
Seven preoperative factors have strong predictive value for postoperative pneumonia in patients undergoing thoracoscopic lung cancer surgery
Highlight box
Key findings
• This study developed a highly accurate model for predicting the incidence of postoperative pneumonia (POP) in patients undergoing thoracoscopic lung resection for lung cancer. A numerical risk stratification for POP was established, assigning points to various factors.
What is known and what is new?
• Age, chronic obstructive pulmonary disease, cardiovascular diseases, cerebrovascular diseases, and diabetes were identified as negative prognostic factors for lung cancer.
• The study emphasized the importance of evaluating these risk factors and implementing preventive measures in high-risk patients.
What is the implication, and what should change now?
• Multidisciplinary collaboration and personalized care were highlighted as crucial in optimizing outcomes for thoracoscopic lung resection patients.
Introduction
Comorbidity, also known as multimorbidity, refers to the condition in which the case has one or more chronic diseases or health problems (1). Comorbidity can affect the treatment outcomes and quality of life for patients with the primary diagnosed disease (2-5). Lung cancer is second most common cancer, but with the highest mortality. Patients with lung cancer often have preexisting comorbidities (6,7), such as age-related conditions and other diseases. Various assessment tools have been developed to predict postoperative risk based on these comorbidities. However, these tools are more suitable for evaluating long-term prognosis rather than short-term outcomes (8,9). Therefore, there is a need for comorbidity assessment tools that can accurately predict short-term prognosis after lung cancer surgery. Furthermore, many patients experience postoperative pneumonia (POP) after lung cancer surgery. The aim of this study was to investigate the impact of preoperative comorbidities on the occurrence of POP in lung cancer patients undergoing thoracoscopic surgery. We present this article in accordance with the TRIPOD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-512/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Tianjin Medical University General Hospital (No. IRB2021-YX-242-01) and Affiliated Hospital of Chifeng University was informed and agreed on this study. Informed consent was taken from all the patients. Data collection was carried out by specialized personnel without any intervention in clinical diagnosis and treatment.
In this study, the patients with lung cancer who underwent thoracoscopic surgery were prospectively enrolled from Tianjin Medical University General Hospital and Affiliated Hospital of Chifeng University from October 2021 to September 2022. Inclusion criteria in this trial were as follows: (I) patients with suspected or diagnosed lung cancer; (II) patients who were prepared for thoracoscopic lung resection; and (III) the patients and their families agreed to participate in the study. This study excluded patients who met the following criteria: (I) cases of secondary lung surgery; (II) cases with non-lung cancer diagnosed by postoperative pathology; (III) cases with critical information missing; and (IV) patients with locally advanced lung cancer which were evaluated after two cycles of neoadjuvant therapy (Figure 1).
The preoperative evaluation and treatment process of all patients were carried out according to the British Thoracic Society surgical selection guidelines (10) and the American College of Chest Physicians’ lung cancer diagnosis and treatment guidelines (3rd ed.) (11). All patients with preoperative comorbidities underwent multidisciplinary consultation, and the comorbidities were well controlled before the surgery. Patients were followed up from the first admission to 3 months after the surgery. Demographic characteristics, such as gender, age, body mass index (BMI), smoking status, and Quality-of-Life Questionnaire (QLQ)-C30 (12) and Lung Cancer Module (LC13) scores, were recorded before the surgery. The code of International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) was extracted from the patients’ medical records as the basis for the diagnosis of the main disease, comorbidity and POP. Based on the level of ICD-10 code, organs with comorbidities, the number and characteristics of the diagnosed comorbidities, were reduced again in the form of artificial clustering according to professional judgment and previous data from the literature. The clustered comorbidity data were formatted in length-width format and factorized. The variable name was made with the binary variable of the comorbidity group (13,14). The pathology report and ICD codes for surgery were also extracted from the first page of medical records which showed tumor and surgical information. All lung resections were performed by thoracoscopy under general anesthesia with a double-lumen endotracheal tube. The surgical procedures included wedge resection, segmental resection, lobectomy, and pneumonectomy. In this study, many patients were diagnosed with multiple nodules in the lung, making it challenging to categorize their surgical procedures. Therefore, the proportion of lung tissue resection was used to describe the surgical characteristics of the patients in a more accurate way (8,9). Pathological reports were used as the basis for tumor classification and staging. All medical data were obtained from the hospital’s electronic medical record system, and QLQ-C30 and LC13 scores were obtained from the phone questionnaire.
