
International expert consensus on diagnosis and treatment of lung cancer complicated by chronic obstructive pulmonary disease
Highlight box
Key findings
• Lung cancer combined by chronic obstructive pulmonary disease (LC-COPD) should be treated for both lung cancer and COPD simultaneously, taking into account their interplay on treatment and monitoring for adverse reactions.
What is known and what is new?
• LC-COPD is a common comorbidity, but there is no well-established consensus on LC-COPD.
• This consensus reports recent advances in LC-COPD, summarizing the common risk factors and mechanisms, screening methods, treatment principles, and detailed treatment strategies, with a particular focus on the mutual influence of lung cancer and COPD on each other’s treatment.
What is the implication, and what should change now?
• The expert panel agrees that special attention should be paid to individuals with LC-COPD, and that both conditions should be treated simultaneously.
Introduction
Lung cancer and chronic obstructive pulmonary disease (COPD) are two major public health problems and concerns. Lung cancer is the cancer with the highest mortality and resulted in 1.8 million deaths worldwide in 2020, accounting for 18% of all cancer deaths (1). According to recent epidemiological survey (2), COPD is already the third leading cause of death worldwide, and its prevalence will continue to rise. It is estimated that more than 5.4 million people will die from COPD by 2060 (3). Most people who develop lung cancer are old and have a history of tobacco smoking, and approximately 40–70% of patients with lung cancer also have COPD (4). Additional information on COPD complication along with lung cancer has been added to the 2021 Global Initiative for Obstructive Lung Disease (GOLD) guidelines. The diagnosis and treatment of lung cancer with COPD (LC-COPD) have attracted extensive attention in the medical field.
As early as 1975, COPD was proposed as a risk factor for lung cancer. Patients with COPD are 3 to 6 times more likely to develop lung cancer than people with normal lung function (5). Approximately 0.8–2.7% of patients with COPD develop lung cancer each year (6), and this association may not be related to smoking (7). COPD has been reported to be an independent risk factor for lung cancer incidence in never smokers (8). Annual COPD death rates are rising in patients with cancer, and this trend is more pronounced in those with lung cancer. COPD is the second most common cause of noncancer deaths in patients with lung cancer (9). Conversely, lung cancer is also an important cause of death in patients with COPD, with nearly 40% of patients with COPD dying within 1 year of being diagnosed with lung cancer. Lung cancer accounts for 33% of all COPD-related deaths (6). Unfortunately, only a small proportion of patients with LC-COPD are properly diagnosed and treated (10): only 7.1% of these patients are accurately and comprehensively diagnosed, and only 28–35% of patients with LC-COPD receive standardized treatment. Studies have confirmed that standardized COPD treatment in conjunction with lung cancer therapy improves the prognosis of patients with LC-COPD (11,12).
COPD can impact the selection of lung cancer treatments and increase the risk of adverse reactions. Studies have indicated that COPD is associated with an increased incidence of postoperative complications and treatment-related adverse reactions in lung cancer patients (13,14). The presence of lung cancer may also overshadow the treatment of COPD, and there may be drug interactions between treatments for these two conditions. The coexistence of lung cancer and COPD can make treatment more complex and challenging. There are not enough data regarding the optimal management and treatment regimens of LC-COPD, and there is no consensus or standardized protocols. Furthermore, there are no well-established diagnosis or treatment guidelines for this condition. Therefore, the Chinese Medical Association Lung Cancer and COPD Groups have developed this consensus document after extensive discussion.
Methods
This consensus was based on the existing high-quality clinical evidence as well as the clinical experience widely recognized by the expert panel. Six experts including Zhou Chengzhi, Zhao Wei, Qin Yinyin, Liang Zhenyu, Li Min and Liu Dan drafted the document. Draft recommendations and strength of the recommendation were submitted to the Preliminary Consensus Expert Panel (PCEP) for consideration and approval with a minimum of 70% agreement for inclusion in the manuscript. The PCEP included experts from oncology, respiratory medicine, radiology, interventional medicine, and thoracic surgery, which was divided into seven groups that discussed and revised the content of each topic. A preliminary consensus has formulated 17 recommendations. The experts who did not participate in the preliminary expert consensus were invited again for further modifications. After repeated revisions, this consensus document was finalized.
The target population is adults (≥18 years of age) with LC-COPD. The target audience of this consensus are clinicians who diagnose and treat patients with LC-COPD in primary, secondary, and tertiary medical institutions, such as oncologists, respiratory specialists, radiation therapists, thoracic surgeons, interventional radiologists, etc. A literature search was conducted the PubMed, EMBASE, Wanfang Data, and China National Knowledge Infrastructure (CNKI) databases for articles published as of March 31, 2023. The keywords used included the following: lung cancer, performance status (PS), comorbidities, complications, adverse events (AEs), chemotherapy, radiotherapy, surgery, interventional therapy, targeted therapy, antiangiogenic therapy, immune checkpoint inhibitors (ICIs), supportive treatment, chronic obstructive pulmonary disease, lung function, antibiotics, inhaled corticosteroids (ICS), long-acting β2-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), and non-invasive ventilation. The levels of evidence and grades of recommendation in this consensus document set based upon the Oxford Centre of Evidence Based Medicine’s Levels of Evidence.
Pathogenic mechanisms
Consensus 1: lung cancer and COPD share common risk factors—smoking, air pollution, occupational dust exposure, and a history of previous lung disease are all risk factors for both lung cancer and COPD (level of evidence: 1a)
Smoking
According to Fang et al. (15), smoking is the most important risk factor for the high incidence of COPD in China. Larger cigarette consumption (pack-years) is associated with a higher risk of COPD. Meanwhile, smoking also significantly increases the risk of lung cancer. A meta-analysis (16) of 19 population-based prospective cohort studies showed that the risk of developing and dying from lung cancer in former smokers was 4.06 times [95% confidence interval (CI): 3.13–5.26] and 4.10 times (95% CI: 3.14–5.36), respectively, than never-smokers, and the risk of developing and dying from lung cancer in current smokers was 13.1 times (95% CI: 9.90–17.30) and 11.5 times (95% CI: 8.21–16.10) the never smokers, respectively. There is also a positive dose–response relationship between cigarette consumption and the risk for developing lung cancer (17).
Air pollution
COPD is related to particulate matter (PM) pollution, exposure to biofuel smoke or secondhand tobacco smoke, and harmful gas pollution (e.g., ozone). For instance, it has been found that an increased PM2.5 (particulate matter <2.5 µm) concentration was significantly associated with an increased prevalence of COPD and a rapid decline in lung function, and the risk of developing COPD was significantly increased when the PM2.5 concentration was >35 µg/m3 (18). A meta-analysis based on more than 25 years of cohort studies showed that PM2.5 exposure was significantly associated with all-cause and specific lung-cancer mortality rates (19). In addition, biofuel smoke has been associated with the development of COPD and lung cancer and disease-related specific mortality (20).
Occupational dust exposure
Occupational exposure to various types of dust, including inorganic dust (e.g., coal dust, silica, and asbestos) and organic dust (e.g., textile dust), may increase the risk of developing COPD (21-23). The higher the exposure dose, concentration, and duration of occupational dusts, the higher the risk of COPD (22). Also, occupational exposures to dust are also strongly associated with the risk of developing and dying from lung cancer. Li et al. (24) reported that the lung cancer mortality rate among coal miners was 1.16 times that of people with no or little exposure to dust (95% CI: 1.03–1.30). For each 100 fiber-years/mL increase in asbestos exposure, the risk of death from lung cancer increased by 1.66-fold (95% CI: 1.53–1.79) (25). Textile dust has also been found to be associated with an increased risk of lung cancer.
Previous lung disease
Chronic bronchitis in patients with COPD is associated with frequent exacerbation [odds ratio (OR): 4.0, 95% CI: 2.7–5.9] and increased mortality [hazard ratio (HR): 2.16; 95% CI: 1.12–4.17] (26). Fan et al. (27) noted that prior chronic bronchitis was associated with an increased risk of lung cancer (HR: 1.50; 95% CI: 1.24–1.81), especially squamous carcinoma (HR: 1.57; 95% CI: 1.19–2.09). A prospective observational study showed that the risk of COPD exacerbation in patients with active asthma was approximately 12.5 times (95% CI: 6.84–22.84) higher than that in nonasthmatic patients (28). Fan et al. (27) found that a history of asthma was associated with an increased risk of small-cell lung cancer (SCLC) (adjusted HR: 2.56; 95% CI: 1.38–4.75).
In addition, a history of prior tuberculosis was significantly associated with the presence of COPD (OR: 3.05, 95% CI: 2.42–3.85) (29), and tuberculosis was a risk factor for developing lung cancer in never-smoking Asian women (OR: 1.31, 95% CI: 1.03–1.66) (30).
Consensus 2: the occurrence and development of COPD and lung cancer are both complex processes involving multiple factors, and may result from the same pathophysiological mechanisms, including but not limited to oxidative stress, chronic inflammation, cellular senescence, telomere shortening, epithelial mesenchymal transition (EMT) genetic susceptibility, and epigenetics (level of evidence: 2a)
Oxidative stress
Inhalation exposure can enhance oxidative stress and cause an oxidative–antioxidant imbalance in the body, resulting in airway and lung tissue injuries, which in turn cause airway epithelial cells reprogramming. The altered innate immunity, mucus over secretion, and cilia dysfunction in the reduced (<2 mm diameter) airway epithelial microenvironment contribute to the onset and progression of COPD (31). Additionally, oxidative stress promotes the occurrence and development of lung cancer by causing DNA damage, inhibiting DNA repair, and promoting cell proliferation (32-35).
Chronic inflammation
Chronic inflammation plays a key role in the pathogenesis of COPD: (I) it can cause the structural damage of the walls of the bronchi and bronchioles and the destruction of the elastic fibers of the lung interstitium; (II) the activated inflammatory cells can induce goblet cell metaplasia and mucus hypersecretion in airway epithelial cells; (III) by triggering the release of macrophage matrix metalloproteinases and neutrophil elastase as well as inactivating α1-antitrypsin, it can cause the destruction of elastin in lung connective tissue, leading to the occurrence of COPD (36); (IV) it can also lead to chronic mitosis, increase DNA damage, and promote bronchoalveolar stem/progenitor cells so as to induce the mutation, proliferation, antiapoptosis, angiogenesis, invasion, and metastasis of tumors, and also the secretion of immunosuppressive factors through inflammatory mediators in the microenvironment, thereby inducing carcinogenesis (37-41).
Cellular senescence
The accumulation of senescent cells such as alveolar epithelial and endothelial cells has been found in the lungs of patients with COPD. Cellular senescence is involved in COPD development through mechanisms such as oxidative stress, telomere shortening, mitochondrial dysfunction, activation of mammalian target of rapamycin (mTOR) signal pathway, reduction in antisenescence compounds, stem cell exhaustion, and DNA repair defects (42). Furthermore, senescent cells secrete a myriad of molecules [collectively called senescence-associated secretory phenotype (SASP)] including inflammatory cytokines and chemokines, thus promoting tumorigenesis (43).
Telomere shortening
A telomere is a region of repetitive DNA sequences at the end of a chromosome. Telomere length has been shown to gradually shorten over time as cells divide. Smoking accelerates age-related telomere shortening, and there is a dose-effect relationship between cumulative tobacco smoke exposure (pack-years) and telomere length, with higher exposures leading to shorter telomere length (44). Telomere protection protein 1 (TPP1) reduction causes telomere attrition and cellular senescence via sirtuin 1 deacetylase in COPD (45). In lung cancer, TPP1 can mediate the telomerase–telomere recruitment pathway, synthesize telomere DNA, maintain telomeres at a relatively stable length, and ensure rapid cell proliferation and cell immortality (46).
Epithelial mesenchymal transition (EMT)
Cigarette smoke induced oxidative stress can promote bronchial epithelial cell EMT through activation of wingless/integrated (Wnt), transforming growth factor β (TGF-β) and other signaling pathways, thus leading to airway remodeling in COPD (47,48). Meanwhile, EMT mediated by TGF-β signaling pathway is also an important process in the occurrence, invasion and metastasis of lung cancer (49,50).
Genetic predisposition
A variety of single-nucleotide polymorphisms (SNPs) associated with COPD and lung cancer have been identified, including SERPINA1 (encoding α1 antitrypsin), matrix metalloproteinase-1(MMP-1), cytochrome P450 subfamily 1 (CYP1A1), epoxide hydrolase 1 (EPHX1), cholinergic receptor, neuronal nicotinic, α-polypeptide 3 (CHRNA3), and CHRNA5 (51). These predisposition genes may be related to the pathogenesis of LC-COPD.
Epigenetics changes
DNA methylation [e.g., coiled-coil domain containing 37 (CCDC37) and microtubule-associated protein 1B (MAP1B)] and non-coding RNA (e.g., miR-21) play important roles in the molecular pathogenesis of both COPD and lung cancer. For example, the miR-21 level is inversely correlated with lung function and is a useful indicator of COPD severity (52). Furthermore, miR-21 also plays an important role in regulating the migration and invasion of NSCLC cells (53).
General strategies
Consensus 3: for patients diagnosed with COPD who have high risk factors for lung cancer, should undergo annually low-dose computed tomography follow-up on the basis of standardized treatment of COPD to allow the early diagnosis of lung cancer should it occur (recommendation category: A; level of evidence: 1a)
The US Preventive Services Task Force (USPSTF) recommends annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 50 to 80 years who have a 20 pack-year history of smoking history and currently smoke or have quit within the past 15 years (54), which can significantly reduce the relative risk (RR) of lung cancer death. The Chinese expert consensus on diagnosis of early lung cancer (2023 Edition) (55) points out that people at high risk of lung cancer are at least 40 to 80 years old and incorporate any of the following risk factors: (I) cumulative smoking index ≥20 pack years; (II) environmental or occupational exposure (radon, silicon, cadmium, arsenic, beryllium, chromium, nickel, asbestos, diesel smoke, soot, radioactive elements); (III) family history of lung cancer in first-degree relatives; (IV) COPD, diffuse pulmonary fibrosis or old pulmonary tuberculosis; (V) previous history of malignant tumor; (VI) long-term inhalation of second-hand smoke (family or indoor workplace, >2 h/d, at least 10 years) or long-term exposure to kitchen oil smoke. Numerous studies have indicated that (56,57) compared to conventional dose CT, LDCT not only reduces the amount of radiation but can also detect tiny lesions. Lung cancer lacks specific symptoms or clinical manifestations in its early stages; LDCT can significantly improve the detection rate of lung nodules, thereby increasing the diagnosis rate of early-stage lung cancer and reducing the case-fatality rate. The risk of developing lung cancer is high (58,59) in people with COPD (manifesting as airflow obstruction or emphysema). Lung cancer screening in the COPD population helps increase the lung cancer diagnosis rate while simultaneously reducing overdiagnosis (60,61). Studies have included this population as a lung cancer screening candidate (58,62) and found that this strategy, when combined with the National Lung Screening Trial (NLST) criteria and the results of emphysema screening, significantly increased the lung cancer detection rate and reduced missed cancer diagnoses. de-Torres et al. (63) explored the impact of screening with LDCT on lung cancer mortality in patients with mild-to-moderate COPD and found that lung cancer incidence and mortality rates were significantly lower in the screening group than in the control group (patients with COPD but not screened for lung cancer). Another study (64) analyzed the prognoses different severities of COPD (mainly moderate-to-severe COPD) and found that 12% of the patients died of lung cancer. However, a secondary analysis of 18463 NLST participants reported that GOLD 3–4 individuals do not benefit from lung cancer screening (65). According to the GOLD 2023 guideline, it is recommended to conduct LDCT lung cancer screening annually for adults aged 50–80 who have a history of smoking 20 packs/year, currently smoking or quit smoking in the past 15 years. However, screening should be stopped for those who have quit smoking for more than 15 years, or have health problems that seriously limit their life expectancy, or have the ability or willingness to perform curative lung surgery. For patients with COPD who never smoke, annual LDCT screening is not recommended, because the potential harm of screening seems to outweigh the potential benefit of finding early lung cancer. Therefore, we recommend annual screening for lung cancer with LDCT in COPD patients with high risk factors so that lung cancer can be diagnosed and treated as early as possible.
Consensus 4: patients diagnosed with lung cancer who have high risk factors for should undergo pulmonary function tests and other relevant examinations as soon as possible to diagnose and treat COPD in time (recommendation category: A; level of evidence: 2a)
The disease course of lung cancer may be related to the timing of the COPD diagnosis and the severity of disease when diagnosed. The “Chinese expert consensus on lung cancer screening and management” (50) defined patients with lung cancer with coexisting COPD as a high-risk lung cancer population and proposed that the presence of COPD is a predictor of poor prognosis in patients with lung cancer. A prospective study conducted by Turner et al. (66) revealed a significant association between lung cancer mortality and emphysema. In one large study (67), patients with COPD were 11 times more likely to develop lung cancer than those without COPD (OR: 11.47, 95% CI: 9.38–14.02). A meta-analysis (68) suggested that the presence of COPD and emphysema are robust predictors of poorer survival in patients with lung cancer, and early disease indications should be considered when monitoring and managing lung cancer. According to Lin et al. (69), COPD staging is valuable in developing clinical treatment plans for patients with LC-COPD, and routine lung function tests and regular follow-up should be carried out in patients with lung cancer to achieve early detection, diagnosis and treatment of COPD.
Therefore, we recommend that patients with lung cancer should undergo pulmonary function tests as soon as possible to rule out the presence of COPD or to achieve an early diagnosis of COPD. Additionally, the changes in lung function should be monitored in patients with lung cancer so as to adjust the treatment plan in a timely fashion, delay the progression of lung cancer, reduce AEs, and improve the prognosis.
Consensus 5: management strategies for the treatments of both lung cancer and COPD should include protocols based on the progression and severity of lung cancer and COPD, individual conditions, and the priorities for interventions (recommendation category: A; level of evidence: 2a)
Smoking (including active and passive smoking) is the primary and most common risk factor for LC-COPD. A study showed that the younger the age of starting smoking, the longer the smoking history and the more smoking, the higher the mortality rate of lung cancer (70). A cohort study with a follow-up of 31 years, showed that among individuals who smoked 15 or more cigarettes a day, a 50% reduction in smoking significantly reduced the risk of lung cancer. The lung function and survival rate of COPD patients improved after 14.5 years of smoking cessation (71). Therefore, smoking cessation is the most effective intervention to prevent the progression of LC-COPD and improve the survival rate (72).
The prognosis of lung cancer worsens with increased COPD severity. Thus, changes in COPD severity should be a concern during antitumor treatment in patients with LC-COPD (73). In a retrospective study by Qin et al. (74), adding antitumor therapy to standardized COPD treatment improved both the quality of life and prognosis in patients with LC-COPD. Wang et al. (11) confirmed that combining COPD treatment with antitumor therapy is better than that of tumor treatment alone in terms of both progression-free survival (PFS) and overall survival (OS). Another study found that there was no significant difference in OS between LC-COPD patients receiving COPD treatment and lung cancer patients without COPD (12). Therefore, greater attention should be paid to the treatment of COPD in patients with LC-COPD. In addition to antitumor therapy, the different COPD grades need to be treated in accordance with GOLD guidelines, and targeted drugs need to be administered in the acute exacerbation and stable phase of COPD, respectively, in order to achieve optimal benefit.
