, L858R or ex 19del. Considering that osimertinib might be less effective for patients with L858R subtype, ramucirumab plus erlotinib should be a viable alternative for this population; however, efficacy of the combination treatment is still unclear for patients with brain metastases. We believe that our study will pave the way for developing the new treatment option for these patients.
Acknowledgments
Funding: This work was supported by “Externally sponsored scientific research” in Eli Lilly and Company to Koichi Takayama.
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-109/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-109/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-109/coif). KT reports honoraria (lecture fee) and research funds from Chugai-Roche and Eli Lilly and Company that are outside of the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol and informed consent documents were approved by the ethical committees of Clinical Research Review Board of Kyoto Prefectural University of Medicine (No. 2022001-3). The other hospitals were informed and agreed with the study. Informed consent will be obtained from all patients. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Le X, Nilsson M, Goldman J, et al. Dual EGFR-VEGF Pathway Inhibition: A Promising Strategy for Patients With EGFR-Mutant NSCLC. J Thorac Oncol 2021;16:205-15. [Crossref] [PubMed]
- Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:1655-69. [Crossref] [PubMed]
- Yoshimura A, Yamada T, Okuma Y, et al. Impact of tumor programmed death ligand-1 expression on osimertinib efficacy in untreated EGFR-mutated advanced non-small cell lung cancer: a prospective observational study. Transl Lung Cancer Res 2021;10:3582-93. [Crossref] [PubMed]
- Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer 2019;19:495-509. [Crossref] [PubMed]
- Farhang Ghahremani M, Goossens S, Haigh JJ. The p53 family and VEGF regulation: "It's complicated". Cell Cycle 2013;12:1331-2. [Crossref] [PubMed]
- Roeper J, Christopoulos P, Falk M, et al. TP53 co-mutations as an independent prognostic factor in 2nd and further line therapy-EGFR mutated non-small cell lung cancer IV patients treated with osimertinib. Transl Lung Cancer Res 2022;11:4-13. [Crossref] [PubMed]
- Nakagawa K, Nadal E, Garon EB, et al. RELAY Subgroup Analyses by EGFR Ex19del and Ex21L858R Mutations for Ramucirumab Plus Erlotinib in Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res 2021;27:5258-71. [Crossref] [PubMed]
- Nishio M, Paz-Ares L, Reck M, et al. RELAY, Ramucirumab Plus Erlotinib (RAM+ERL) in Untreated Metastatic EGFR-Mutant NSCLC (EGFR+ NSCLC): Association Between TP53 Status and Clinical Outcome. Clin Lung Cancer 2023;24:415-28. [Crossref] [PubMed]
- Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cel lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873-82. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018; Epub ahead of print. [Crossref]
- Jiang T, Zhang Y, Li X, et al. EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs alone in patients with EGFR-mutant NSCLC and multiple brain metastases. Eur J Cancer 2019;121:98-108. [Crossref] [PubMed]
- Chen F, Chen N, Yu Y, et al. Efficacy and Safety of Epidermal Growth Factor Receptor (EGFR) Inhibitors Plus Antiangiogenic Agents as First-Line Treatments for Patients With Advanced EGFR-Mutated Non-small Cell Lung Cancer: A Meta-Analysis. Front Oncol 2020;10:904. [Crossref] [PubMed]