The study end point was whether POP occurred in patients with lung cancer after thoracoscopic surgery. We used the most common definition (15) of pneumonia established by the U.S. Centers for Disease Control and Prevention, according to Horan et al. (16). Pneumonia was diagnosed in postoperative patients within 30 days after surgery with the following criteria: (I) at least two chest imaging examinations were performed within 30 days after surgery, with new or progressive and persistent pulmonary infiltrates, consolidation, or cavitation. (II) With at least one of the following conditions: (i) fever (body temperature >38 ℃) without other clear reasons; (ii) peripheral white blood cell counts >12×109/L or <4×109/L; and (iii) aged ≥70 years with mental changes without other clear reasons. (III) With at least two of the following features: (i) new purulent sputum or changes in sputum character; (ii) increased respiratory secretions and the requirement for additional sputum suctions; (iii) new cough, dyspnea, and increased respiratory rate; (iv) preexisting cough, dyspnea, and gas exchange deteriorating; and (v) increased oxygen demand or the need of mechanical ventilation support.
Statistical analysis
In this study, demographic characteristics, oncology characteristics, lung resection proportion, and comorbidity characteristics were used as predictive variables, and the occurrence of POP was used as the response variable to establish a prediction model. Averages and standard errors for continuous attributes and absolute value and frequency distributions for categorical attributes were used to define the based features of the study population. To compare numerical data, the Student’s t-test and Wilcoxon rank testing were utilized. To compare variations in the percentages of categorical variables, χ2 or Fisher’s exact test was employed. Bilateral P values <0.05 were considered statistically significant. Prior to regression modeling, the collinearity of candidate variables was investigated by correlation analysis. No strongly correlated variables were found. Univariate logarithmic probability regression was used to select potential predictors of POP, and variables with P<0.05 were retained as candidate variables for logistic regression with multivariate variables. A nomogram was drawn based on the model, and 1,000 bootstrap resampling was performed for internal verification to evaluate the prediction accuracy of the model. Discrimination and calibration were used to evaluate the model’s performance. The area under the curve (AUC) analysis was used to evaluate the discrimination ability of the model. For clinical validity, decision curve analysis (DCA) was used to quantify the net benefits under different probability thresholds. All statistical analyses were conducted using R version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/; accessed on October 31, 2022). The R packages used in this study mainly included the following types: tidyverse, compareGroups, rms, stats, MASS, pROC, ROCR, rmda, etc.
Results
In this study, a total of 1,927 individuals were clinically diagnosed with lung cancer, and 1,229 patients who were scheduled to undergo thoracoscopic surgery were enrolled between October 2021 and September 2022. In total, 196 cases (15.95%) had POP (Table 1). We found a high incidence of having POP in lung cancer surgical patients. This may be due to the large number of elderly patients and multiple preoperative comorbidities in our cohort, as well as other unidentified factors. Among the included patients, seven patients received neoadjuvant therapy, two patients were diagnosed with stage Via lung cancer in whom pleural nodules were found intraoperatively and metastases (M1a) was suggested pathologically, 16 patients with severe POP required tracheal intubation and mechanical ventilation; they were cured within 2 weeks after surgery, of which 13 patients were in remission within 2 weeks after surgery, and the other three patients were in serious condition with secondary lung infection, but all of them were successfully cured and discharged from hospital within 2 months after surgery. Fortunately, there were no deaths during the study period.