In practice, lung cancer treatment regimens are developed according to differences in the patient Eastern Cooperative Oncology Group (ECOG) PS score, which can be directly affected by lung comorbidities. Some common etiologies and comorbidities [e.g., large airway lesions, pulmonary embolism, idiopathic pulmonary fibrosis (IPF), and acute exacerbation of COPD (AECOPD)] have a serious impact on the PS score and can seriously affect the treatment decision-making and even survival if not treated in time (75). Zhou et al. (76) proposed that patients with LC-COPD may not be able to receive optimal antitumor therapy due to poor lung function and complex comorbidities caused by COPD. In a real-world study conducted in South Korea (77), 113 of 8,014 patients with NSCLC were found have pulmonary emboli (PE); the results showed that the mortality rate was 4.1 times higher in patients who did not receive conventional anticoagulation therapy than in those who did.
Therefore, the control and treatment of comorbidities or complications is critically important for patients with LC-COPD, and lung cancer and its comorbidities represent both the primary and secondary aspects, respectively, of the “contradiction“ in treatment decision-making. In most cases, lung cancer is the primary contradiction; at a specific stage of the disease, however, comorbidities also need to be urgently managed, and thus the primary and secondary contradictions can transform into one another. Clinically, both conditions should be fully considered at any time. The acute or primary contradiction should be the top priority, while the secondary contradiction should also be taken into consideration. The strategy of “treatments for both lung cancer and COPD” may break the vicious circle and maximize clinical benefit.
Consensus 6: lung cancer and COPD are both chronic progressive diseases with heterogeneity. Changes in the pathological type, gene status, immune status, and lung function should be dynamically monitored, whenever possible, in patients with LC-COPD (recommendation category: A; level of evidence: 2a)
Zhou et al. pointed out in the first edition of the International Consensus on Severe Lung Cancer (78) that dynamic and accurate monitoring can provide timely identification of those patients with lung cancer likely to benefit from treatment. The majority of lung cancer display high heterogeneity, and the gene and/or tumor status may change after systemic antineoplastic therapy. Therefore, subsequent treatments based solely on the pathological findings and gene status in the initial specimen may not be appropriate (79,80). Li et al. (81) found that a considerable number of patients die of disease progression or acquired drug resistance. Therefore, it is important to dynamically monitor the pathological type and gene status of the tumor throughout the course of treatments so as to be able to adjust the medications in a timely manner. Meanwhile, tailored therapy guided by circulating tumor cells (CTC) (82) and circulating tumor DNA (ctDNA) monitoring has significant clinical value (83). Noninvasive ctDNA analysis using next-generation sequencing (NGS) can dynamically monitor the clonal evolution of tumors and reveal potential resistance mechanisms. Xu et al. (84) confirmed that serum exosomal miRNAs may be used as novel biomarkers in the liquid biopsy for NSCLC meningeal metastases. Liquid biopsy has the advantages of low invasiveness and good reproducibility; as a complementary means of tissue biopsy, it will make dynamic detection possible and more comprehensive (85-87).
The prognosis of lung cancer has been found to be positively correlated with the severity of COPD (73), so attention needs to be paid to the progression and stages of COPD. The NLST has shown that lung cancer with altered lung function is more aggressive (60). Therefore, changes in lung function should be dynamically monitored during tumor treatment. In the “Guidelines for the diagnosis and management of chronic obstructive pulmonary disease” (2021 revised version) (36), the patients with a forced expiratory volume in 1 second (FEV1) and forced volume vital capacity (FVC) ratio of 60–80% should receive a follow-up test 3 months later to identify whether the FEV1:FVC ratio is still below 70%; follow-up study of the lung function should be carried out 12–16 weeks after discharge.
It is therefore recommended that pathological biopsy, genetic testing and immune status testing should be reperformed as frequently as possible in patients with LC-COPD afflicted with progressive disease during drug treatment so as to identify change in tumor pathological type, gene mutations and immune status to allow adjustment of the medications in a timely manner. Lung ventilation should be assessed before treatment and after every 2 courses of drug therapy (or at least every 3 months). For patients with moderate-to-severe COPD who have abnormal lung compliance and severe irreversible airflow obstruction, a lung diffusion test should also be performed during pulmonary ventilation testing. The combination of these two tests will help inform a rational adjustment of COPD medications.
Consensus on clinical applications
Consensus 7: COPD is stable during lung cancer treatment. Bronchodilator-based inhalation therapy is recommended, which should be regularly assessed and adjusted for individual patients according to clinical symptoms, lung function, risk of acute exacerbation, comorbidities, and peripheral blood eosinophil count. In addition, nonpharmacological treatments (e.g., smoking cessation, avoiding exposure to harmful factors, vaccination, respiratory rehabilitation and oxygen therapy) should also be carried out (recommendation category: A; level of evidence: 1a)
Inhaled medications (ICS, LABA and LAMA)
(I) Drug selection for stable COPD. A large amount of evidence has demonstrated that regular treatment of COPD in patients with LC-COPD on the basis of antitumor therapy can benefit patients in terms of lung function (88-91), quality of life (89,92) and postoperative complications (88,90,91), OS (11,12), and PFS (11,92). One study showed that patients receiving regular treatment for coexisting lung cancer and COPD had similar survival time compared with patients without COPD (12). Therefore, regular inhalation therapy should be initiated once a diagnosis of COPD is confirmed, regardless of the lung cancer stage. Based on the symptom score [or COPD Assessment Test (CAT) score and/or Modified Medical Research Council (mMRC) score], risk of acute exacerbation, lung function, and peripheral blood eosinophil count, single bronchodilator, LABA + LAMA or LABA + LAMA + ICS can be used (36,93,94).
Patients with COPD often have multiple comorbidities that increase the risk of AECOPD, while concomitant lung cancer is associated with a significantly higher risk of exacerbation than any other comorbidities. The risk for developing COPD exacerbation in patients with lung cancer is 1.85 times higher than those without (95), suggesting that patients with LC-COPD may require more aggressive initial treatment (e.g., LABA + LAMA or LABA + LAMA + ICS). Concomitant COPD also contributes to a worse prognosis of lung cancer (96). We know from the NETT trial that patients might benefit from maximum pre-optimization of any medical treatment in very severe forms of emphysema just before surgery: this could be translated in any type of treatment for lung cancer including stereotactic ablative radiotherapy (SABR) or systemic therapy (97,98). Large clinical studies have shown that triple therapy (ICS + LABA + LAMA; e.g., budesonide + glycopyrrolate + formoterol, and fluticasone furoate + vilanterol + umeclidinium) significantly reduces the risk of future exacerbations and all-cause mortality (99,100) in patients with COPD at high risk of acute exacerbation, suggesting triple inhalation therapy has significant potential benefit in patients with LC-COPD, although more evidence is needed.
(II) Selection of inhalation devices. At present, inhalation devices for patients with COPD can be divided into three common types: pressurized metered dose inhalers (pMDIs), soft mist inhalers (SMIs), and dry-powder inhaler (DPIs). The inhaler device selection should be individualized, taking into account the patient’s ability to use the inhaler, peak inhalation flow rate (PIFR), and level of hand-mouth coordination (101). Furthermore, several factors should be considered. Firstly, assess the availability of the drug in the device and the patient’s satisfaction with the device. Minimize the use of multiple device types and avoid unnecessary device switching without proper justification, information, education, and follow-up. Shared decision making is crucial, and the patient’s cognition, dexterity, and strength should be taken into account. If a patient cannot use a specific device, alternatives should be considered. Factors like size, portability, and cost should be taken into account, and smart inhalers may aid adherence and technique. Lastly, physicians should only prescribe devices they are familiar with. More information is available on the website of Aerosol Drug Management Improvement Team (ADMIT) (available at www.inhalers4u.org).
(III) Medication evaluation and adjustment. The long-term follow-up and management processes of “review-assessment-adjustment” for COPD should also be applied in patients with LC-COPD. If the initial treatment is effective, the original treatment regimen can be maintained. Otherwise, the treatment regimen should be tailored to suit the patient’s needs, depending on whether the poor response is defined as “no improvement in dyspnea” or “a high incidence of exacerbations” (36,93,94).
Nonpharmacological treatments
Nonpharmacological treatments for COPD include the following: (I) avoiding exposure to harmful factors (36,102,103), which involves quitting smoking, avoiding exposure to biofuels and fumes, etc.; (II) patient education (36,93,103), including basic knowledge of COPD, the importance of compliance with and methods for the long-term regular inhalation of drugs, requisite skills for relieving breathing problems, timing of visiting the hospital, knowledge about respiratory rehabilitation, management of acute exacerbations, etc.; (III) pulmonary rehabilitation (36,93); (IV) oxygen therapy and respiratory support (103-105); (V) psychological intervention and palliative care (36,103,106); (VI) nutritional support (103,107); (VII) vaccination: influenza vaccine, pneumococcal vaccine, acellular pertussis (Tdap) vaccination; shingles vaccine (over 50 years) (93); (VIII) and others, including airway intervention, and surgical treatment (36,93,103).
Consensus 8: when AECOPD occurs during lung cancer treatment, the triggers should be removed, and based on the assessment of acute exacerbation, appropriate treatment should be given according to the patient’s condition, such as inhalation of short-acting bronchodilators (β2 agonists and/or anticholinergics), followed by the appropriate use of systemic corticosteroids, mucolytics and antibacterials; noninvasive or invasive mechanical ventilation may be applied if necessary (recommendation category: A; level of evidence: 1a)
Diagnosis and differential diagnosis
When a patient with LC-COPD experience acutely worsening respiratory symptoms, such as dyspnea, increased sputum volume, and purulent sputum, a differential diagnosis should be performed first, and then any comorbidities such as tumor progression, pleural effusion, airway obstruction, (obstructive) pneumonia, pulmonary embolism, pneumothorax, heart failure and arrhythmia may then be taken into account. After the above conditions are ruled out, a diagnosis of AECOPD can be made and proper management applied.
Principles of management
The management principle of AECOPD is to remove predisposing factors, minimize the impact of this acute exacerbation, and prevent the occurrence of another acute exacerbation. Depending on the severity of AECOPD and comorbidities (e.g., lung cancer), outpatient or inpatient service may be offered. Patients with mild-to-moderate exacerbations may be treated on an outpatient basis with bronchodilators, mucolytics, glucocorticoids, and/or antimicrobials; hospitalization is required during severely acute exacerbations, and admission to an intensive care unit (ICU) is required as soon as possible if the condition is life-threatening. Inpatients with AECOPD should be given respiratory support such as oxygen therapy, noninvasive mechanical ventilation, and invasive mechanical ventilation as needed, and fluid balance and nutrition should be monitored (36,93).
Use of bronchodilators
Inhaled short-acting β agonists (SABAs; e.g., salbutamol and terbutaline) alone or in combination with short-acting muscarinic-antagonist (SAMAs; e.g., ipratropium bromide) if preferred. Aerosol delivery from a metered-dose inhaler or home nebulizer therapy may be offered in the outpatient or home settings, whereas nebulization is preferred for inpatients. Patients who require mechanical ventilation can be treated with nebulization by connecting a nebulizer to the ventilator, as described in the manufacturer’s instructions. After 12 to 24 hours of treatment with β2 agonists and anticholinergic drugs, the combination of theophylline may be cautiously considered when the condition does not improve well, with monitoring for adverse reactions. Maintenance inhalation therapy with inhaled long-acting bronchodilators or in combination with inhaled corticosteroids can be resumed when the condition stabilizes (36,93).
Selection and treatment course of antibiotics
Antibacterial therapy for AECOPD has the following indications: (I) presence of all 3 major symptoms of increased dyspnea, increased sputum volume and sputum purulence (the so-called Anthonisen type 1 exacerbations); (II) purulent sputum and another major symptom (Anthonisen type 2 exacerbations); and (III) requirement of invasive or noninvasive mechanical ventilation (36,108). The duration of antibacterial therapy is 5–7 days (36). The following possibilities should be considered if the efficacy is poor: (I) whether the antimicrobial regimen is appropriate to the underlying pathogens; (II) whether there are factors affecting infection control, such as mucus clearance disorders; (III) whether the etiologies of infection have been repeatedly tested for drug-resistant bacteria or special pathogen infections; and (IV) whether there are uncontrolled comorbidities and complications (36), especially factors associated with lung cancer, based on the impacts of tumor status, antitumor therapy, glucocorticoid therapy, other immunity-related factors, or other special pathogen infections.
Systemic corticosteroids
In patients with moderate-to-severe AECOPD, systemic corticosteroids can improve FEV1 and oxygenation status as well as shorten recovery time and hospital stay, with the recommended drug and dose being prednisolone at 40 mg/day for 5 days, and systemic glucocorticoids can also be replaced or partially replaced with aerosol inhaled hormones (94). Notably, high doses of systemic corticosteroids during ICIs or corticosteroids during the ICIs-enabled phase has been reported to be associated with significant reductions in objective response rate (ORR), PFS, and OS. Therefore, high doses of corticosteroids or use of systemic corticosteroids during the ICIs-enabled phase should be avoided.
Respiratory support
In the presence of hypoxemia, controlled oxygen therapy or high-flow nasal cannula (HFNC) oxygen therapy can be used, with the desired peripheral oxygen saturation (SpO2) being 88–92% (with hypercapnia) or >92% (without hypercapnia). When AECOPD is complicated by type II respiratory failure, noninvasive mechanical ventilation may be the preferred modality of respiratory support (109). Invasive mechanical ventilation may be necessary if respiratory failure continues to progressively worsen, as in cases of altered consciousness and/or life-threatening acid-base imbalance despite aggressive pharmacologic treatment and noninvasive ventilation. The decision to initiate mechanical ventilation should thoroughly considered based on tumor stage, possible improvement of the disease, the wishes of patients and their families, and local health care conditions. Mode selection and parameter setting during mechanical ventilation should consider factors related to lung cancer, such as pleural effusion, airway obstruction, atelectasis, and lobectomy.
Adverse drug reactions and interactions in the treatment of LC-COPD
During the treatment of lung cancer, certain antitumor drugs can affect cardiac repolarization and induce a prolonged QT interval, while others can be cardiotoxic; in addition, other COPD drugs may also cause prolongation of the QT interval. Thus, the concomitant use of these drugs can increase the risk of fatal torsade de pointes (TdPs). Close electrocardiogram (ECG) and blood electrolyte monitoring is also required when the condition necessitates concomitant use. In addition, the concomitant use of certain COPD drugs with antitumor drugs can augment the toxicity of chemotherapy drugs and therefore should be avoided or used with caution. These drugs are described below.
- Drugs that can lead to QT interval prolongation (110-112): (i) COPD medications: bronchodilators, including β-adrenergic receptors (even selective β2 receptor agonists are associated with this risk); and antimicrobial drugs, including macrolides, quinolones, voriconazole, posaconazole, etc. (ii) Lung cancer medications: chemotherapy drugs, including pemetrexed, erythromycin, doxorubicin, etc.; small-molecule kinase inhibitors, including anlotinib, osimertinib, etc.; monoclonal antibodies, including nivolumab, pembrolizumab, etc. and antiemetics, including granisetron, dolasetron, ondansetron, etc.
- Drugs that augment the toxicity of antitumor drugs: (i) voriconazole, which augments the toxicity of doxorubicin (combined use can prolong the QT interval), docetaxel (increases the risk of bone marrow suppression, fever, and diarrhea), vincristine (increases blood concentration, leading to neurotoxicity and other serious adverse reactions), and ceritinib (increases its blood concentration), etc.; (ii) itraconazole, which augments the toxicity of erlotinib [maximum concentration (Cmax) ↑↑, area under the curve (AUC) ↑], gefitinib (Cmax ↑, AUC ↑), and irinotecan (the plasma concentrations of irinotecan and its metabolites will be increased, with unknown levels; patients must not be treated with irinotecan during and 2 weeks after itraconazole treatment).
Consensus 9: chemotherapy for patients with LC-COPD: the clinical stage and pathological type of lung cancer, PS score, and COPD status should be considered. Platinum-based dual drug therapy or nonplatinum monotherapy may be selected for patients with stable COPD (recommendation category: A; level of evidence: 1a)
The postoperative adjuvant chemotherapy regimen can be selected according to PS score and COPD status in patients with surgically resectable NSCLC complicated with COPD
(I) Patients with stage IB-IIIA lung cancer and a PS score of 0–1 along with good COPD control (GOLD COPD group A) are recommended to undergo postoperative adjuvant chemotherapy with platinum-based regimens (113-115). (II) Patients with stage II–IIIA lung cancer and a PS score of ≥2 or poor COPD control (GOLD COPD groups B, C, and D) are recommended to consideradjuvant platinum-based dual drug chemotherapy once the patients can tolerate chemotherapy (115).
The optimal mode of neoadjuvant chemotherapy for patients with surgically resectable NSCLC complicated by COPD remains controversial, and the improvement of survival after preoperative neoadjuvant chemotherapy is not significant (116) (level of evidence: II; grade of recommendation: B).
Regimens can be selected according to the PS score and COPD status in patients with advanced, non–driver mutation nonsquamous NSCLC complicated by COPD
Platinum-containing two-drug combination regimens are recommended for chemotherapy in patients with a PS score of 0–1 and good COPD control (GOLD COPD group A), and its efficacy is significantly better than vinorelbine and gemcitabine monotherapy, with only slightly increased toxicity incidence and toxicity-related mortality (117,118). Among the platinum-based anticancer drugs, carboplatin (119), cisplatin, and loplatin (120) are appropriate choices. Compared with single-drug chemotherapy, the survival of patients with cisplatin-based double-drug chemotherapy has not been significantly prolonged and the incidence of side effects is high, so the former is not recommended as the standard first-line treatment. Drugs that can be used in combination with platinum mainly include pemetrexed (121), paclitaxel (122,123), gemcitabine (124,125), or docetaxel (126), among which pemetrexed plus platinum-based doublet chemotherapy has a high safety profile (127,128). The efficacy of platinum-containing dual-agent chemotherapy is similar to that of the non-platinum-containing dual-agent chemotherapy, but has a high safety profile. Therefore, carboplatin plus pemetrexed dual-agent chemotherapy regimen is recommended in this population. Non–platinum-based dual-agent chemotherapy regimens including gemcitabine plus vinorelbine (123), and gemcitabine plus docetaxel (122,124) may be considered in patients in whom platinum is contraindicated or for some reason has to be used with extreme caution.
For patients with a PS score of 2 or poor COPD control (GOLD COPD group B), carboplatin plus pemetrexed can be selected when tolerated (129,130). Single-agent chemotherapy is recommended for patients who cannot tolerate side effects such as severe anemia and granulocytopenia. Compared with supportive care, single-agent chemotherapy can prolong survival and improve quality of life. The currently available drugs include pemetrexed, paclitaxel, gemcitabine, vinorelbine, and docetaxel (131).
Patients with a PS score of 3–4 and very poor control of COPD status (GOLD COPD group E) are not recommended to receive cytotoxic chemotherapy. Symptomatic treatment or participation in relevant clinical trials is recommended.
In term of maintenance chemotherapy regimens, if the patient achieves disease control [complete response (CR), partial response (PR), and stable disease (SD)] after 4–6 cycles of first-line chemotherapy, with a good PS score and chemotherapy tolerance, maintenance therapy can be applied. Maintenance therapy can be performed with pemetrexed (127,132-134).
Regimens can be selected based on the PS score and COPD status in patients with advanced non-driver mutation squamous lung cancer complicated by COPD
Platinum-containing dual-drug chemotherapy regimens are recommended for patients with a PS score of 0–1 and good COPD control (GOLD COPD group A), and its efficacy is significantly better than vinorelbine and gemcitabine monotherapy, with only slightly increased toxicity incidence and toxicity-related mortality (117-119). Among the platinum-based anticancer drugs, cisplatin, carboplatin (120), loplatin (135), and nedaplatin (136) are appropriate. Compared with cisplatin-containing dual-agent chemotherapy, carboplatin- or nedaplatin-containing dual-agent chemotherapy has been found to significantly prolong survival in patients with squamous cell carcinoma, with significantly lower incidence of toxicities. Therefore, carboplatin- or nedaplatin-containing dual-agent chemotherapy is recommended as the standard first-line treatment for this population (121,136,137). The drugs used in combination with platinum mainly include paclitaxel (122,123), gemcitabine (124,125), docetaxel (126), paclitaxel liposomes or albumin-bound paclitaxel (ABP) (132), among which ABP-containing chemotherapy regimens are associated with a low incidence of treatment-related adverse reactions (138). Therefore, the carboplatin plus ABP dual-agent chemotherapy regimen is recommended. The efficacy of platinum-containing dual-agent chemotherapy is similar to that of the non-platinum-containing dual-agent chemotherapy, but has a high safety profile (129). Therefore, non–platinum-based dual-agent chemotherapy regimens including gemcitabine plus vinorelbine (124), and gemcitabine plus docetaxel (127) may be considered in patients in whom platinum is contraindicated or has to be used with extreme caution.
For patients with a PS score of 2 or poor COPD control (GOLD COPD group B), carboplatin plus paclitaxel can be selected when tolerated (129,130). Single-agent chemotherapy is recommended for patients who cannot tolerate side effects of severe anemia or granulocytopenia. Compared with supportive care, single-agent chemotherapy can prolong survival and improve the quality of life. The currently available drugs include gemcitabine, vinorelbine, paclitaxel, and docetaxel (131).
Patients with a PS score of 3–4 and very poor control of COPD status (GOLD COPD group E) are not recommended to receive cytotoxic chemotherapy. Symptomatic treatment or participation in relevant clinical trials is recommended instead.
In terms of maintenance chemotherapy, if the patient achieves disease control (CR, PR, and SD) after 4–6 cycles of first-line chemotherapy, with a good PS score and chemotherapy tolerance, maintenance therapy can be applied. Maintenance therapy can be performed with gemcitabine (133,139) or docetaxel continuation.
Regimens can be selected based on the PS score and COPD status in patients with SCLC complicated by COPD
(I) The regimens for patients with limited-stage SCLC (LS-SCLC) complicated with COPD are described below.
For patients with a PS score of 0–2 and good COPD control (GOLD COPD group A), concurrent chemotherapy plus radiotherapy is the standard treatment, and the recommended chemotherapy regimen is etoposide plus cisplatin or chest radiotherapy (140,141).
For patients with a PS score of 3–4 (caused by SCLC rather than COPD) and good COPD control (GOLD COPD group A), individualized chemotherapy regimens (e.g., single-agent chemotherapy regimens or reduced-dose combination chemotherapy regimens) should be carefully selected after thorough consideration of all relevant factors. If the PS score decreases to 2 points or lower after treatment, a combination with radiotherapy (131) may be considered.
In the case of patients with a PS score of 3–4 (not caused by SCLC) and poor COPD control status (GOLD COPD groups B and E), it is not recommended they receive cytotoxic chemotherapy. Symptomatic treatment or participation in relevant clinical trials is recommended.
(II) The regimens for patients with extensive-stage SCLC (ES-SCLC) complicated with COPD are described below.
For patients with a PS score of 0–2 and good COPD control (GOLD COPD group A), etoposide plus cisplatin in combination with atezolizumab or durvalumab is recommended. Patients with low programmed death-ligand 1(PD-L1) expression can also benefit from this regimen (142-144).
For of patients with a PS score of 3–4 (caused by SCLC rather than COPD) and good COPD control (GOLD COPD group A), platinum-containing dual-drug chemotherapy regimens are recommended. The recommended platinum treatments include cisplatin (145), carboplatin (146), and loplatin (147). The drugs used in combinations with platinum mainly include etoposide (145) and irinotecan (148).
It is not recommended that patients with a PS score of 3–4 (not caused by SCLC) and poor COPD control status (GOLD COPD groups B and E) receive cytotoxic chemotherapy. Symptomatic treatment or participation in relevant clinical trials is recommended.
Consensus 10: targeted therapy for patients with LC-COPD—genetic testing is recommended based on the stages and pathological types, and targeted therapy is performed according to the testing results. When there are multiple drugs available for first-line or later-line targeted therapy, a targeted drug with low pulmonary toxicity should be selected whenever possible (recommendation category: A; level of evidence: 1a)
Targeted drug recommendation for patients with LC-COPD: improve driver gene detection, select appropriate targeted drugs according to driver gene of patients, and treat COPD simultaneously
There is no evidence for interactions among ICS, LABA, LAMA, or tyrosine kinase inhibitors (TKIs), or evidence for TKI-induced AECOPD.
Zhou et al. (78) investigated the benefits and risks of chemotherapy in driver gene-negative patients with advanced with LC-COPD and PS scores >2 and reported, in the guidelines for severe lung cancer, that the use of EGFR-TKIs and ALK-TKIs prolongs the survival in these patients and could be well tolerated. Therefore, targeted therapy can be feasible in patients with severe COPD; however, if AECOPD occurs and the patient has to receive endotracheal intubation and intensive care unit (ICU) treatments, the application of anticancer therapy should also consider the expected outcomes and the willingness of the family.
It is therefore recommended that efforts be made to detect epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) fusion, ROS proto-oncogene 1, receptor tyrosine kinase (ROS1) fusion, Kirsten rat sarcoma (KRAS), B-type Raf kinase (BRAF), Neurotrophic Receptor Tyrosine Kinase (NTRK) 1/2/3, mesenchymal-epithelial transition (MET) exon 14 skipping mutation, and Rearranged During Transfection (RET) rearrangements in biopsy tissues or surgical specimens from patients with stage IB–IV NSCLC complicated by COPD. Targeted therapy may be carried out on the basis of driver genotyping results. If the tissue specimen cannot be readily harvested, ctDNA analysis for driver genes is recommended, although false negativeness may be a concern. According to the targeted therapy guided by the driver gene (specific drug selection was shown in Table 1), and COPD was treated at the same time.
Table 1
| Driver gene types | Setting | Recommending |
|---|---|---|
| EGFR mutation | EGFR mutation after complete resection in stage IB–II | Osimertinib |
| Positive EGFR-sensitive gene mutation after stage IIA–III surgery | Icotinib, osimertinib | |
| EGFR 19del/L858R mutation in stage IV | Osimertinib, erlotinib, afatinib, gefitinib, icotinib, dacomitinib, almonertinib | |
| EGFR rare mutations (G719X, S768I, L861Q) | Osimertinib, almonertinib | |
| T790M mutation | Osimertinib | |
| EGFR exon 20 insertion mutations | Amivantamab, or mobocertinib after chemotherapy | |
| ALK fusion | ALK fusion positive in stage IV | Crizotinib, ceritinib, alectinib, ensartinib, brigatinib, lorlatinib |
| ROS1 fusion | ROS1 fusion positive in stage IV | Crizotinib, ceritinib, entrectinib |
| MET 14 exon skipping | – | Capmatinib, tepotinib, crizotinib, savolitinib |
| Rare mutations | – | |
| BRAF V600 | – | Dabrafenib + trametinib |
| RET rearrangements | – | Cabozantinib, pralsetinib, selpercatinib |
| NTRK | – | Larotrectinib and entrectinib |
ALK, anaplastic lymphoma kinase; BRAF, B-type Raf kinase; EGFR, epidermal growth factor receptor; MET, mesenchymal-epithelial transition; NTPK, neurotrophic receptor tyrosine kinase; RET, rearranged during transfection; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase; 19del, 19 exon deletion.
After resistance to targeted therapy occurs in patients with LC-COPD, it is decided whether to conduct another biopsy to evaluate the mechanism of drug resistance according to the patients’ presence or lack of symptoms and the range of progression. Follow-up treatment is selected according to the first-line treatment and the mechanism of drug resistance (specific drug selection was shown in Tables 2,3). The decision to perform puncture biopsy should be made to assess whether the patient can tolerate invasive procedures. ctDNA should be used as an alternative in cases where tissue specimens cannot be readily harvested. If the patient is suffering from AECOPD, AECOPD should be treated first, mechanical ventilation should be required for critical cases, and drug-resistant diagnosis and treatment of tumor should be suspended until AECOPD becomes stable.
Table 2
| Driver gene types | Setting | Recommending |
|---|---|---|
| EGFR mutation | First-line osimertinib treatment | Osimertinib plus adjuvant local therapy |
| First-line treatment with erlotinib, afatinib, and gefitinib (T790M−) | Original EGFR-TKI plus adjuvant local therapy; osimertinib is preferred for patients with brain metastases or meningeal invasion | |
| First-line treatment with erlotinib, afatinib, gefitinib (T790M+) | Osimertinib plus adjuvant local therapy | |
| ALK fusion | First-line crizotinib treatment | Alectinib, ceritinib, brigatinib, lorlatinib, or ensartinib plus adjuvant local therapy |
| First-line treatment with alectinib, ceritinib, brigatinib, lorlatinib | Original ALK-TKI plus adjuvant local therapy | |
| ROS1 fusion | First-line treatment with crizotinib, ceritinib, entrectinib | Original ALK-TKI or lorlatinib, plus adjuvant local |
ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase; TKI, tyrosine kinase inhibitor.
Table 3
| Driver gene types | Setting | Recommending |
|---|---|---|
| EGFR19del/L858R | EGFR T790M mutation after first- or second-generation EGFR-TKIs | Osimertinib, almonertinib, furmonertinib |
| EGFR T790M mutation-negative after first- or second-generation EGFR-TKIs | Chemotherapy, anti-VEGF treatment | |
| Third-generation EGFR-TKIs | Chemotherapy, anti-VEGF treatment | |
| ALK fusion | First-line treatment with first-generation ALK-TKIs | Alectinib, ceritinib, brigatinib, ensartinib |
| First-line treatment with second-generation ALK-TKIs | Lorlatinib | |
| ROS1 fusion | Crizotinib, ceritinib, entrectinib | Lorlatinib |
ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase; TKIs, tyrosine kinase inhibitors; VEGF, vascular endothelial growth factor; 19del, 19 exon deletion.
TKI-induced interstitial lung disease
The risk factors for TKI interstitial lung disease (TKI-ILD) should be actively screened before medication is administered for LC-COPD. A severe underlying ILD is a high-risk factor for TKI-ILD, and TKIs should be used with caution in such patients should use TKI as appropriate. The possible occurrence of TKI-ILD during the period that the medication is administered must be closely monitored.
(I) There is no significant difference in the incidence of TKI-induced ILDs. The selection of targeted therapy drugs for patients with LC-COPD should also be based on the principles of lung cancer treatment. Nevertheless, the relevant risk factors must be identified before treatment with medication, and the potential occurrence of drug-related lung injury must be closely monitored.
Research has demonstrated that there is no significant difference in the incidence of interstitial pneumonia caused by gefitinib, erlotinib, or afatinib (149), and there are no reported studies which suggest that the third-generation EGFR-TKI has a higher risk of causing ILD than do the first- and second-generation EGFR-TKIs. Based on the reported data, the incidence rates of ILD caused by gefitinib, erlotinib, and afatinib are approximately 1–2%. ADAURA and FLAURA studies (150,151) reported that the incidence of osimertinib-related ILD was 3% and 4%, respectively. Patients who have previously received EGFR-TKI treatment appear to have a higher probability of developing ILD after osimertinib readministration (152). For patients receiving targeted therapy for ALK/ROS1 fusion-positive NSCLC, the incidence of pneumonitis is reportedly 2.68%, 4.11%, 1.62%, and 1.62%, after crizotinib, brigatinib, alectinib, and ceritinib treatment, respectively; however, no significant difference in the incidence of pneumonia caused by multiple ALK-TKIs has been reported (153). In summary, the selection of targeted therapy drugs for patients with LC-COPD should still be based on the principle of lung cancer treatment. However, COPD increases the risk of TKI-associated ILD. Patients may also be more likely to develop drug-related interstitial pneumonia due to severe COPD and a PS score of ≥2 (154). Therefore, the relevant risk factors must be taken into account before starting the medication, and the potential occurrence of drug-associated lung injury must be closely monitored during drug administration.
(II) The risk factors for TKI-induced ILD must be identified and screened before administration of medication, and TKIs should be used with caution in patients with LC-COPD with severe ILD.
COPD, PS score ≥2, male gender, age >60 years, history of smoking, pre-existing ILD, pulmonary infection, tumor therapy within 1 year, history of radiotherapy or chemotherapy, and combined or sequenced with immunotherapy are risk factors for TKI-induced ILD (155-158). Performing routine blood tests, blood biochemistry, chest CT, and pulmonary function tests before medication initiation is recommended, and special attention should be paid to lung function indicators [mainly FEV1, FVC, and diffusing lung capacity for carbon monoxide (DLCO)], infection indicators [e.g., white blood cell count, neutrophil count and proportion, and procalcitonin (PCT)], as well as inflammatory indicators (e.g., interleukin 6 (IL-6)]. TKI should be considered according to the patient’s individual situation and willingness in patients with LC-COPD with severe underlying ILD (159).
(III) Any new or worsened respiratory symptoms should be closely observed, and PS scores should be dynamically assessed. Chest CT, routine blood test, blood biochemistry, lung function tests, and all other requisite tests should be performed more frequently in these patients than in patients receiving conventional tumor treatment, and the occurrence and progression of TKI-induced ILD should be closely monitored.
For patients with high-risk factors, the benefits and risks should be evaluated more closely during the medication period than in those receiving conventional antitumor treatment. The PS score should be dynamically assessed. The occurrence or worsening of any clinical symptoms, such as chest tightness and shortness of breath, should be closely observed, and serum inflammatory markers, infection indicators, high-resolution CT, and lung function should be monitored. Patients may be at a high risk of experiencing for TKI adverse reactions 24 days to 3 months after the initiation of antitumor therapy (160,161). During the medication treatment, targeted therapy-induced pneumonia should be suspected if there is new onset of cough, fever, dyspnea, and hypoxemia or worsening of existing respiratory symptoms accompanied by shadows in the lungs. The possibility of radiation-induced pneumonia should be ruled out in patients receiving the combination of targeted therapy with radiotherapy by reviewing the previous radiotherapy time, radiation dose, irradiation field, and margins. For patients who can tolerate bronchoscopy, bronchoalveolar lavage fluid (BALF) may be collected and sent for metagenomic NGS, microbial smear preparation, microbial culture, quantification of hemosiderin-laden macrophage, analysis of total and differential cell counts, brush cytology, and bronchoscopic biopsy so as to exclude infection, alveolar hemorrhage, and tumor progression (162). Cardiogenic pulmonary edema should be identified in patients with underlying cardiac diseases. In addition, change in serum Krebs von den Lungen 6 (KL-6) has a certain predictive value for EGFR-TKI-induced fatal ILD (163).
(IV) In patients with confirmed or highly suspected drug-induced ILD, TKI therapy should be discontinued and treatment of drug-induced ILD should be initiated. The treatment is typically based on glucocorticoids, the doses of which can be adjusted according to the severity of ILD. Respiratory support for the different types may be offered according to the oxygenation level.
In patients with confirmed or highly suspected TKI-induced ILD, discontinuation of TKI should be considered based on the disease severity.
TKI-induced ILD is graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0 in the following fashion: grade 1 (G1) = asymptomatic; G2 = symptomatic and affecting instrumental activities of daily living; G3 = severe symptoms, limited self-care capacity, and a requirement of oxygen therapy; G4 = life-threatening respiratory failure requiring emergency intervention with endotracheal intubation or tracheostomy; and G5 = death. There is no imaging classification for TKI-induced ILD only, but reference can be made to the American Clinical Association’s immune-related adverse event pulmonary adverse reaction classification: G1: confined to a single lobe or less than 25% of the lung parenchyma; G2: involvement of more than one lobe or 25–50% of the lung parenchyma; G3: involvement of all lobes or 50% of the lung parenchyma; G4: life-threatening respiratory failure requiring emergency intervention with endotracheal intubation or tracheostomy (164,165). For asymptomatic patients (G1), there is no need to stop the medication, although the condition should be closely monitored; for patients experiencing G2 ILD and above, the medication should be discontinued immediately, and ILD should be treated (Table 4).
Table 4
| Grade | Symptom | Activity ability | Imaging focus range | Treatment |
|---|---|---|---|---|
| 1 | Asymptomatic; only clinical or diagnostic observations | Normal | <25% | No intervention needed |
| 2 | Symptomatic | Limiting instrumental ADL | 25–50% | Medical intervention indicated |
| 3 | Severe symptoms | Limiting self care ADL | 51–75% | Oxygen indicated |
| 4 | Life-threatening respiratory compromise | Lying in bed | >75% | Urgent intervention indicated (e.g., intubation and ventilation) |
| 5 | Death | – | – | – |
ADL, activities of daily living; TKI-ILD, tyrosine kinase inhibitor- interstitial lung disease.
There is no consensus on the specific treatment of TKI-induced ILD, although glucocorticoids can alleviate lung inflammation and delay the progression of EGFR-TKI-associated ILD (166).
According to the China’s Expert Consensus on the Diagnosis and Treatment of Anticancer Drug-Induced Interstitial Lung Disease, for G2 TKI-induced ILD, the initial dose of prednisolone is 0.5–1 mg/kg/d, which should be maintained for 2–4 weeks and then slowly reduced after the symptoms and signs have resolved, and total treatment duration of at least 6 weeks. For G3 TKI-induced ILD, the initial dose of prednisolone is 1–2 mg/kg/d, which should be maintained and then gradually reduced after the symptoms and signs have resolved, and total treatment duration of at least 8 weeks. For G4 TKI-induced ILD, methylprednisolone pulse therapy at 500–1,000 mg/day is applied for 3 days, after which the dose is reduced to 1–2 mg/kg/day; this can be maintained for 2–4 weeks and then slowly reduced after the symptoms and signs have resolved (164), and total treatment duration of at least 8–10 weeks. Hormone-associated side effects including infection, gastrointestinal bleeding, electrolyte imbalance, and osteoporosis should be prevented whenever possible and otherwise managed. For patients with imaging manifestations of fibrosis, antifibrotic therapy may be considered after the acute phase, with pirfenidone and nintedanib available as alternative options. Patients with resting hypoxemia with an oxygen saturation level below 89% after activity due to respiratory symptoms and hence limited activity should receive oxygen therapy (167). A coexisting infection should be treated appropriately, and symptomatic supportive treatment should not be ignored. In critically ill patients with severe hypoxemia and acute respiratory distress syndrome (ARDS), mechanical ventilation should be considered after carefully weighing the benefits and risks, which should include considering the prognosis of lung cancer and other lung diseases.