Table 1
| Characteristics | Pneumonia | P value | |
|---|---|---|---|
| No, n=1,033 (84.1) | Yes, n=196 (15.9) | ||
| Gender | <0.001 | ||
| Female | 716 (69.3) | 83 (42.3) | |
| Male | 317 (30.7) | 113 (57.7) | |
| Age (years) | 58.45 (11.70) | 64.73 (10.00) | <0.001 |
| BMI (kg/m2) | 23.89 (3.85) | 25.43 (4.46) | <0.001 |
| Smoke index (cigarettes-year) | 75.30 (178.60) | 376.58 (396.80) | <0.001 |
| Pathological type | 0.001 | ||
| A | 956 (92.5) | 166 (84.7) | |
| S | 66 (6.4) | 27 (13.8) | |
| O | 11 (1.1) | 3 (1.5) | |
| TNM | 0.306 | ||
| 0 | 5 (0.5) | 0 (0.0) | |
| I | 866 (83.8) | 159 (81.1) | |
| II | 143 (13.8) | 34 (17.3) | |
| III | 18 (1.7) | 2 (1.0) | |
| IV | 1 (0.1) | 1 (0.5) | |
| Tumor location | 0.21 | ||
| Left upper lobe | 222 (21.5) | 44 (22.4) | |
| Left lower lobe | 140 (13.6) | 34 (17.3) | |
| Left lung | 89 (8.6) | 19 (9.7) | |
| Right middle lobe | 61 (5.9) | 3 (1.5) | |
| Right lower lobe | 118 (11.4) | 22 (11.2) | |
| Right upper lobe | 253 (24.5) | 49 (25.0) | |
| Right lung | 150 (14.5) | 25 (12.8) | |
| Lung resection (%) | 18.98 (6.78) | 19.83 (6.72) | 0.11 |
| Physical function | 98.01 (3.98) | 96.41 (5.20) | <0.001 |
| Role function | 97.00 (6.41) | 95.58 (7.38) | 0.006 |
| Emotional function | 87.52 (7.07) | 88.18 (6.64) | 0.226 |
| Cognitive function | 74.86 (16.39) | 75.94 (15.67) | 0.398 |
| Social function | 82.93 (11.67) | 82.91 (11.75) | 0.981 |
| Fatigue | 4.76 (7.57) | 6.35 (7.80) | 0.008 |
| Insomnia | 43.14 (40.27) | 36.56 (39.02) | 0.035 |
| Appetite loss | 12.78 (16.21) | 10.71 (15.61) | 0.101 |
| Constipation | 1.48 (7.03) | 3.23 (9.89) | 0.003 |
| Financial difficulties | 16.36 (16.67) | 16.16 (16.70) | 0.875 |
| Coughing | 2.19 (8.65) | 3.74 (11.08) | 0.029 |
| Hemoptysis | 0.03 (0.60) | 0.23 (2.24) | 0.018 |
| Dyspnea | 6.29 (10.69) | 14.28 (13.78) | <0.001 |
Data are presented as n (%) or mean (SD). POP, postoperative pneumonia; BMI, body mass index; A, adenocarcinoma; S, squamous carcinoma; O, other types of carcinoma; TNM, tumor-node-metastasis; SD, standard deviation.
In addition, 1,025 (83.40%) patients had comorbid conditions. The total number of comorbidity diagnosed in all samples was 2,929. We clustered over 700 comorbidities into 22 groups based on onset frequency, characteristics, and systemic organs involved. This subgrouping helped screen variables clearly and ensured clinical significance, sufficient sample size, and appropriate confidence intervals (CIs) for each comorbidity group. Using comorbidity groups as predictors simplified and expedited the prediction of postoperative POP risk. The number of comorbidity diagnosed in each sample was counted as the “comorbidity burden” of the sample. The maximum comorbidity burden of all samples was 14 (patients had 14 of the 22 comorbidity groups), and the minimum comorbidity burden was 0 (patient had no comorbidity diagnosed) (Table 2).