Consensus 11: anti-angiogenic therapies for patients with LC-COPD, the combinations of anti-angiogenic drugs should be based on the specific pathological types and clinical stages of lung cancer, and the indications and contraindications of specific anti-angiogenic drugs; in addition, the adverse reactions must be closely monitored (recommendation category: B; level of evidence: 2a)
For patients with advanced nonsquamous NSCLC complicated COPD, antiangiogenic drugs may be used in combination or as a third-line monotherapy
In patients negative for driver genes, bevacizumab combined with chemotherapy may be considered if the chemotherapy is tolerable. Studies have shown that bevacizumab combined with chemotherapy can achieve an increase in PFS and the ORR in elderly patients compared with chemotherapy alone (168-170).
For patients positive for driver genes, bevacizumab combined with small-molecule TKI has been shown to achieve a PFS benefit in elderly patients. Compared with patients under 75 years, patients aged ≥75 years benefited even more from erlotinib plus bevacizumab treatment (171,172); however, no significant OS benefit has been observed. A retrospective study has shown that the combination of pembrolizumab with anlotinib increased both PFS and OS in patients with NSCLC and EGFR mutations who had failed previous treatment compared with pembrolizumab monotherapy. There is also no significant difference in PFS and/or OS between elderly and nonelderly patients (173).
Anlotinib monotherapy can be used as a third-line treatment option (174).
In patients with advanced squamous NSCLC complicated by COPD, combinations with Endostar (rh-endostatin) or anlotinib monotherapy may be considered
The subgroup data of a phase III clinical study showed that, compared with chemotherapy alone, recombinant human endostatin (Endostar) combined with chemotherapy significantly showed a significant benefit in elderly patients with advanced squamous cell carcinoma (175).
Anlotinib monotherapy can be used in the third-line settings. A placebo-controlled, randomized, double-blind, multicenter phase III clinical trial showed that anlotinib treatment achieved OS benefit in elderly patients with advanced squamous cell carcinoma (compared with the control group) (176).
Anlotinib plus chemotherapy can be used in patients with ES-SCLC complicated with COPD
Many studies have shown that elderly patients with ES-SCLC can benefit from anlotinib-based combinations (177,178). Notably, patients with LC-COPD are notably at higher risk of developing AEs after antiangiogenic drug treatment. For instance, COPD is associated with increased inflammatory cytokines and vascular endothelial cell damage, which can increase the risk of venous thromboembolic events (179). In addition, patients with COPD are prone to cardiovascular and cerebrovascular diseases (e.g., hypertension) due to various factors, including pulmonary ventilation dysfunction, pulmonary vascular endothelial dysfunction, and respiratory tract inflammation (180). LC-COPD increases the likelihood of adverse events associated with antiangiogenic drugs. Therefore, the blood pressure of patients with LC-COPD who are using antiangiogenic drugs should be dynamically monitored, and special attention should be paid to their urine protein content. For the management of bleeding and thrombosis, high-risk patients must be ruled out before using antiangiogenic drugs, and the grades of bleeding and thrombosis should be dynamically monitored during drug use.
Consensus 12: immunotherapy for patients with LC-COPD—clinical stage and pathological type of lung cancer, PS score and COPD status should be considered. When COPD is stable, immunotherapy monotherapy or immunotherapy-based combinations can be applied, during which time the immunotherapy-associated adverse reactions must be closely monitored (recommendation category: A; level of evidence: 2a)
The development of individualized immunotherapy regimens for patients with LC-COPD should be based on the diagnosis and staging of the lung cancer, the diagnosis and grading of COPD, detailed organ function assessment before treatment, close monitoring during treatment, and regular follow-up after treatment
The treatment of lung cancer has entered a new era of immunotherapy. A growing body of research has demonstrated that ICIs can achieve disease control and prolongation of survival in most patients with driver-negative NSCLC and in patients with extensive SCLC. Lung cancer and COPD share a common pathophysiological basis, including susceptibility genes, immune abnormalities, chronic inflammatory damage, and oxidative stress (51,181-183). The imbalance in the immune microenvironment is a result of the chronic inflammation in COPD leading to the continuous production of cytokines. Moreover, TGF-β1 signaling also induces the expression of PD-L1 in regulatory T cells (Tregs), resulting in immune imbalance and immune escape in T cells (184-186), which provide a basis for patients with comorbidities to receive immunotherapy.
Retrospective clinical studies have confirmed that the clinical efficacy of immunotherapy in patients with LC-COPD is better than that in patients with lung cancer alone, and COPD is an independent prognostic factor for improved outcomes after immunotherapy in patients with NSCLC. A report summarizing nine studies suggests that lung cancer patients with COPD may benefit more than those without COPD, with better PFS, OS, and ORR (187). Three studies reported that patients with COPD and NSCLC receiving ICI achieved longer PFS (76,188,189). Stratified analysis revealed that the PFS was better in smokers or ex-smokers than in nonsmokers (P=0.0359), and further stratified analysis among ex-smokers showed that both PFS (P=0.0491) and OS (359 versus 146 days; P=0.0350) were superior in patients with COPD than in patients without COPD (188). Biton et al. (189) found that annual exposure to tobacco smoke in the general population was associated with better PFS and OS. Subgroup analysis of the patients with coexisting COPD showed that PFS and OS were also more favorable in the high-smoke-exposure subgroup than in the low-smoke-exposure subgroup. A study on the role of pembrolizumab treatment reported that PFS, OS, and ORR were significantly more favorable in patients with advanced NSCLC and COPD than in those without COPD (190).
There is still no consensus on the correlation between the severity of COPD airflow restriction and the efficacy of immunotherapy. Biton et al. (189) found no impact of COPD grade on the efficacy of immunotherapy (P=0.8). However, Zhou et al. (76) found that patients with NSCLC with moderate-to-severe COPD tended to have longer PFS than did those without COPD, with Shin et al. (190) reporting that PFS was longer in patients with NSCLC and mild COPD.
Patients with LC-COPD should be assessed and monitored before, during, and after treatment with lung CT, pulmonary function test, SpO2 test, and cytokines
In addition to its benefits, immunotherapy may also cause immune-related AEs (irAEs) (191). Two prospective studies (192,193) on the changes in lung function after the use of ICIs in patients with LC-COPD consistently showed that neoadjuvant immunotherapy for NSCLC did not worsen lung function; rather, FEV1, FVC%, and DLCO were improved after the treatment. Checkpoint inhibitor pneumonitis (CIP), an ICI-associated lung injury with various clinical and imaging manifestations, is a critical adverse reaction that causes ICI-related deaths. In a retrospective study, factors including age ≥70 years, history of smoking, decreased underlying lung function, lung cancer, and a history of pulmonary radiation therapy are associated with the development of CIP (194). Stratified analysis in a clinical study revealed that patients with LC-COPD had a higher incidence of CIP after treatment with ICIs than those without COPD (195). Therefore, patients with LC-COPD should be regularly monitored during treatment with lung CT, pulmonary function test, SpO2 test and cytokines. For patients with lung cancer complicated with GOLD grade 3–4 COPD, the above examinations are particularly important. Even if there is no obvious change in clinical symptoms, carrying out the above examinations every two cycle is recommended so as to achieve the early detection and intervention of early-stage CIP and to prevent CIP from becoming a serious AE (SAE).
The indications for immunotherapy in patients with LC-COPD should still refer to the guidelines released by the US National Comprehensive Cancer Network and the American Society of Clinical Oncology
ICIs can stimulate lymphocytes to kill tumor cells. Several studies have evaluated the roles of ICIs (196), and the data suggest that ICIs improves OS in patients with LC-COPD (compared to chemotherapy). Vokes et al. (197) found that in patients with LC-COPD, the 5-year survival rate in the immunotherapy group was 16%, which was higher than that of the chemotherapy group (5%). Based on the current preliminary conclusions that patients with LC-COPD respond well to immunotherapy, the indications of immunotherapy in this population are not limited by the comorbidities.
For patients with acute exacerbation of moderate-to-severe COPD, COPD should be managed first, and immunotherapy may be carried out after the symptoms have improved and the PS score is ≤2
There is insufficient evidence to support the assertion that patients with acute respiratory dysfunction or a PS score of ≥3 due to acute exacerbation of moderate-to-severe COPD can benefit from immunotherapy. The common principles of systemic drug therapy for lung cancer and treatments for COPD should be followed; that is, priority should be given to the management of acute and critical COPD-related conditions, and immunotherapy for lung cancer can be carried out as appropriate after the PS score is improved to ≤2.
For patients with LC-COPD, if there is no AECOPD and the COPD is GOLD grade 1 or 2, there is no need to adjust the immunotherapy regimen; in contrast, if the COPD is GOLD grade 3 or 4, dual immunotherapy, immunotherapy plus anti-angiogenic therapy, and immunotherapy plus radiotherapy should be cautiously adopted; immunotherapy monotherapy is recommended for patients with PD-L1 ≥50%
Retrospective studies have shown that the incidence of CIP in patients with lung cancer and COPD was higher than that in patients with lung cancer without COPD (198,199). In randomized controlled trials, the incidence of adverse reactions was significantly higher in the immunotherapy plus radiotherapy group, the PD-L1 inhibitor plus cytotoxic T-lymphocyte–associated protein 4 (CTL-4) inhibitor (dual immunotherapy) group, and the immunotherapy plus antiangiogenic therapy group than in the single immunotherapy group (200-203). For patients with GOLD grade 3–4 COPD, especially those with positive or strongly positive PD-L1 expression, restriction to a single immunotherapy regimen can be considered to reduce the risk of serious adverse reactions (e.g., CIP). Immunization plus chest radiotherapy or sequential radiotherapy is associated with a significantly increased risk of lung injury and therefore should be used with particular caution in patients with LC-COPD with poor pulmonary reserve.
When systemic glucocorticoids are used in patients with COPD, the medication should be standardized according to the updated GOLD guidelines to minimize the negative impacts of off-label drug use, overdose, and extended use on the efficacy of immunotherapy
In a clinical study (204), inhaled corticosteroids were shown to be beneficial to improving lung function. In patients with early-stage diseases, they improved surgical tolerance and reduced postoperative complications; in nonsurgical patients, they could improve the quality of life without interfering with the efficacy of the immunotherapy. A study on the effect of systemic glucocorticoids on the efficacy of immunotherapy in patients with lung cancer revealed that baseline use of prednisone (10 mg) weakened the efficacy of PD-L1 and programmed cell death protein 1 (PD-1) inhibitors, resulting in poorer ORR, PFS and OS compared to patients who received 0 to <10 mg of prednisone (205). Therefore, the use of glucocorticoids must be considered standard in the treatment of COPD; for patients who are scheduled to receive immunotherapy, the dosage of systemic glucocorticoids may be appropriately adjusted or discontinued once COPD is stable.
For patients with LC-COPD, if there are indications for antimicrobial therapy, the application of drugs and the course of anti-infection therapy must be standardized
In patients with lung cancer complicated by obstructive pneumonia or with COPD complicated by infection, antimicrobial treatment is required. However, a prospective multicenter study found that in patients with advanced tumors treated with PD-L1/PD-1 inhibitors, OS was significantly worse in the group using antibiotics 30 days prior to ICI therapy than in the group receiving concurrent antibiotics during immunotherapy, regardless of PD-L1 expression (206). Therefore, antibiotics must be used in a standardized manner to avoid poor immunotherapy efficacy caused by off-label drug use and/or unreasonable doses and time courses.
Consensus 13: interventional treatment for patients with early-stage lung cancer combined with COPD: if patients are not suitable for surgery or radical radiotherapy due to reduced lung function, interventional treatment should be considered according to the specific conditions, such as tumor location, size, and clinical stage (recommendation category: A; level of evidence: 2a)
In patients with early-stage lung cancer combined with COPD co-morbidity, radioactive particle implantation may be used in patients who do not tolerate surgery or radical radiotherapy due to lung function and other organ functions. The studies of image-guided percutaneous radioactive particle implantation for brachytherapy of lung cancer were carried out earlier. The introduction of CT-guided radioactive particle implantation in 2002 has greatly improved the accuracy of particle implantation in the treatment of lung cancer. For patients who cannot receive radical treatment, the CT-guided technique introduces radioactive particle implantation with a higher safety profile than first-line chemotherapy, as well as higher local control and one-year survival rates. A study comparing lobectomy with sublobar resection combined with particle implantation in 167 cases of early-stage (stage Ib) lung cancer showed that patients with sublobar resection had significantly worse preoperative pulmonary function, but the recurrence rate at about 1 year and four-year mortality rate were not inferior to lobectomy, which is a worthwhile treatment for patients with early-stage lung cancer combined with COPD (207). For the operation method of particle implantation, new technologies such as 3D printing templates and robotics have also been studied and applied. There are currently no definite indications for patients with LC-COPD, and the indications of radioactive particle implantation for NSCLC are as follows: (I) inoperable due to cardiopulmonary dysfunction and/or old age; (II) tumor recurrence after surgery or external radiation therapy or refusal of surgery and external radiation therapy by the patients; (III) residual tumor or tumor progression after surgery, radiotherapy, or chemotherapy; (IV) tumor boundaries that cannot be defined by thermal ablation or surgical treatment; (V) a fair general condition of the patient [Karnofsky Performance Status (KPS) score >60 points], an expected survival time of >6 months, and a tumor diameter ≤7 cm (208).
Patients with early-stage lung cancer combined with COPD who do not tolerate or receive radical surgery or radiotherapy can be treated with radiofrequency ablation (RFA), microwave ablation (MWA), photodynamic therapy, cryoablation (CRYO), etc. Ablation techniques have characteristics and advantages such as minimally invasive, effective and repeatable treatment.
In 2000, RFA was first reported for the treatment of lung tumors (209). In December 2007, the FDA approved RFA for the treatment of lung tumors. Since 2009, the NCCN guidelines for NSCLC and the Chinese Code of Practice for the Treatment of Primary Lung Cancer have recommended RFA can be used for the treatment of patients with early-stage lung cancer that cannot tolerate surgery. A study by Simon et al. reported survival rates of 78%, 57%, and 27% at 1, 2, and 5 years, respectively, for stage I NSCLC patients with lesions less than 3 cm in diam (210).
MWA is another commonly used method for thermal ablation of lung tumors. In contrast to RFA, MWA does not require grounding and the temperature within the tumor can be measured by a separate thermocouple placed on the microwave probe. Theoretically, MWA has a stronger thermal coagulation effect on tumor cells and is more efficient, ablating a larger area and requiring less time compared to RFA. The National Cancer Database study (211) showed that the OS at 1, 2, 3, and 5 years in the thermal ablation treatment group were: 85%, 65.2%, 47.8%, and 24.6%, respectively, compared with 86.3%, 64.5%, 45.9%, and 26.1% in the SBRT group. There was no statistically significant difference between the two groups.
CRYO applied to the radical treatment of early-stage lung cancer have also been reported. A retrospective study (212) showed that 45 patients with inoperable stage I NSCLC had a 5-year disease-free survival rate of 87.9% and a local recurrence rate of 36.2% after treatment with CRYO. Another retrospective study reported that after 25 cases of stage I NSCLC treated with CRYO, the OS at 1 and 3 years were 100% and 63%, respectively, and the local control rates at 1 and 3 years were 71% and 37%. The application of CRYO in early-stage lung cancer lacks probative evidence of high evidence level, and its efficacy is inferior to thermal ablation technique in terms of local control rate analysis, and is not recommended as a preferred radical interventional treatment.
For patients with early-stage lung cancer with COPD who cannot receive surgery or radical radiotherapy, the appropriate ablation technique can be selected according to the location, size and COPD classification of the lesion. The selection of ablation method is typically based to the location of the tumors: for tumors located in the middle and outer thirds of the lung, CRYO, MWA, or RFA may be considered; for central lung cancer that is adjacent to the airway, pleura, or chest wall, CRYO is preferred (213,214), especially for lesions less than 30 mm in diameter, which can achieve better results.
Consensus 14: interventional palliative treatment for patients with advanced lung cancer and COPD: appropriate interventional palliative treatment can be selected based on the pathological type, stage, lesion location, and clinical symptoms of the tumor to improve the quality of life of the patient (recommendation category: A; level of evidence: 2a)
Most of the intermediate and advanced lung cancers are treated based on systemic therapy, but some patients may suffer from acute or severe lung cancer due to lesion invasion or combined COPD. Combined interventional therapy can relieve airway obstruction and impaired respiratory function, which can save lives and improve survival quality.
For intermediate and advanced lung cancer combined with COPD, feasible interventional palliative treatment can include argon plasma coagulation, photodynamic therapy, and CRYO
The advantage of these techniques is that it can be performed multiple times in a minimally invasive manner to achieve rapid tumor shrinkage and can achieve the efficacy of delaying disease progression. Palliative ablation is indicated in patients with LC-COPD in whom the maximum tumor diameter is >5 cm or whose number of lesions on 1 side is >3 (>5 bilaterally). During assessment, preoperative contrast-enhanced CT is used to observe the location of the lesions and their relationship with the adjacent visceral organs, blood vessels, and bronchi. Laboratory tests include routine blood and urine tests, coagulation test, liver and kidney function tests, determination of tumor markers, blood typing, ECG, and cardiac ultrasound. In the pathology examinations the diagnosis of the pathology should be confirmed before surgery (215).
Strict postoperative imaging follow-up is critically important for the assessing the treatment response and detecting local recurrence as early as possible. Radiographic findings hours and days after ablation vary depending on the ablation modality. Tumors treated with RFA or MWA usually shrink within minutes, and concentric circles with varying degrees of attenuation (i.e., the “cockade phenomenon”) appear around the tumor. There is no obvious change in tumor size within minutes of CRYO (216). The efficacy of tumor ablation for tumors is typically assessed according to the Response Evaluation Criteria In Solid Tumors (RECIST). However, since coagulative necrosis occurs at the ablation site, the lesion size on imaging will be larger than that before the procedure because the ablation area includes both normal lung tissue and the lesions. Without proper knowledge of the imaging findings prior to ablation, this apparent change can easily be misinterpreted as disease progression or infection. Therefore, evaluating the therapeutic response based on lesion diameter alone can easily be misleading (217). The functional imaging [mainly contrast-enhanced CT and positron emission tomography (PET)-CT] may have a role in evaluating the actual response of lung cancer to ablation, as the residual tumors may be enhanced or hypermetabolic. However, post ablation hyperemia and inflammation will affect the enhancement on imaging. Thus, evaluation of the response after ablation should also consider the size and enhancement of the lesions, thus enabling a comprehensive evaluation based on both anatomical and functional imaging. Chest contrast-enhanced CT should be arranged monthly the first three months after ablation, during which time the patient should be examined by the technical operator. CT performed 1 month after ablation can become a new baseline imaging session for assessing the condition. Three months after ablation, contrast-enhanced CT or PET-CT and tumor marker measurements should be repeated every 3 months; after 1 year, contrast-enhanced CT should be repeated every 6 months; after the third year, contrast-enhanced CT should be repeated every 12 months (218,219).
The use of tracheobronchial stents in clinical practice has provided a direct method for reducing respiratory obstruction and maintaining airway patency in patients with lung cancer complicated by severe airway stenosis, improving the quality of life to a remarkable extent
The indications for tracheobronchial stents are the following: (I) patients who cannot tolerate thoracotomy due to poor lung function and/or old age; (II) patients with peripheral lung cancer involving the pleura and chest wall that cannot be completely resected; (III) patients with lung cancer that has shrunk and become stable after multi-modality treatment but not disappeared; and (IV) patients with localized lung cancer indicated for surgical resection, which, however, is refused by the patient.
The exclusion criteria for tracheobronchial stent treatment are the following: (I) bilateral or unilateral multiple lesions; (II) a mass close to a large mediastinal vessel, with the expectation that the puncture will inevitably injure the large vessel; (III) severe pulmonary hypofunction and a maximum ventilation volume of the lungs of <39%; (IV) severe cough and repeated dyspnea who are noncompliant with treatment; and (V) patients with advanced tumors, obvious cachexia, or bleeding tendency.
Consensus 15: interventional lung volume reduction for patients with LC-COPD: it should be integrated according to COPD classification, lung function, as well as tumor location, size and clinical stage (recommendation category: B; level of evidence: 2a)
Bronchoscopic lung volume reduction (BLVR) has become the preferred treatment after lung volume reduction surgery (LVRS). It has been reported that the lung function of patients with NSCLC complicated with COPD recovered to a suitable level after BLVR, which enabled the successful surgical resection of lung cancer. The currently available BLVR techniques include one-way valves and coils inserted into the bronchi, biological occlusion agents injected into the bronchi, thermal ablation of the airways, and decompression of the bullae by placement of airway stents. The selection process for surgical and transbronchial intervention for COPD can be seen in Figure 1.
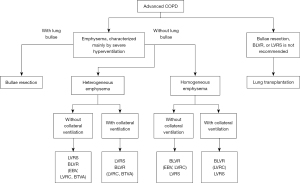
For patients without collateral ventilation, an endobronchial valve may be a safe alternative
The use of an endobronchial valve (EBV) allows for the drainage of gas and secretions while blocking the air entrance, eventually turning the hyperinflated emphysematous tissue distal to the valve into atelectasis, which is quite similar to the effectiveness of a lung volume reduction operation. Compared with surgery, BLVR has lower morbidity and mortality (220-222). The main complications of BLVR include pneumothorax, pneumonia, COPD exacerbation, hemoptysis, and valve displacement. Two types of valves are available: duckbill and umbrella (223,224).
Bronchoscopic thermal vapor ablation is a well-recognized method of minimally invasive lung volume reduction in patients with severe emphysema
Preclinical data suggest that BTVA has great potential for the minimally invasive ablation of lung cancer lesions. BTVA transfers heat energy in a targeted manner (225). It induces a localized inflammatory response in the targeted lung segment, resulting in fibrosis and contraction of the segment, which is followed by a decrease in lung volume. Subsequently, the heat energy can be transferred to other sites (226). Homogeneous necrotic regions with subsegment anatomical boundaries have been delineated in canine models (227,228).
Consensus 16: radiotherapy for patients with LC-COPD: lung cancer may become inoperable due to decreased lung function in some patients with LC-COPD, and appropriate multidisciplinary treatment including radiotherapy can be considered according to the location, size, clinical stage, and other specific conditions of the tumors. Assessment and monitoring of lung function should be given high priority when administering radiotherapy (recommendation category: A; level of evidence: 2a)
(I) SBRT is an alternative treatment option for patients with early-stage lung cancer combined with COPD.
COPD has been associated with a worse prognosis in patients with NSCLC (208,229-231), especially in those with emphysema and pulmonary fibrosis (232). However, no high-level evidence related to radiotherapy in patients with LC-COPD has been available to guide the clinical practice of radiotherapy in these patients.
Whether COPD increases the incidence of radiation pneumonia in patients with lung cancer remains controversial (233-240). Despite the lack of prospective research data, many retrospective analyses have consistently confirmed that SBRT is safe and tolerable in patients with coexisting COPD or emphysema (232,233,235,238,241). Therefore, COPD is not a contraindication to radiotherapy. For early-stage NSCLC, SBRT has been shown to confer a survival benefit in patients with severe COPD (GOLD stages 3–4) (242). Palma et al. reported a lower 30-day mortality (0% vs. 10%) in patients with severe COPD treated with SBRT compared with those treated with surgery (243). Therefore, we recommend SBRT in patients with LC-COPD, especially in patients with early-stage NSCLC and FEV1 <30% (244). Conventional radiotherapy may be considered in patients with locally advanced NSCLC for whom are not suitable for surgical treatment or SBRT is not suitable. Research has shown that conventional radiotherapy does notably increase the risk of radiation pneumonitis (236) or death (245) in patients with LC-COPD.
(II) Patients with LC-COPD receiving radiotherapy should pay attention to lung function assessment and monitoring.
Most of the relevant studies have shown that poor lung function does not increase the risk of radiation pneumonitis (236,246) and that poor baseline lung function does not predict increased pulmonary toxicity after SBRT (247). Therefore, poor baseline lung function and low baseline FEV1 and/or DLCO should not be used to exclude patients with NSCLC from SBRT (247,248). Retrospective studies have reported no significant decline (246,249) or only a slight decrease (250) in lung function after SBRT. A further study reported that the GOLD COPD stage was inversely correlated with a decline in respiratory function after SBRT; that is, the decline in respiratory function after radiotherapy in patients with a higher GOLD stage was not obvious (244). Similarly, another study showed significant reductions in FEV1 and FVC in patients with normal function or mild-to-moderate COPD but not in patients with severe COPD (GOLD stages 3–4), along with a lower rate of long-term decline in lung function ≥2 one year after SBRT (251).
In contrast, conventional chest radiotherapy is associated with decreased lung function. Tang et al. retrospectively analyzed the outcomes of curative-intent radiotherapy in patients with NSCLC and COPD (n=587) or ILD (n=34) and observed increased breathlessness and oxygen requirements after radiotherapy in patients with severe/very severe COPD and ILD (252). Borst et al. (253) measured pulmonary function in 34 patients with inoperable NSCLC before radiotherapy and at 3 and 18 months of follow-up. It was found that the pulmonary function parameters (FEV1 and diffusion capacity [T(lcoc)]) significantly decreased at 3 months, especially in patients with COPD. The decline in lung function was positively correlated with the average lung dose, and no recovery in pulmonary function was seen at 18 or 36 months after RT. However, such decline in lung function increases the shortness of breath by only 1–2 grades in most patients. Only 5% of the patients developed severe dyspnea (254), and no significant change in mortality was observed (255).
Therefore, it is important to assess and monitor lung function during radiotherapy in patients with LC-COPD. Pulmonary function and emphysema stages should be carefully assessed prior to radiotherapy, in potential the possible presence of underlying ILD. The risks and benefits of radiotherapy should be carefully weighed. In addition, multiparameter models for prediction (239) as well as imaging techniques including perfusion imaging, functional imaging, and 4D-CT (256,257) can be used to guide the radiotherapy-field setting and dose restriction, which may in turn further reduce radiotherapy-related lung injury.
The radiotherapy parameters should be effectively adjusted in patients with LC-COPD.
When conventional radiotherapy is applied in patients with LC-COPD, intensity-modulated conformal radiation therapy of the primary lesion plus involved field irradiation is recommended (258), and the radiation dose and volume in normal lungs should be minimized. There is currently no consensus on the dose limits of radiotherapy for LC-COPD. Based on the available data, we recommend that the percentage of pulmonary volume irradiated to >20 Gy (V20) should be ≤25% (240) and the mean lung dose (MLD) should be ≤14 Gy so as to reduce the incidence of radiation pneumonia. Research has shown that V20 ≤21% may be more favorable in maintaining lung function and reducing the occurrence of dyspnea. If there are comorbid pulmonary interstitial lesions, the benefits and toxicity of radiotherapy should be carefully weighed. The radiation dose to the lungs should be further limited to V20 ≤20% (259) and MLD ≤12.3 Gy (260) if radiotherapy is deemed necessary. If SBRT is applied, the Radiation Therapy Oncology Group (RTOG) recommends that V20 should be <10% that used in the normal setting; if the underlying pulmonary disease is comorbid, we recommend minimizing V20. In addition, the radiation dose to the lungs should also be adjusted according to any previous or current chemotherapy, immunotherapy, or lobectomy, as well as other affecting factors. The patients should be closely observed to enable timely detection and early treatment of radiation pneumonia, and grade ≥3 radiation pneumonia should be avoided. In addition, more sophisticated radiotherapy techniques such as proton and carbon ion radiotherapy (261-264) may further reduce pulmonary toxicity and thus help configure the treatment landscape of lung cancer.
Consensus 17: surgical treatment for patients with LC-COPD, complete preoperative cardiopulmonary function tests, adequate intraoperative and postoperative risk assessment, and proper perioperative management should be carried out. Appropriate minimally invasive surgery may be performed on the basis of a multidisciplinary team discussion (recommendation category: A; level of evidence: 2a)
Preoperative assessment
Surgical radical resection of tumors is the best treatment to cure lung cancer. However, about one-third of patients with LC-COPD may not be suitable for lung cancer surgery due to their poor physical conditions (265). Studies have shown that compared with lobectomy by thoracotomy, video-assisted thoracoscopic surgery (VATS)-lobectomy was associated with a lower incidence of pulmonary complications for patients with LC-COPD (266,267). Another study showed that mortality rate after VATS-lobectomy in poor lung function was similar to that of patients with normal lung function (268). Therefore, minimally invasive surgery may be preferred in order to decrease the mortality and morbidity risks for patients with LC-COPD. Several recent prospective studies have demonstrated that lung sparing procedures (wedge and/or anatomical segmentectomy) is both effective and safe for lung cancer patients with small lesions (<2 cm in size) (269-271). According to the Chinese expert consensus on multidisciplinary perioperative airway management (2018 version) (272), coexisting COPD is a preoperative risk factor, and risk assessment (including preoperative pulmonary function tests) should be performed for these patients.
Lung function test
(I) Lung ventilation and diffusion functions. According to the expert consensus on perioperative airway management in thoracic surgery (273-278), appropriate surgical methods can be selected based on FEV1 (Table 5).
Table 5
| FEV1 (L) | Recommended feasible techniques |
|---|---|
| >2 | Pneumonectomy |
| 0.8–2 | Lobectomy |
| <0.8 | Segmental or wedge lung resection |
FEV1, a forced expiratory volume in 1 second.
There are subtle differences in the interpretation of surgical risk for pulmonary diffusion function by several national expert consensus (Table 6) (273-282).
Table 6
| Surgical risks | DLCOPred% | |
|---|---|---|
| Domestic | Foreign | |
| Low | >80% | >60% |
| Medium | 40–80% | 30–60% |
| High | <40% | <30% |
DLCO, diffusing lung capacity for carbon monoxide; Pred, predicted
(II) Perioperative risk assessment. According to the guidelines of the American College of Chest Physicians (ACCP) and Perioperative Medicine (second Edition) (274,283), FEV1 and DLCO must be measured before surgery in all patients with lung cancer requiring surgery, and the predicted postoperative FEV1 (ppoFEV1) and predicted postoperative DLCO (ppoDLCO) must be calculated based on the extent of lung tissue to be resected. The calculated values provide a risk assessment for patients who are about to undergo surgery (Figure 2). Predictions of postoperative lung function are calculated based on the following equation: (i) lung lobectomy: ppo PFV1 = preoperative FEV1 value × Q%, where Q% is the proportion of the remaining lung after resection (calculated by alternative methods if the resected lung segment is obstructed); (ii) pulmonary resection (by radionuclide lung perfusion scan to measure the total perfusion fraction of the resected lung): ppoFEV1= FEV1 × (1 − total perfusion fraction of the resected lung).
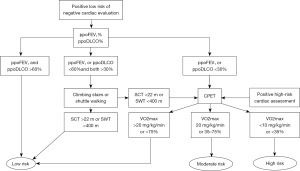
In addition, radical pneumonectomy may also be considered in patients who demonstrate acceptable exercise capacity (stair climbing test >22 m) with a ppo FEV1 as low as 30%.
(III) Comprehensive examination of cardiopulmonary function. For patients with LC-COPD carrying high surgical risk, COPD management and pulmonary rehabilitation should be carried out first before a second assessment so as to minimize the surgical risk. In addition, it is recommended that surgery be performed after the following comprehensive examination conditions are met: no carbon dioxide retention is found during blood gas analysis, echocardiography indicates good cardiac function [with an ejection fraction (EF) >50%], and peak expiratory flow (PEF) >300 L/min.
Perioperative management and postoperative support
Medical management, smoking cessation, and pulmonary rehabilitation are three major strategies for improving the prognosis of patients with LC-COPD after surgery (264,273). Various drugs including bronchodilators, ICSs, and antibiotics, help alleviate symptoms and prevent exacerbations in patients with COPD, thereby improving perioperative safety (273,284,285).
Bronchodilators
Kobayashi et al. (284) in their retrospective study revealed that use of inhaled tiotropium 2 weeks before surgery significantly improved lung function indicators (e.g., FEV1 and FVC) in patients with LC-COPD, thus creating opportunities for patients with poor lung function and intolerant of surgical treatment to receive surgery. Leiro-Fernández et al. (285) also found in a prospective study that double bronchodilation (LABA + LAMA) during the perioperative period in patients with LC-COPD might also improve lung function, promote postoperative recovery and reduce the risk of surgery.
Glucocorticoids
Bolukbas (91) added ICSs to LABA/LAMA in patients with LC-COPD and found that the predicted FEV1% was significantly increased while the incidence of postoperative pulmonary complications was significantly reduced, indicating that perioperative combination with ICS not only effectively improved lung function but it also reduced postoperative pulmonary complications such as lung infection in patients with LC-COPD.
Antimicrobial agents
Yamada et al. (286) reported that, for patients with LC-COPD, Staphylococcus aureus and Gram-negative bacilli should be targets for postoperative prophylactic antibiotic selection so as to prevent postoperative pneumonia and avoid the negative effects of potential pathogenic bacteria. If postoperative pulmonary infection develops, respiratory specimens should first be collected for etiological identification; in addition, the risk factors for sepsis and multidrug-resistant pathogen infections should be assessed. Based on these risk factors and the results of antimicrobial susceptibility tests, antimicrobial agents should be rationally selected and administered, with the principle of escalation and de-escalation being followed (287).
Smoking cessation
Chinese guidelines for perioperative airway management in thoracic surgery (2020 edition) recommend at least 4 weeks of preoperative smoking cessation (288). Studies (289,290) showed that smoking cessation significantly reduced the incidence of postoperative complications, and the effect of each week of smoking cessation increased by 19%. In addition, Smoking cessation for ≥4 weeks reduced the RR by 20% and the risk of postoperative pulmonary complications by 23% compared to smoking cessation for <4 weeks. Preoperative smoking cessation for 8 weeks reduced the risk of pulmonary complications by 46% (RR =0.54, 95% CI: 0.35–0.85).
Respiratory rehabilitation
The effectiveness of preoperative pulmonary rehabilitation has been demonstrated in patients with LC-COPD undergoing resection of lung cancer (291-293). In 27 patients with impaired lung function, Divisi et al. (293) observed a significant increase in FEV1 after pulmonary rehabilitation 4 weeks before surgery. Other study (294) also revealed that, for patients with moderate-to-severe COPD complicated by lung cancer, FEV1 and FVC significantly increased after comprehensive perioperative treatment. In addition, Zheng et al. (295) concluded that, in addition to COPD, advanced tumors were indications for respiratory rehabilitation; moreover, pulmonary rehabilitation reduced postoperative complications and shortened the hospital stay (296,297). These findings highlight the importance of preoperative pulmonary rehabilitation in patients with lung cancer, as it helps to reduce the functional limitations related to surgery and improves the postoperative recovery.
In summary, we recommend that patients with LC-COPD should quit smoking at least 4 weeks before surgery, and use inhaled LABA or LAMA 2 weeks before surgery, combined with ICS and intravenously administered theophylline if necessary and then use prophylactic antibiotics after surgery, which may be supplemented by respiratory rehabilitation, so as to improve both the surgical safety and prognosis of outcome.
Multidisciplinary treatment
The diagnosis and treatment of LC-COPD is a highly complex process, involving multiple factors such as the clinical stage, pathological findings, tumor heterogeneity, disease progression and individual case differences. Experts from multiple departments, including oncology, thoracic surgery, respiratory and critical care medicine, radiotherapy, pathology, medical imaging, anesthesiology, nutrition, and bronchoscopy should be called upon to develop a multidisciplinary treatment (MDT) protocol that comprehensively weighs the benefits and risks of the treatment. In addition, when there are disagreements with regard to the staging, diagnosis, or treatment of a patient with LC-COPD, MDT can help to improve the precision of the diagnosis and treatment. Zhi et al. (281) also suggested in the “Chinese standard for diagnosis and treatment of primary lung cancer” (2015 edition) that the management of patients with lung cancer should be based on both MDT and individualized treatment an attempt to improve the patient’s quality of life and prolong survival by developing a well-designed and rational MDT protocol that combines surgery, chemotherapy, radiotherapy, and molecularly-targeted therapy based on patients’ physical condition, extent of tumor invasion, and tumor progression. Data from the Surveillance, Epidemiology and End Results (SEER) database show that pulmonologist involvement in the care of patients with early NSCLC and COPD may both increase the surgical resection rate and reduce the risk of death (298). For patients diagnosed with lung cancer, MDT based on systemic therapy should be adopted, and individualized treatment strategies should be formulated according to the pathological type and molecular genetic characteristics of the tumors as well as the physical status of the patients so as to maximize the patients’ survival time, curb disease progression, and improve the quality of life (299). According to the US National Comprehensive Cancer Network (NCCN) guidelines (300), the management of lung cancer require the involvement of MDT to improve treatment benefits and reduce complications. In addition, MDT can improve survival and adherence to evidence-based guidelines as well as the timeliness of care for patients with lung cancer. Therefore, the importance of MDT should be emphasized to further optimize the management of patients with LC-COPD.
Conclusions
In conclusion, Patients with LC-COPD should receive simultaneous treatment for both conditions, with careful consideration given to the potential interplay between the two diseases and the possibility of adverse reactions to treatment.
Acknowledgments
The authors appreciate the academic support from The Society for Translational Medicine and AME Lung Cancer Collaborative Group.
Funding: None.