Table 2
| Characteristics | Pneumonia | P value | |
|---|---|---|---|
| No, n=1,033 (84.1) | Yes, n=196 (15.9) | ||
| Respiratory system disease | <0.001 | ||
| No | 779 (77.9) | 76 (38.8) | |
| Yes | 254 (24.6) | 120 (61.2) | |
| Cardiac disease | 0.354 | ||
| No | 908 (87.9) | 167 (89.3) | |
| Yes | 125 (12.1) | 29 (14.8) | |
| Vascular disease | 0.06 | ||
| No | 707 (68.5) | 114 (58.2) | |
| Yes | 325 (31.5) | 82 (41.8) | |
| Nervous system disease | <0.001 | ||
| No | 1,009 (97.7) | 182 (92.9) | |
| Yes | 38 (3.68) | 22 (11.2) | |
| Mental and behavior disorder | 0.743 | ||
| No | 1,022 (98.9) | 195 (99.5) | |
| Yes | 11 (1.1) | 1 (0.5) | |
| Diabetes | 0.023 | ||
| No | 908 (87.9) | 160 (81.6) | |
| Yes | 125 (12.1) | 36 (18.4) | |
| Thyroid benign disease | 0.217 | ||
| No | 951 (92.1) | 186 (94.9) | |
| Yes | 82 (7.9) | 10 (5.1) | |
| Other endocrine and metabolic abnormalities | 0.827 | ||
| No | 984 (95.3) | 188 (95.9) | |
| Yes | 49 (4.7) | 8 (4.1) | |
| Digestive and abdominal diseases | 0.028 | ||
| No | 974 (94.3) | 176 (89.8) | |
| Yes | 59 (5.7) | 20 (10.2) | |
| Hepatobiliary disease | 0.215 | ||
| No | 971 (94.0) | 179 (91.3) | |
| Yes | 62 (6.0) | 17 (8.7) | |
| Urinary system diseases | 0.848 | ||
| No | 1,012 (98.0) | 191 (97.4) | |
| Yes | 21 (2.0) | 5 (2.6) | |
| Gynecological diseases | 0.369 | ||
| No | 1,022 (98.8) | 196 (100.0) | |
| Yes | 11 (1.1) | 0 (0.0) | |
| Other malignancies | 0.102 | ||
| No | 969 (93.8) | 177 (90.3) | |
| Yes | 64 (6.2) | 19 (9.7) | |
| Other benign disease | 0.624 | ||
| No | 927 (89.7) | 173 (88.3) | |
| Yes | 106 (10.3) | 23 (11.7) | |
| Stomatological and ENT diseases | 0.289 | ||
| No | 977 (94.6) | 181 (92.3) | |
| Yes | 56 (5.4) | 15 (7.7) | |
| Injuries/sequela | 0.386 | ||
| No | 1,010 (97.8) | 189 (96.4) | |
| Yes | 23 (2.2) | 7 (3.6) | |
| Congenital disease | 1 | ||
| No | 1,028 (99.5) | 195 (99.5) | |
| Yes | 5 (0.5) | 1 (0.5) | |
| Arthritis | 1 | ||
| No | 983 (95.2) | 186 (94.9) | |
| Yes | 50 (4.8) | 10 (5.1) | |
| Dermatopathy | 1 | ||
| No | 1,030 (99.7) | 195 (99.5) | |
| Yes | 3 (0.3) | 1 (0.5) | |
| Abnormal performance | 0.028 | ||
| No | 970 (93.9) | 175 (89.3) | |
| Yes | 63 (6.1) | 21 (10.7) | |
| Personal history | 0.45 | ||
| No | 610 (59.1) | 122 (62.2) | |
| Yes | 423 (40.9) | 74 (37.8) | |
| Comorbidities burden | 2.22 (1.89) | 3.26 (2.19) | <0.001 |
Data are presented as n (%) or mean (SD). POP, postoperative pneumonia; ENT, ear, nose, and throat; SD, standard deviation.
The potential predictors of POP were screened by univariate logistic regression (Table 3). The final variables used in prediction were determined by multivariate logistic regression (Table 4). As a result, age [odds ratio (OR), 1.045; 95% CI: 1.025–1.065; P<0.001], smoke index (OR, 1.004; 95% CI: 1.003–1.005; P<0.001), physical function (OR, 0.946; 95% CI: 0.910–0.986; P=0.007), respiratory diseases (OR, 5.918; 95% CI: 4.030–8.811; P<0.001), and nervous system diseases (OR, 2.233; 95% CI: 1.066–4.541; P=0.029) were selected. The incidence of POP was higher in patients with advanced age, high smoke index, poor physical function, with respiratory diseases, and nervous system diseases.