Footnote
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-339/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-339/coif). Yong Song serves as an Editor-in-Chief of Translational Lung Cancer Research from September 2020 to August 2022. RB reports payment from AZ, BI, NOVARTIS, EISAI, MSD, OTSUKA, LILLY, ROCHE, Amgen, GSK, Seagen, Pierre Fabre Italfarmaco, outside the present manuscript. Nicolas Girard reports receiving research grants or support from Abbvie, Amgen, AstraZeneca, Beigene, Boehringer Ingelheim, Bristol-Myers-Squibb, Daiichi-Sankyo, Eli-Lilly, Gilead, Hoffmann-La Roche, Janssen, LeoPharma, Merck, Merck Sharp & Dohme, Novartis, Sivan; receiving payment from Abbvie, Amgen, AstraZeneca, Beigene, Bristol Myers Squibb, Daiichi-Sankyo, Eli-Lilly, Gilead, Hoffmann - La Roche, Ipsen, Janssen, LeoPharma, Medtronic, Merck Sharp & Dohme, Novartis, Pierre Fabre, Pfizer, Sanofi, and Takeda; having participation on a data safety monitoring board for Roche; having leadership role in the International Thymic Malignancy Interest Group; and having employment of a family member with AstraZeneca. AP received consulting or advisory role from Roche/Genentech, Bristol Myers Squibb, AstraZeneca, MSD Oncology, Pfizer, Boehringer Ingelheim, Janssen, Novartis, Daiichi Sankyo Europe GmbH, Bayer; payment or honoraria for speakers’ bureau form AstraZeneca, Janssen, Boehringer Ingelheim, Daiichi Sankyo Europe GmbH, MSD Oncology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Guan WJ, Zheng XY, Chung KF, et al. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet 2016;388:1939-51. [Crossref] [PubMed]
- Liang ZY, Chen RC. Revision to the guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021): process and perspective. Zhonghua Jie He He Hu Xi Za Zhi 2021;44:165-6. [Crossref] [PubMed]
- Miao JL, Cai JJ, Qin XF, et al. Analysis of the Clinicopathological Characteristics and Risk Factors in Patients with Lung Cancer and Chronic Obstructive Pulmonary Disease. Biomed Res Int 2018;2018:8398156. [Crossref] [PubMed]
- Caplin M, Festenstein F. Relation between lung cancer, chronic bronchitis, and airways obstruction. Br Med J 1975;3:678-80. [Crossref] [PubMed]
- Machida H, Inoue S, Shibata Y, et al. The Incidence and Risk Analysis of Lung Cancer Development in Patients with Chronic Obstructive Pulmonary Disease: Possible Effectiveness of Annual CT-Screening. Int J Chron Obstruct Pulmon Dis 2021;16:739-49. [Crossref] [PubMed]
- Ahn SV, Lee E, Park B, et al. Cancer development in patients with COPD: a retrospective analysis of the National Health Insurance Service-National Sample Cohort in Korea. BMC Pulm Med 2020;20:170. [Crossref] [PubMed]
- Park HY, Kang D, Shin SH, et al. Chronic obstructive pulmonary disease and lung cancer incidence in never smokers: a cohort study. Thorax 2020;75:506-9. [Crossref] [PubMed]
- Zheng Y, Huang Y, Zheng X, et al. Deaths from COPD in patients with cancer: a population-based study. Aging (Albany NY) 2021;13:12641-59. [Crossref] [PubMed]
- Zhang J, Zhou JB, Lin XF, et al. Prevalence of undiagnosed and undertreated chronic obstructive pulmonary disease in lung cancer population. Respirology 2013;18:297-302. [Crossref] [PubMed]
- Wang F, Xie XH, Lin XQ, et al. Exploration of the treatment model for patients with advanced non-small cell lung cancer complicated with chronic obstructive pulmonary disease based on real-world data. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:450-4. [Crossref] [PubMed]
- Ajimizu H, Ozasa H, Sato S, et al. Survival impact of treatment for chronic obstructive pulmonary disease in patients with advanced non-small-cell lung cancer. Sci Rep 2021;11:23677. [Crossref] [PubMed]
- Xu W, Zhu J, Li L, et al. The Prognostic Role of Chronic Obstructive Pulmonary Disease for Lung Cancer After Pulmonary Resection. J Surg Res 2022;275:137-48. [Crossref] [PubMed]
- Qi C, Sun SW, Xiong XZ. From COPD to Lung Cancer: Mechanisms Linking, Diagnosis, Treatment, and Prognosis. Int J Chron Obstruct Pulmon Dis 2022;17:2603-21. [Crossref] [PubMed]
- Fang X, Wang X, Bai C. COPD in China: the burden and importance of proper management. Chest 2011;139:920-9. [Crossref] [PubMed]
- Ordóñez-Mena JM, Schöttker B, Mons U, et al. Quantification of the smoking-associated cancer risk with rate advancement periods: meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med 2016;14:62. [Crossref] [PubMed]
- Chen ZM, Peto R, Iona A, et al. Emerging tobacco-related cancer risks in China: A nationwide, prospective study of 0.5 million adults. Cancer 2015;121:3097-106. [Crossref] [PubMed]
- Liu S, Zhou Y, Liu S, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax 2017;72:788-95. [Crossref] [PubMed]
- Pope CA 3rd, Coleman N, Pond ZA, et al. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ Res 2020;183:108924. [Crossref] [PubMed]
- Qiu AY, Leng S, McCormack M, et al. Lung Effects of Household Air Pollution. J Allergy Clin Immunol Pract 2022;10:2807-19. [Crossref] [PubMed]
- Murgia N, Gambelunghe A. Occupational COPD-The most under-recognized occupational lung disease? Respirology 2022;27:399-410. [Crossref] [PubMed]
- Grahn K, Gustavsson P, Andersson T, et al. Occupational exposure to particles and increased risk of developing chronic obstructive pulmonary disease (COPD): A population-based cohort study in Stockholm, Sweden. Environ Res 2021;200:111739. [Crossref] [PubMed]
- Blanc PD, Iribarren C, Trupin L, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax 2009;64:6-12. [Crossref] [PubMed]
- Li L, Jiang M, Li X, et al. Association between Coalmine Dust and Mortality Risk of Lung Cancer: A Meta-Analysis. Biomed Res Int 2021;2021:6624799. [Crossref] [PubMed]
- Lenters V, Vermeulen R, Dogger S, et al. A meta-analysis of asbestos and lung cancer: is better quality exposure assessment associated with steeper slopes of the exposure-response relationships? Environ Health Perspect 2011;119:1547-55. [Crossref] [PubMed]
- Lahousse L, Seys LJM, Joos GF, et al. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur Respir J 2017;50:1602470. [Crossref] [PubMed]
- Fan YG, Jiang Y, Chang RS, et al. Prior lung disease and lung cancer risk in an occupational-based cohort in Yunnan, China. Lung Cancer 2011;72:258-63. [Crossref] [PubMed]
- Silva GE, Sherrill DL, Guerra S, et al. Asthma as a risk factor for COPD in a longitudinal study. Chest 2004;126:59-65. [Crossref] [PubMed]
- Byrne AL, Marais BJ, Mitnick CD, et al. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis 2015;32:138-46. [Crossref] [PubMed]
- Wong JYY, Zhang H, Hsiung CA, et al. Tuberculosis infection and lung adenocarcinoma: Mendelian randomization and pathway analysis of genome-wide association study data from never-smoking Asian women. Genomics 2020;112:1223-32. [Crossref] [PubMed]
- Christenson SA, Smith BM, Bafadhel M, et al. Chronic obstructive pulmonary disease. Lancet 2022;399:2227-42. [Crossref] [PubMed]
- Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer 2015;90:121-7. [Crossref] [PubMed]
- Qin L, Guitart M, Curull V, et al. Systemic Profiles of microRNAs, Redox Balance, and Inflammation in Lung Cancer Patients: Influence of COPD. Biomedicines 2021;9:1347. [Crossref] [PubMed]
- Barreiro E, Fermoselle C, Mateu-Jimenez M, et al. Oxidative stress and inflammation in the normal airways and blood of patients with lung cancer and COPD. Free Radic Biol Med 2013;65:859-71. [Crossref] [PubMed]
- Mateu-Jiménez M, Sánchez-Font A, Rodríguez-Fuster A, et al. Redox Imbalance in Lung Cancer of Patients with Underlying Chronic Respiratory Conditions. Mol Med 2016;22:85-98. [Crossref] [PubMed]
- Chronic Obstructive Pulmonary Disease Group of Chinese Thoracic Society. Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021). Zhonghua Jie He He Hu Xi Za Zhi 2021;44:170-205. [Crossref] [PubMed]
- Sekine Y, Katsura H, Koh E, et al. Early detection of COPD is important for lung cancer surveillance. Eur Respir J 2012;39:1230-40. [Crossref] [PubMed]
- Xia Y, Zha J, Curull V, et al. Gene expression profile of epithelial-mesenchymal transition in tumours of patients with nonsmall cell lung cancer: the influence of COPD. ERJ Open Res 2022;8:00105-2022. [Crossref] [PubMed]
- Mateu-Jimenez M, Curull V, Pijuan L, et al. Systemic and Tumor Th1 and Th2 Inflammatory Profile and Macrophages in Lung Cancer: Influence of Underlying Chronic Respiratory Disease. J Thorac Oncol 2017;12:235-48. [Crossref] [PubMed]
- Tang J, Ramis-Cabrer D, Curull V, et al. B Cells and Tertiary Lymphoid Structures Influence Survival in Lung Cancer Patients with Resectable Tumors. Cancers (Basel) 2020;12:2644. [Crossref] [PubMed]
- Tang J, Ramis-Cabrer D, Curull V, et al. Immune Cell Subtypes and Cytokines in Lung Tumor Microenvironment: Influence of COPD. Cancers (Basel) 2020;12:1217. [Crossref] [PubMed]
- Wang BN, Zhang JX, Bai C. Research progress in the relationship between cellular senescence and chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 2021;44:59-63. [Crossref] [PubMed]
- Liu P, Li F, Lin J, et al. m(6)A-independent genome-wide METTL3 and METTL14 redistribution drives the senescence-associated secretory phenotype. Nat Cell Biol 2021;23:355-65. [Crossref] [PubMed]
- Morlá M, Busquets X, Pons J, et al. Telomere shortening in smokers with and without COPD. Eur Respir J 2006;27:525-8. [Crossref] [PubMed]
- Ahmad T, Sundar IK, Tormos AM, et al. Shelterin Telomere Protection Protein 1 Reduction Causes Telomere Attrition and Cellular Senescence via Sirtuin 1 Deacetylase in Chronic Obstructive Pulmonary Disease. Am J Respir Cell Mol Biol 2017;56:38-49. [Crossref] [PubMed]
- Zhu J, Liu W, Chen C, et al. TPP1 OB-fold domain protein suppresses cell proliferation and induces cell apoptosis by inhibiting telomerase recruitment to telomeres in human lung cancer cells. J Cancer Res Clin Oncol 2019;145:1509-19. [Crossref] [PubMed]
- Aghapour M, Raee P, Moghaddam SJ, et al. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am J Respir Cell Mol Biol 2018;58:157-69. [Crossref] [PubMed]
- Hou W, Hu S, Li C, et al. Cigarette Smoke Induced Lung Barrier Dysfunction, EMT, and Tissue Remodeling: A Possible Link between COPD and Lung Cancer. Biomed Res Int 2019;2019:2025636. [Crossref] [PubMed]
- Lee HW, Jose CC, Cuddapah S. Epithelial-mesenchymal transition: Insights into nickel-induced lung diseases. Semin Cancer Biol 2021;76:99-109. [Crossref] [PubMed]
- Bronte G, Bravaccini S, Bronte E, et al. Epithelial-to-mesenchymal transition in the context of epidermal growth factor receptor inhibition in non-small-cell lung cancer. Biol Rev Camb Philos Soc 2018;93:1735-46. [Crossref] [PubMed]
- Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer 2013;13:233-45. [Crossref] [PubMed]
- He S, Li L, Sun S, et al. A Novel Murine Chronic Obstructive Pulmonary Disease Model and the Pathogenic Role of MicroRNA-21. Front Physiol 2018;9:503. [Crossref] [PubMed]
- Mateu-Jimenez M, Curull V, Rodríguez-Fuster A, et al. Profile of epigenetic mechanisms in lung tumors of patients with underlying chronic respiratory conditions. Clin Epigenetics 2018;10:7. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962-70. [Crossref] [PubMed]
- Society CT. Chinese expert consensus on diagnosis of early lung cancer (2023 Edition). Zhonghua Jie He He Hu Xi Za Zhi 2023;46:1-18. [Crossref] [PubMed]
- Chen LA. Early diagnosis and treatmet of lung cancer: the responsibility of respiratory physician. Zhonghua Jie He He Hu Xi Za Zhi 2018;41:753-4. [Crossref] [PubMed]
- Li WM, Zhao S, Liu LX. The Methods and Clinical Significance of Early Diagnosis of Lung Cancer. Sichuan Da Xue Xue Bao Yi Xue Ban 2017;48:331-5.
- Sanchez-Salcedo P, Wilson DO, de-Torres JP, et al. Improving selection criteria for lung cancer screening. The potential role of emphysema. Am J Respir Crit Care Med 2015;191:924-31. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380-6. [Crossref] [PubMed]
- Young RP, Duan F, Chiles C, et al. Airflow Limitation and Histology Shift in the National Lung Screening Trial. The NLST-ACRIN Cohort Substudy. Am J Respir Crit Care Med 2015;192:1060-7. [Crossref] [PubMed]
- Gould MK. Lung Cancer Screening in Individuals with Chronic Obstructive Pulmonary Disease. Finding the Sweet Spot. Am J Respir Crit Care Med 2015;192:1027-8. [Crossref] [PubMed]
- Lowry KP, Gazelle GS, Gilmore ME, et al. Personalizing annual lung cancer screening for patients with chronic obstructive pulmonary disease: A decision analysis. Cancer 2015;121:1556-62. [Crossref] [PubMed]
- de-Torres JP, Casanova C, Marín JM, et al. Exploring the impact of screening with low-dose CT on lung cancer mortality in mild to moderate COPD patients: a pilot study. Respir Med 2013;107:702-7. [Crossref] [PubMed]
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-12. [Crossref] [PubMed]
- Young RP, Ward RC, Scott RJ, et al. Airflow limitation and mortality during cancer screening in the National Lung Screening Trial: why quantifying airflow limitation matters. Thorax 2023;78:690-7. [Crossref] [PubMed]
- Turner MC, Chen Y, Krewski D, et al. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med 2007;176:285-90. [Crossref] [PubMed]
- Powell HA, Iyen-Omofoman B, Baldwin DR, et al. Chronic obstructive pulmonary disease and risk of lung cancer: the importance of smoking and timing of diagnosis. J Thorac Oncol 2013;8:6-11. [Crossref] [PubMed]
- Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology 2016;21:269-79. [Crossref] [PubMed]
- Lin XF, Wang Q, Bai CX, et al. A retrospective study of diagnosis and treatment of chronic obstructive pulmonary disease in patients with lung cancer. Zhonghua Yi Xue Za Zhi 2011;91:2815-8.
- Li T, He X, Chen Y. Mechanism of lung cancer and chronic obstructive pulmonary disease. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2017;42:1212-6. [Crossref] [PubMed]
- Tønnesen P. Smoking cessation and COPD. Eur Respir Rev 2013;22:37-43. [Crossref] [PubMed]
- Caini S, Del Riccio M, Vettori V, et al. Quitting Smoking At or Around Diagnosis Improves the Overall Survival of Lung Cancer Patients: A Systematic Review and Meta-Analysis. J Thorac Oncol 2022;17:623-36. [Crossref] [PubMed]
- Wang W, Dou S, Dong W, et al. Impact of COPD on prognosis of lung cancer: from a perspective on disease heterogeneity. Int J Chron Obstruct Pulmon Dis 2018;13:3767-76. [Crossref] [PubMed]
- Qin YY, Zhang DH, Lin XQ, et al. Clinical analysis of 36 cases of advanced non-small cell lung cancer (NSCLC) with performance status (PS) scores between 2 and 4. Zhonghua Zhong Liu Za Zhi 2017;39:855-61. [Crossref] [PubMed]
- Qin YY, Zhou CZ, Zhu Z, et al. Case report: dermatomyositis associated with lung cancer with heterogeneous morphology. J Thorac Dis 2017;9:E1110-7. [Crossref] [PubMed]
- Zhou J, Chao Y, Yao D, et al. Impact of chronic obstructive pulmonary disease on immune checkpoint inhibitor efficacy in advanced lung cancer and the potential prognostic factors. Transl Lung Cancer Res 2021;10:2148-62. [Crossref] [PubMed]
- Sun JM, Kim TS, Lee J, et al. Unsuspected pulmonary emboli in lung cancer patients: the impact on survival and the significance of anticoagulation therapy. Lung Cancer 2010;69:330-6. [Crossref] [PubMed]
- Zhou C, Li S, Liu J, et al. International consensus on severe lung cancer-the first edition. Transl Lung Cancer Res 2021;10:2633-66.
- Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. Blood 2012;120:1067-76. [Crossref] [PubMed]
- Almendro V, Marusyk A, Polyak K. Cellular heterogeneity and molecular evolution in cancer. Annu Rev Pathol 2013;8:277-302. [Crossref] [PubMed]
- Li L, Jiang L, Wang Y, et al. Combination of Metformin and Gefitinib as First-Line Therapy for Nondiabetic Advanced NSCLC Patients with EGFR Mutations: A Randomized, Double-Blind Phase II Trial. Clin Cancer Res 2019;25:6967-75. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov 2021;11:858-73. [Crossref] [PubMed]
- Wang Y, Li L, Han R, et al. Clinical analysis by next-generation sequencing for NSCLC patients with MET amplification resistant to osimertinib. Lung Cancer 2018;118:105-10. [Crossref] [PubMed]
- Xu Q, Ye L, Huang L, et al. Serum Exosomal miRNA Might Be a Novel Liquid Biopsy to Identify Leptomeningeal Metastasis in Non-Small Cell Lung Cancer. Onco Targets Ther 2021;14:2327-35. [Crossref] [PubMed]
- Liang W, Zhao Y, Huang W, et al. Liquid biopsy for early stage lung cancer. J Thorac Dis 2018;10:S876-81. [Crossref] [PubMed]
- Santarpia M, Liguori A, D'Aveni A, et al. Liquid biopsy for lung cancer early detection. J Thorac Dis 2018;10:S882-97. [Crossref] [PubMed]
- Russo A, De Miguel Perez D, Gunasekaran M, et al. Liquid biopsy tracking of lung tumor evolutions over time. Expert Rev Mol Diagn 2019;19:1099-108. [Crossref] [PubMed]
- Shin SH, Shin S, Im Y, et al. Effect of perioperative bronchodilator therapy on postoperative pulmonary function among lung cancer patients with COPD. Sci Rep 2021;11:8359. [Crossref] [PubMed]
- Hu B, Zhang L, Yu R, et al. Clinical Efficacy of Inhaled Therapy with Symbicort Turbulaler in the Treatment of Patients with Lung Cancer Complicated with Chronic Obstructive Pulmonary Disease and the Effect on the Quality of Life. Progress in Modern Biomedicine 2017;17:5686-9.
- Azuma Y, Sano A, Sakai T, et al. Prognostic and functional impact of perioperative LAMA/LABA inhaled therapy in patients with lung cancer and chronic obstructive pulmonary disease. BMC Pulm Med 2021;21:174. [Crossref] [PubMed]
- Bölükbas S, Eberlein M, Eckhoff J, et al. Short-term effects of inhalative tiotropium/formoterol/budenoside versus tiotropium/formoterol in patients with newly diagnosed chronic obstructive pulmonary disease requiring surgery for lung cancer: a prospective randomized trial. Eur J Cardiothorac Surg 2011;39:995-1000. [Crossref] [PubMed]
- Qin Y, Zhou C, Zhang X, et al. Clinical Research of Patients with Primary Bronchogenic Carcinoma Complicated with Chronic Obstructive Pulmonary Disease. Chin J Respir Crit Care Med 2013;12:65-8.
- Disease PoPSfCDaToCOP. Standards for clinical practice in diagnosis and treatment of chronic obstructive pulmonary disease. Guo Ji Hu Xi Za Zhi 2022:401-9.
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Diseas (2023 REPORT). Available online: https://goldcopd.org/2023-gold-report/
- Westerik JA, Metting EI, van Boven JF, et al. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res 2017;18:31. [Crossref] [PubMed]
- Raviv S, Hawkins KA, DeCamp MM Jr, et al. Lung cancer in chronic obstructive pulmonary disease: enhancing surgical options and outcomes. Am J Respir Crit Care Med 2011;183:1138-46. [Crossref] [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881-93. [Crossref] [PubMed]
- Rabe KF, Martinez FJ, Ferguson GT, et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N Engl J Med 2020;383:35-48. [Crossref] [PubMed]
- Lipson DA, Barnhart F, Brealey N, et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N Engl J Med 2018;378:1671-80. [Crossref] [PubMed]
- Inhalation Therapy and Respiratory Rehabilitation Group, Respiratory Diseases Committee of China Association of Medical Equipment. Chinese Chronic Obstructive Pulmonary Disease Coalition. Chinese expert consensus on standard application of inhalation device in stable chronic airway disease patients. Zhonghua Jie He He Hu Xi Za Zhi 2019;42:241-53. [Crossref] [PubMed]
- A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report. The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives. JAMA 2000;283:3244-54.
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Diseas (2022 REPORT). Available online: https://goldcopd.org/gold-reports/
- Shah NM, D'Cruz RF, Murphy PB. Update: non-invasive ventilation in chronic obstructive pulmonary disease. J Thorac Dis 2018;10:S71-9. [Crossref] [PubMed]
- Respiratory & Critical Care Medicine Group of Chinese Thoracic Society. Respiratory & Critical Care Medicine Committee of Chinese Association of Chest Physician. Zhonghua Jie He He Hu Xi Za Zhi 2019;42:83-91. [Crossref] [PubMed]
- Au DH, Udris EM, Engelberg RA, et al. A randomized trial to improve communication about end-of-life care among patients with COPD. Chest 2012;141:726-35. [Crossref] [PubMed]
- Collins PF, Elia M, Stratton RJ. Nutritional support and functional capacity in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respirology 2013;18:616-29. [Crossref] [PubMed]
- Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196-204. [Crossref] [PubMed]
- Keenan SP, Sinuff T, Burns KE, et al. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ 2011;183:E195-214. [Crossref] [PubMed]
- An S, Chen Z, Fu G, et al. Analysis on new anti-tumor drugs induced cardiotoxicity. Clinical Medication Journal 2021;19:46-51.
- Yuan D, Yu G, Wang D. Research progress on the effect of tyrosine kinase inhibitor on QT interval and its clinical significance. Chin J Clin Pharmacol Ther 2017;22:825-30.
- Lin Y, Yu H, Fang F, et al. Cancer treatment and QT interval prolongation. Chinese Journal of the Frontiers of Medical Science 2017;9:1-6. (Electronic Version).
- Park HJ, Park HS, Cha YJ, et al. Efficacy of adjuvant chemotherapy for completely resected stage IB non-small cell lung cancer: a retrospective study. J Thorac Dis 2018;10:2279-87. [Crossref] [PubMed]
- Wisnivesky JP, Smith CB, Packer S, et al. Survival and risk of adverse events in older patients receiving postoperative adjuvant chemotherapy for resected stages II-IIIA lung cancer: observational cohort study. BMJ 2011;343:d4013. [Crossref] [PubMed]
- Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol 2015;26:1573-88. [Crossref] [PubMed]
- Lim E, Harris G, Patel A, et al. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380-8. [Crossref] [PubMed]
- Santos FN, de Castria TB, Cruz MR, et al. Chemotherapy for advanced non-small cell lung cancer in the elderly population. Cochrane Database Syst Rev 2015;2015:CD010463. [Crossref] [PubMed]
- Lee YG, Lee JH, Kim SH, et al. Comparative analysis between combination and single-agent chemotherapy for elderly patients with advanced non-small cell lung cancer: A nationwide population-based outcome study. Lung Cancer 2018;122:88-93. [Crossref] [PubMed]
- Davidoff AJ, Tang M, Seal B, et al. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2191-7. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Gridelli C, Morabito A, Cavanna L, et al. Cisplatin-Based First-Line Treatment of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: Joint Analysis of MILES-3 and MILES-4 Phase III Trials. J Clin Oncol 2018;36:2585-92. [Crossref] [PubMed]
- Pujol JL, Breton JL, Gervais R, et al. Gemcitabine-docetaxel versus cisplatin-vinorelbine in advanced or metastatic non-small-cell lung cancer: a phase III study addressing the case for cisplatin. Ann Oncol 2005;16:602-10. [Crossref] [PubMed]
- Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011;378:1079-88. [Crossref] [PubMed]
- Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol 2007;18:317-23. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 2003;21:3016-24. [Crossref] [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [Crossref] [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [Crossref] [PubMed]
- Zukin M, Barrios CH, Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 2013;31:2849-53. [Crossref] [PubMed]
- Morabito A, Gebbia V, Di Maio M, et al. Randomized phase III trial of gemcitabine and cisplatin vs. gemcitabine alone in patients with advanced non-small cell lung cancer and a performance status of 2: the CAPPA-2 study. Lung Cancer 2013;81:77-83. [Crossref] [PubMed]
- Oncology Society of Chinese Medical Association. Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer (2022 edition). Zhonghua Zhong Liu Za Zhi 2022;44:457-90. [Crossref] [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [Crossref] [PubMed]
- Gridelli C, de Marinis F, Thomas M, et al. Final efficacy and safety results of pemetrexed continuation maintenance therapy in the elderly from the PARAMOUNT phase III study. J Thorac Oncol 2014;9:991-7. [Crossref] [PubMed]
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55. [Crossref] [PubMed]
- Qin S, Cheng Y, Luo L, et al. A randomized, controlled, multicenter phase III study to compare lobaplatin/paclitaxel versus carboplatin/paclitaxel as the first-line treatment in Chinese patients with advanced non-small cell lung cancer:Subgroup analysis of the patients over 60 years old. Chinese Clinical Oncology 2019;24:732-7.
- Shukuya T, Yamanaka T, Seto T, et al. Nedaplatin plus docetaxel versus cisplatin plus docetaxel for advanced or relapsed squamous cell carcinoma of the lung (WJOG5208L): a randomised, open-label, phase 3 trial. Lancet Oncol 2015;16:1630-8. [Crossref] [PubMed]
- Santos FN, Cruz MR, Riera R. Chemotherapy for Advanced Non-Small-Cell Lung Cancer in Elderly Patients. JAMA Oncol 2016;2:1645-6. [Crossref] [PubMed]
- Wang Z, Huang C, Yang JJ, et al. A randomised phase II clinical trial of nab-paclitaxel and carboplatin compared with gemcitabine and carboplatin as first-line therapy in advanced squamous cell lung carcinoma (C-TONG1002). Eur J Cancer 2019;109:183-91. [Crossref] [PubMed]
- Brodowicz T, Krzakowski M, Zwitter M, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer 2006;52:155-63. [Crossref] [PubMed]
- Sundstrøm S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 2002;20:4665-72. [Crossref] [PubMed]
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012;30:1692-8. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Liu SV, Reck M, Mansfield AS, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol 2021;39:619-30. [Crossref] [PubMed]
- Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29:2215-22. [Crossref] [PubMed]
- Okamoto H, Watanabe K, Nishiwaki Y, et al. Phase II study of area under the plasma-concentration-versus-time curve-based carboplatin plus standard-dose intravenous etoposide in elderly patients with small-cell lung cancer. J Clin Oncol 1999;17:3540-5. [Crossref] [PubMed]
- Cheng Y, Fan Y, Liu X, et al. Randomized controlled trial of lobaplatin plus etoposide vs. cisplatin plus etoposide as first-line therapy in patients with extensive-stage small cell lung cancer. Oncol Lett 2019;17:4701-9. [Crossref] [PubMed]
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85-91. [Crossref] [PubMed]
- Ding PN, Lord SJ, Gebski V, et al. Risk of Treatment-Related Toxicities from EGFR Tyrosine Kinase Inhibitors: A Meta-analysis of Clinical Trials of Gefitinib, Erlotinib, and Afatinib in Advanced EGFR-Mutated Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:633-43. [Crossref] [PubMed]
- Herbst RS, Wu YL, John T, et al. Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non-Small-Cell Lung Cancer: Updated Results From the Phase III Randomized ADAURA Trial. J Clin Oncol 2023;41:1830-40. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Suh CH, Park HS, Kim KW, et al. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: Meta-analysis of 153 cohorts with 15,713 patients: Meta-analysis of incidence and risk factors of EGFR-TKI pneumonitis in NSCLC. Lung Cancer 2018;123:60-9. [Crossref] [PubMed]
- Suh CH, Kim KW, Pyo J, et al. The incidence of ALK inhibitor-related pneumonitis in advanced non-small-cell lung cancer patients: A systematic review and meta-analysis. Lung Cancer 2019;132:79-86. [Crossref] [PubMed]
- Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 2008;177:1348-57. [Crossref] [PubMed]
- Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2006;24:2549-56. [Crossref] [PubMed]
- Gemma A, Kudoh S, Ando M, et al. Final safety and efficacy of erlotinib in the phase 4 POLARSTAR surveillance study of 10 708 Japanese patients with non-small-cell lung cancer. Cancer Sci 2014;105:1584-90. [Crossref] [PubMed]
- Oshima Y, Tanimoto T, Yuji K, et al. EGFR-TKI-Associated Interstitial Pneumonitis in Nivolumab-Treated Patients With Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:1112-5. [Crossref] [PubMed]
- Gemma A, Kusumoto M, Sakai F, et al. Real-World Evaluation of Factors for Interstitial Lung Disease Incidence and Radiologic Characteristics in Patients With EGFR T790M-positive NSCLC Treated With Osimertinib in Japan. J Thorac Oncol 2020;15:1893-906. [Crossref] [PubMed]
- Passaro A, Leighl N, Blackhall F, et al. ESMO expert consensus statements on the management of EGFR mutant non-small-cell lung cancer. Ann Oncol 2022;33:466-87. [Crossref] [PubMed]
- Müller NL, White DA, Jiang H, et al. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer 2004;91:S24-30. [Crossref] [PubMed]
- Cohen MH, Williams GA, Sridhara R, et al. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist 2003;8:303-6. [Crossref] [PubMed]
- Wuyts WA, Dooms C, Verleden GM. The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2013;187:777. [Crossref] [PubMed]
- Kawase S, Hattori N, Ishikawa N, et al. Change in serum KL-6 level from baseline is useful for predicting life-threatening EGFR-TKIs induced interstitial lung disease. Respir Res 2011;12:97. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Chinese Society of Lung Cancer, Chinese Anti-Cancer Association. EGFR-TKI ADR Management Chinese Expert Consensus. Zhongguo Fei Ai Za Zhi 2019;22:57-81. [Crossref] [PubMed]
- Tani T, Naoki K, Asakura T, et al. Successful treatment of non-small-cell lung cancer with afatinib and a glucocorticoid following gefitinib- and erlotinib-induced interstitial lung disease: A case report. Mol Clin Oncol 2016;5:488-90. [Crossref] [PubMed]
- Lim RK, Humphreys C, Morisset J, et al. Oxygen in patients with fibrotic interstitial lung disease: an international Delphi survey. Eur Respir J 2019;54:1900421. [Crossref] [PubMed]
- Lionel F, Perol D, Souquet PJ, et al. Bevacizumab as first-line treatment in advanced non-squamous non-small cell lung cancer (NSCLC) in patients aged over 65 years in France: Final results of the AVANTAGE study. Ann Oncol 2018;29:518.
- Kozuki T, Nogami N, Hataji O, et al. Open-label, multicenter, randomized phase II study on docetaxel plus bevacizumab or pemetrexed plus bevacizumab for treatment of elderly (aged ≥75 years) patients with previously untreated advanced non-squamous non-small cell lung cancer: TORG1323. Transl Lung Cancer Res 2020;9:459-70. [Crossref] [PubMed]
- Wozniak AJ, Kosty MP, Jahanzeb M, et al. Clinical outcomes in elderly patients with advanced non-small cell lung cancer: results from ARIES, a bevacizumab observational cohort study. Clin Oncol (R Coll Radiol) 2015;27:187-96. [Crossref] [PubMed]
- Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019;20:625-35. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Chen Y, Yang Z, Wang Y, et al. Pembrolizumab Plus Chemotherapy or Anlotinib vs. Pembrolizumab Alone in Patients With Previously Treated EGFR-Mutant NSCLC. Front Oncol 2021;11:671228. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Wang J, Sun Y, Liu Y, et al. Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi 2005;8:283-90. [Crossref] [PubMed]
- Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018;118:654-61. [Crossref] [PubMed]
- Xu H, Cao D, Jie F, et al. The efficacy and safety of anlotinib for subsequent line treatment of small cell lung cancer: a systematic review and meta-analysis. Tumori 2023;109:203-14. [Crossref] [PubMed]
- Song PF, Xu N, Li Q. Efficacy and Safety of Anlotinib for Elderly Patients with Previously Treated Extensive-Stage SCLC and the Prognostic Significance of Common Adverse Reactions. Cancer Manag Res 2020;12:11133-43. [Crossref] [PubMed]
- Li F, Su D, Ma J. Study on the related factors of postoperative deep vein thrombosis in COPD patients complicated with lung cancer. Chinese Journal of the Frontiers of Medical Science 2018;10:71-4. (Electronic Version).
- Ren F, Hao T, Zhu L, et al. Clinical study on the renin ⁃ angiotensin ⁃ aldosterone system in patients with COPD induced hypertension. J Mol Diagn Ther 1648-51;2020:56.
- Kuźnar-Kamińska B, Mikuła-Pietrasik J, Książek K, et al. Lung cancer in chronic obstructive pulmonary disease patients: importance of cellular senescence. Pol Arch Intern Med 2018;128:462-8. [Crossref] [PubMed]
- Nader CP, Cidem A, Verrills NM, et al. Protein phosphatase 2A (PP2A): a key phosphatase in the progression of chronic obstructive pulmonary disease (COPD) to lung cancer. Respir Res 2019;20:222. [Crossref] [PubMed]
- Sekine Y, Hata A, Koh E, et al. Lung carcinogenesis from chronic obstructive pulmonary disease: characteristics of lung cancer from COPD and contribution of signal transducers and lung stem cells in the inflammatory microenvironment. Gen Thorac Cardiovasc Surg 2014;62:415-21. [Crossref] [PubMed]
- Zeneyedpour L, Dekker LJM, van Sten-van T, Hoff JJM, et al. Neoantigens in Chronic Obstructive Pulmonary Disease and Lung Cancer: A Point of View. Proteomics Clin Appl 2019;13:e1800093. [Crossref] [PubMed]
- Wang DC, Shi L, Zhu Z, et al. Genomic mechanisms of transformation from chronic obstructive pulmonary disease to lung cancer. Semin Cancer Biol 2017;42:52-9. [Crossref] [PubMed]
- Wu K, Wang J, Zhao L, et al. The prognosis of non-small cell lung cancer combined with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Clin Respir J 2020;14:389-96. [Crossref] [PubMed]
- Lin M, Huang Z, Chen Y, et al. Lung cancer patients with chronic obstructive pulmonary disease benefit from anti-PD-1/PD-L1 therapy. Front Immunol 2022;13:1038715. [Crossref] [PubMed]
- Mark NM, Kargl J, Busch SE, et al. Chronic Obstructive Pulmonary Disease Alters Immune Cell Composition and Immune Checkpoint Inhibitor Efficacy in Non-Small Cell Lung Cancer. Am J Respir Crit Care Med 2018;197:325-36. [Crossref] [PubMed]
- Biton J, Ouakrim H, Dechartres A, et al. Impaired Tumor-Infiltrating T Cells in Patients with Chronic Obstructive Pulmonary Disease Impact Lung Cancer Response to PD-1 Blockade. Am J Respir Crit Care Med 2018;198:928-40. [Crossref] [PubMed]
- Shin SH, Park HY, Im Y, et al. Improved treatment outcome of pembrolizumab in patients with nonsmall cell lung cancer and chronic obstructive pulmonary disease. Int J Cancer 2019;145:2433-9. [Crossref] [PubMed]
- Arnaud-Coffin P, Maillet D, Gan HK, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer 2019;145:639-48. [Crossref] [PubMed]
- Provencio M, Calvo V, Romero A, et al. Treatment Sequencing in Resectable Lung Cancer: The Good and the Bad of Adjuvant Versus Neoadjuvant Therapy. Am Soc Clin Oncol Educ Book 2022;42:1-18. [Crossref] [PubMed]
- Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504-14. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1607-16. [Crossref] [PubMed]
- Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017;28:2377-85. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959-65. [Crossref] [PubMed]
- Atchley WT, Alvarez C, Saxena-Beem S, et al. Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer: Real-World Incidence, Risk Factors, and Management Practices Across Six Health Care Centers in North Carolina. Chest 2021;160:731-42. [Crossref] [PubMed]
- Deng H, Deng J, Lin X, et al. A Risk-Scoring Model for Severe Checkpoint Inhibitor-Related Pneumonitis: A Case-Control Study. Clin Drug Investig 2023;43:347-57. [Crossref] [PubMed]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020-31. [Crossref] [PubMed]
- Deng H, Zhou C. From CheckMate 227 to CheckMate 9LA: rethinking the status of chemotherapy in the immunotherapy era-chemo-free or chemo-reform? Transl Lung Cancer Res 2021;10:1924-7. [Crossref] [PubMed]
- Socinski MA, Nishio M, Jotte RM, et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J Thorac Oncol 2021;16:1909-24. [Crossref] [PubMed]
- Gomatou G, Tzilas V, Kotteas E, et al. Immune Checkpoint Inhibitor-Related Pneumonitis. Respiration 2020;99:932-42. [Crossref] [PubMed]
- Maes A, DePetrillo P, Siddiqui S, et al. Pharmacokinetics of Co-Suspension Delivery Technology Budesonide/Glycopyrronium/Formoterol Fumarate Dihydrate (BGF MDI) and Budesonide/Formoterol Fumarate Dihydrate (BFF MDI) Fixed-Dose Combinations Compared With an Active Control: A Phase 1, Randomized, Single-Dose, Crossover Study in Healthy Adults. Clin Pharmacol Drug Dev 2019;8:223-33. [Crossref] [PubMed]
- Ricciuti B, Dahlberg SE, Adeni A, et al. Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol 2019;37:1927-34. [Crossref] [PubMed]
- Pinato DJ, Howlett S, Ottaviani D, et al. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol 2019;5:1774-8. [Crossref] [PubMed]
- Birdas TJ, Koehler RP, Colonias A, et al. Sublobar resection with brachytherapy versus lobectomy for stage Ib nonsmall cell lung cancer. Ann Thorac Surg 2006;81:434-8; discussion 438-9. [Crossref] [PubMed]
- Agarwal JP, Pilar A, Mummudi N, et al. Stereotactic body radiation therapy for medically inoperable early-stage lung cancer: Tata Memorial Hospital perspective and practice recommendations. Indian J Cancer 2020;57:18-24. [Crossref] [PubMed]
- Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000;174:57-9. [Crossref] [PubMed]
- Simon TG, Beland MD, Machan JT, et al. Charlson Comorbidity Index predicts patient outcome, in cases of inoperable non-small cell lung cancer treated with radiofrequency ablation. Eur J Radiol 2012;81:4167-72. [Crossref] [PubMed]
- Vyfhuis MAL, Mohindra P, Simone CB 2nd. Stereotactic Body Radiation Therapy versus Thermal Ablation for Early Stage Non-Small Cell Lung Cancer. Radiology 2019;290:574-5. [Crossref] [PubMed]
- Russkamp NF, Ruemmler R, Roewe J, et al. Experimental design of complement component 5a-induced acute lung injury (C5a-ALI): a role of CC-chemokine receptor type 5 during immune activation by anaphylatoxin. FASEB J 2015;29:3762-72. [Crossref] [PubMed]
- Ablation Expert Committee of the Chinese Society of Clinical Oncology. Society of Tumor Ablation Therapy of the Chinese Anti-Cancer Association; Tumor Ablation Committee of the Chinese College of Interventionalists. Clinical practice guidelines: image-guided thermal ablation of primary and metastatic lung tumors (2021 edition). Zhonghua Nei Ke Za Zhi 2021;60:1088-105. [Crossref] [PubMed]
- Abtin FG, Eradat J, Gutierrez AJ, et al. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics 2012;32:947-69. [Crossref] [PubMed]
- Rose SC, Dupuy DE, Gervais DA, et al. Research reporting standards for percutaneous thermal ablation of lung neoplasms. J Vasc Interv Radiol 2009;20:S474-85. [Crossref] [PubMed]
- Anderson EM, Lees WR, Gillams AR. Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol 2009;32:478-83. [Crossref] [PubMed]
- Steinke K, King J, Glenn D, et al. Radiologic appearance and complications of percutaneous computed tomography-guided radiofrequency-ablated pulmonary metastases from colorectal carcinoma. J Comput Assist Tomogr 2003;27:750-7. [Crossref] [PubMed]
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121:3491-8. [Crossref] [PubMed]
- Palussiere J, Lagarde P, Aupérin A, et al. Percutaneous lung thermal ablation of non-surgical clinical N0 non-small cell lung cancer: results of eight years’ experience in 87 patients from two centers. Cardiovasc Intervent Radiol 2015;38:160-6. [Crossref] [PubMed]
- Taton O, Heinen V, Bondue B, et al. Long-Term Follow-Up of Intralobar Bullae After Endobronchial Valve Treatment for Emphysema. Int J Chron Obstruct Pulmon Dis 2022;17:1735-42. [Crossref] [PubMed]
- Ing AJ, Jayapadman A, Kim WV, et al. Reversal of collateral ventilation using endoscopic polymer foam in COPD patients undergoing endoscopic lung volume reduction with endobronchial valves: A controlled parallel group trial. Respirology 2022;27:1064-72. [Crossref] [PubMed]
- Train SE, Shafiq M. Old Dog, New Trick: Using Endobronchial Valves to Manage Persistent Air Leak Resulting From Endobronchial Valve Placement. J Bronchology Interv Pulmonol 2023;30:76-7. [Crossref] [PubMed]
- Hamedani H, Ma K, DiBardino D, et al. Simultaneous Imaging of Ventilation and Gas Exchange with Hyperpolarized (129)Xe MRI for Monitoring Patients with Endobronchial Valve Interventions. Am J Respir Crit Care Med 2022;205:e48-50. [Crossref] [PubMed]
- Saccomanno J, Ruwwe-Glösenkamp C, Neumann K, et al. Impact of Ventilation Modes on Bronchoscopic Chartis Assessment Outcome in Candidates for Endobronchial Valve Treatment. Respiration 2022;101:408-16. [Crossref] [PubMed]
- Steinfort DP, Christie M, Antippa P, et al. Bronchoscopic Thermal Vapour Ablation for Localized Cancer Lesions of the Lung: A Clinical Feasibility Treat-and-Resect Study. Respiration 2021;100:432-42. [Crossref] [PubMed]
- Marchese R, Lo Nigro C, Scaduto F. Use of bronchoscopic steam thermal ablation (BTVA) in a clinically compromised patient. J Cardiothorac Surg 2022;17:16. [Crossref] [PubMed]
- Gompelmann D, Shah PL, Valipour A, et al. Bronchoscopic Thermal Vapor Ablation: Best Practice Recommendations from an Expert Panel on Endoscopic Lung Volume Reduction. Respiration 2018;95:392-400. [Crossref] [PubMed]
- Petrella F. The Role of Surgery in Lung Cancer Treatment. Cancers (Basel) 2020;12:2777. [Crossref] [PubMed]
- Shah S, Blanchette CM, Coyle JC, et al. Survival associated with chronic obstructive pulmonary disease among SEER-Medicare beneficiaries with non-small-cell lung cancer. Int J Chron Obstruct Pulmon Dis 2019;14:893-903. [Crossref] [PubMed]
- Rancati T, Ceresoli GL, Gagliardi G, et al. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol 2003;67:275-83. [Crossref] [PubMed]
- Wang P, Zhang D, Guo XG, et al. Clinical characteristics and risk factors affecting outcomes of elderly patients with non-small cell lung cancer complicated by chronic obstructive pulmonary disease. Nan Fang Yi Ke Da Xue Xue Bao 2017;37:889-94. [Crossref] [PubMed]
- Kim H, Yoo H, Pyo H, et al. Impact Of Underlying Pulmonary Diseases On Treatment Outcomes In Early-Stage Non-Small Cell Lung Cancer Treated With Definitive Radiotherapy. Int J Chron Obstruct Pulmon Dis 2019;14:2273-81. [Crossref] [PubMed]
- Inoue T, Shiomi H, Oh RJ. Stereotactic body radiotherapy for Stage I lung cancer with chronic obstructive pulmonary disease: special reference to survival and radiation-induced pneumonitis. J Radiat Res 2015;56:727-34. [Crossref] [PubMed]
- Ishijima M, Nakayama H, Itonaga T, et al. Patients with severe emphysema have a low risk of radiation pneumonitis following stereotactic body radiotherapy. Br J Radiol 2015;88:20140596. [Crossref] [PubMed]
- Kimura T, Matsuura K, Murakami Y, et al. CT appearance of radiation injury of the lung and clinical symptoms after stereotactic body radiation therapy (SBRT) for lung cancers: are patients with pulmonary emphysema also candidates for SBRT for lung cancers? Int J Radiat Oncol Biol Phys 2006;66:483-91. [Crossref] [PubMed]
- Wang J, Cao J, Yuan S, et al. Poor baseline pulmonary function may not increase the risk of radiation-induced lung toxicity. Int J Radiat Oncol Biol Phys 2013;85:798-804. [Crossref] [PubMed]
- Saito T, Nakayama H, Yamada T, et al. Is severe emphysema, as defined by quantitative CT measurement, a negative risk factor of radiation fibrosis? Br J Radiol 2018;91:20170921. [Crossref] [PubMed]
- Yamamoto T, Kadoya N, Sato Y, et al. Prognostic Value of Radiation Pneumonitis After Stereotactic Body Radiotherapy: Effect of Pulmonary Emphysema Quantitated Using CT Images. Clin Lung Cancer 2018;19:e85-90. [Crossref] [PubMed]
- Zhou Z, Song X, Wu A, et al. Pulmonary emphysema is a risk factor for radiation pneumonitis in NSCLC patients with squamous cell carcinoma after thoracic radiation therapy. Sci Rep 2017;7:2748. [Crossref] [PubMed]
- Kimura T, Togami T, Takashima H, et al. Radiation pneumonitis in patients with lung and mediastinal tumours: a retrospective study of risk factors focused on pulmonary emphysema. Br J Radiol 2012;85:135-41. [Crossref] [PubMed]
- Kang N, Shin SH, Noh JM, et al. Treatment modality and outcomes among early-stage non-small cell lung cancer patients with COPD: a cohort study. J Thorac Dis 2020;12:4651-60. [Crossref] [PubMed]
- Louie AV, Rodrigues G, Hannouf M, et al. Withholding stereotactic radiotherapy in elderly patients with stage I non-small cell lung cancer and co-existing COPD is not justified: outcomes of a Markov model analysis. Radiother Oncol 2011;99:161-5. [Crossref] [PubMed]
- Palma D, Lagerwaard F, Rodrigues G, et al. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys 2012;82:1149-56. [Crossref] [PubMed]
- Takeda A, Kunieda E, Ohashi T, et al. Severe COPD is correlated with mild radiation pneumonitis following stereotactic body radiotherapy. Chest 2012;141:858-66. [Crossref] [PubMed]
- Huang JY, Jian ZH, Ndi Nfor O, et al. The Impact of Coexisting Asthma, Chronic Obstructive Pulmonary Disease and Tuberculosis on Survival in Patients with Lung Squamous Cell Carcinoma. PLoS One 2015;10:e0133367. [Crossref] [PubMed]
- Hara Y, Takeda A, Eriguchi T, et al. Stereotactic body radiotherapy for chronic obstructive pulmonary disease patients undergoing or eligible for long-term domiciliary oxygen therapy. J Radiat Res 2016;57:62-7. [Crossref] [PubMed]
- Stanic S, Paulus R, Timmerman RD, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early- stage peripheral non-small cell lung cancer: an analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 2014;88:1092-9. [Crossref] [PubMed]
- Henderson M, McGarry R, Yiannoutsos C, et al. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:404-9. [Crossref] [PubMed]
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009;4:838-44. [Crossref] [PubMed]
- Guckenberger M, Kestin LL, Hope AJ, et al. Is there a lower limit of pretreatment pulmonary function for safe and effective stereotactic body radiotherapy for early-stage non-small cell lung cancer? J Thorac Oncol 2012;7:542-51. [Crossref] [PubMed]
- Takeda A, Enomoto T, Sanuki N, et al. Reassessment of declines in pulmonary function ≥1 year after stereotactic body radiotherapy. Chest 2013;143:130-7. [Crossref] [PubMed]
- Tang C, Mistry H, Bayman N, et al. Outcomes of curative-intent radiotherapy in non-small cell lung cancer (NSCLC) patients with chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD). Radiother Oncol 2021;160:78-81. [Crossref] [PubMed]
- Borst GR, De Jaeger K, Belderbos JS, et al. Pulmonary function changes after radiotherapy in non-small-cell lung cancer patients with long-term disease-free survival. Int J Radiat Oncol Biol Phys 2005;62:639-44. [Crossref] [PubMed]
- Choi NC, Kanarek DJ. Toxicity of thoracic radiotherapy on pulmonary function in lung cancer. Lung Cancer 1994;10:S219-30. [Crossref] [PubMed]
- Moon SW, Park MS, Kim YS, et al. Combined pulmonary fibrosis and emphysema and idiopathic pulmonary fibrosis in non-small cell lung cancer: impact on survival and acute exacerbation. BMC Pulm Med 2019;19:177. [Crossref] [PubMed]
- Bahig H, Campeau MP, Lapointe A, et al. Phase 1-2 Study of Dual-Energy Computed Tomography for Assessment of Pulmonary Function in Radiation Therapy Planning. Int J Radiat Oncol Biol Phys 2017;99:334-43. [Crossref] [PubMed]
- Dong Y, Kumar H, Tawhai M, et al. In Silico Ventilation Within the Dose-Volume is Predictive of Lung Function Post-radiation Therapy in Patients with Lung Cancer. Ann Biomed Eng 2021;49:1416-31. [Crossref] [PubMed]
- Morimoto M, Nishino K, Wada K, et al. Elective Nodal Irradiation for Non-small Cell Lung Cancer Complicated With Chronic Obstructive Pulmonary Disease Affects Immunotherapy After Definitive Chemoradiotherapy. Anticancer Res 2020;40:6957-70. [Crossref] [PubMed]
- Wu L, Zhao S, Huang H, et al. Treatment outcomes of patients with stage III non-small cell lung cancer and interstitial lung diseases receiving intensity-modulated radiation therapy: A single-center experience of 85 cases. Thorac Cancer 2022;13:1583-91. [Crossref] [PubMed]
- Liu YF, Zhang Z, Rinsurongkawong W, et al. Driver mutations to predict for poorer outcomes in non-small cell lung cancer patients treated with concurrent chemoradiation and consolidation durvalumab. J Clin Oncol 2021;39: [Crossref]
- Baumann BC, Mitra N, Harton JG, et al. Comparative Effectiveness of Proton vs Photon Therapy as Part of Concurrent Chemoradiotherapy for Locally Advanced Cancer. JAMA Oncol 2020;6:237-46. [Crossref] [PubMed]
- Nakajima M, Yamamoto N, Hayashi K, et al. Carbon-ion radiotherapy for non-small cell lung cancer with interstitial lung disease: a retrospective analysis. Radiat Oncol 2017;12:144. [Crossref] [PubMed]
- Sugane T, Baba M, Imai R, et al. Carbon ion radiotherapy for elderly patients 80 years and older with stage I non-small cell lung cancer. Lung Cancer 2009;64:45-50. [Crossref] [PubMed]
- Karube M, Yamamoto N, Nakajima M, et al. Single-Fraction Carbon-Ion Radiation Therapy for Patients 80 Years of Age and Older With Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;95:542-8. [Crossref] [PubMed]
- Liang H, Yang C, Gonzalez-Rivas D, et al. Sleeve lobectomy after neoadjuvant chemoimmunotherapy/chemotherapy for local advanced non-small cell lung cancer. Transl Lung Cancer Res 2021;10:143-55. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: a propensity score-matched analysis. Eur J Cardiothorac Surg 2014;45:640-5. [Crossref] [PubMed]
- Bongiolatti S, Gonfiotti A, Vokrri E, et al. Thoracoscopic lobectomy for non-small-cell lung cancer in patients with impaired pulmonary function: analysis from a national database. Interact Cardiovasc Thorac Surg 2020;30:803-11. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Stamatis G, Leschber G, Schwarz B, et al. Survival outcomes in a prospective randomized multicenter Phase III trial comparing patients undergoing anatomical segmentectomy versus standard lobectomy for non-small cell lung cancer up to 2 cm. Lung Cancer 2022;172:108-16. [Crossref] [PubMed]
- Trzaska-Sobczak M, Skoczyński S, Pierzchała W. Pulmonary function tests in the preoperative evaluation of lung cancer surgery candidates. A review of guidelines. Kardiochir Torakochirurgia Pol 2014;11:278-82. [Crossref] [PubMed]
- Che G, Wu Q, Qiu Y, et al. Chinese expert consensus on multidisciplinary perioperative airway management (2018 version). Chin J Clin Thorac Cardiovasc Surg 2018;25:545-9.
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Degani-Costa LH, Faresin SM, dos Reis Falcão LF. Preoperative evaluation of the patient with pulmonary disease. Braz J Anesthesiol 2014;64:22-34. [Crossref] [PubMed]
- Roy PM. Preoperative pulmonary evaluation for lung resection. J Anaesthesiol Clin Pharmacol 2018;34:296-300. [Crossref] [PubMed]
- Wang T, Li D, Cui Y, et al. Chinese expert consensus on perioperative lung protection in thoracic surgery (2019 version). Chin J Clin Thorac Cardiovasc Surg 2019;26:835-42.
- Expert consensus on prevention and treatment of perioperative pulmonary complications in thoracic surgery. Chinese Journal of Thoracic and Cardiovascular Surgery 2009;25:217-8.
- Sun B, Song Z. How do surgeons interpret and apply the pulmonary function report. Chinese Journal of Practical Surgery 2011;31:180-2.
- Quan Z. Evaluation of pulmonary function test on risk and postoperative complications of abdominal surgery. Chinese Journal of Practical Surgery 2004;24:142-4.
- Zhi X, Yang Y, Wang C, et al. Chinese Standard for Diagnosis and Treatment of Primary Lung Cancer (2015 edition): surgical part. Chinese Journal of the Frontiers of Medical Science(Electronic Version) 2015:28-31.
- Zhang W, Chen M, Li H, et al. Hypoxia preconditioning attenuates lung injury after thoracoscopic lobectomy in patients with lung cancer: a prospective randomized controlled trial. BMC Anesthesiol 2019;19:209. [Crossref] [PubMed]
- Swamiappan R, Cereda M. 9 - Pulmonary Risk Assessment. In: Newman MF, Fleisher LA, Ko C, et al., editors. Perioperative Medicine (Second Edition). St. Louis (MO): Elsevier; 2022:83-100.
- Kobayashi S, Suzuki S, Niikawa H, et al. Preoperative use of inhaled tiotropium in lung cancer patients with untreated COPD. Respirology 2009;14:675-9. [Crossref] [PubMed]
- Leiro-Fernández V, Priegue Carrera A, Fernández-Villar A. Efficacy of Double Bronchodilation (LABA+LAMA) in Patients with Chronic Obstructive Pulmonary Disease (COPD) and Lung Cancer. Arch Bronconeumol 2016;52:622-3. [Crossref] [PubMed]
- Yamada Y, Sekine Y, Suzuki H, et al. Trends of bacterial colonisation and the risk of postoperative pneumonia in lung cancer patients with chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2010;37:752-7. [Crossref] [PubMed]
- Gautam SS, O'Toole RF. Convergence in the Epidemiology and Pathogenesis of COPD and Pneumonia. COPD 2016;13:790-8. [Crossref] [PubMed]
- Zhi X, Liu L. Chinese guidelines for perioperative airway management in thoracic surgery (2020 edition). Chinese Journal of Clinical Thoracic and Cardiovascular Surgery 2021;28:251-62.
- Mills E, Eyawo O, Lockhart I, et al. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med 2011;124:144-154.e8. [Crossref] [PubMed]
- Wei S, Lai X, Han Y, et al. Effect of smoking cessation duration before surgery on postoperative pulmonary complications: a meta-analysis. Chin J Health Manage 2018;32-7.
- Stefanelli F, Meoli I, Cobuccio R, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg 2013;44:e260-5. [Crossref] [PubMed]
- Mujovic N, Mujovic N, Subotic D, et al. Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci 2014;10:68-75. [Crossref] [PubMed]
- Divisi D, Di Francesco C, Di Leonardo G, et al. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2013;43:293-6. [Crossref] [PubMed]
- Liu Y. Perioperative pulmonary function changes and management in patients with moderate and severe COPD complicated with lung cancer. Journal of Aerospace Medicine 2016;27:874-5.
- Zheng Z, Hu J, Liu N. Progress in the research of respiratory rehabilitation therapy 2017. Chinese Journal of Practical Internal Medicine 2018;38:393-6.
- Nagarajan K, Bennett A, Agostini P, et al. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact Cardiovasc Thorac Surg 2011;13:300-2. [Crossref] [PubMed]
- Vagvolgyi A, Rozgonyi Z, Kerti M, et al. Effectiveness of pulmonary rehabilitation and correlations in between functional parameters, extent of thoracic surgery and severity of post-operative complications: randomized clinical trial. J Thorac Dis 2018;10:3519-31. [Crossref] [PubMed]
- Deepak JA, Ng X, Feliciano J, et al. Pulmonologist involvement, stage-specific treatment, and survival in adults with non-small cell lung cancer and chronic obstructive pulmonary disease. Ann Am Thorac Soc 2015;12:742-51. [Crossref] [PubMed]
- Shi Y, Sun Y, Yu J, et al. China Experts Consensus on the Diagnosis and Treatment of Advanced Stage Primary Lung Cancer (2016 Version). Zhongguo Fei Ai Za Zhi 2016;19:1-15. [Crossref] [PubMed]
- The NCCN Non-Small Cell Lung Cancer clinical practice guidelines in oncology (version 3.2023). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450.
(English Language Editor: J. Gray)