Table 3
| Variables | Univariable analysis | |
|---|---|---|
| OR (95% CI) | P value | |
| Gender | ||
| Female | Ref. | |
| Male | 0.326 (0.238–0.444) | <0.001 |
| Age (years) | 1.057 (1.041–1.075) | <0.001 |
| BMI (kg/m2) | 1.099 (1.058–1.141) | <0.001 |
| Smoke index (cigarettes-year) | 1.004 (1.004–1.005) | <0.001 |
| Pathological type | ||
| A | Ref. | |
| S | 2.356 (1.442–3.758) | <0.001 |
| O | 1.571 (0.353–5.093) | 0.492 |
| Tumor location | ||
| Left upper lobe | Ref. | |
| Left lower lobe | 1.190 (0.704–2.050) | 0.524 |
| Left lung | 1.281 (0.661–2.451) | 0.457 |
| Right middle lobe | 0.296 (0.069–0.882) | 0.053 |
| Right lower lobe | 1.119 (0.598–2.084) | 0.724 |
| Right upper lobe | 1.163 (0.696–1.984) | 0.574 |
| Right lung | 1.458 (0.831–2.586) | 0.192 |
| Lung resection (%) | 1.019 (0.996–1.043) | 0.111 |
| Physical function | 0.928 (0.899–0.958) | <0.001 |
| Role function | 0.971 (0.951–0.992) | 0.006 |
| Emotional function | 1.014 (0.992–1.036) | 0.227 |
| Cognitive function | 1.005 (0.995–1.014) | 0.398 |
| Social function | 1.000 (0.987–1.014) | 0.981 |
| Fatigue | 1.026 (1.007–1.045) | 0.008 |
| Appetite loss | 0.992 (0.983–1.002) | 0.102 |
| Constipation | 1.025 (1.008–1.041) | 0.005 |
| Financial difficulties | 1.000 (0.991–1.009) | 0.876 |
| Coughing | 1.016 (1.001–1.031) | 0.032 |
| Hemoptysis | 1.120 (1.001–1.281) | 0.053 |
| Dyspnea | 1.052 (1.040–1.065) | <0.001 |
| Respiratory system disease | ||
| No | Ref. | |
| Yes | 4.843 (3.524–6.693) | <0.001 |
| Cardiac disease | ||
| No | Ref. | |
| Yes | 1.262 (0.803–1.927) | 0.297 |
| Vascular disease | ||
| No | Ref. | |
| Yes | 1.567 (1.144–2.140) | 0.005 |
| Nervous system disease | ||
| No | Ref. | |
| Yes | 3.311 (1.886–5.687) | 0.001 |
| Mental and behavioral disorder | ||
| No | Ref. | |
| Yes | 0.477 (0.026–2.471) | 0.479 |
| Diabetes | ||
| No | Ref. | |
| Yes | 1.635 (1.076–2.434) | 0.019 |
| Thyroid benign disease | ||
| No | Ref. | |
| Yes | 0.624 (0.299–1.169) | 0.171 |
| Other endocrine and metabolic abnormalities | ||
| No | Ref. | |
| Yes | 0.855 (0.370–1.736) | 0.687 |
| Digestive and abdominal diseases | ||
| No | Ref. | |
| Yes | 1.876 (1.079–3.142) | 0.021 |
| Hepatobiliary disease | ||
| No | Ref. | |
| Yes | 1.488 (0.826–2.548) | 0.165 |
| Urinary system diseases | ||
| No | Ref. | |
| Yes | 1.262 (0.418–3.140) | 0.645 |
| Other malignancies | ||
| No | Ref. | |
| Yes | 1.626 (0.928–2.729) | 0.077 |
| Other benign disease | ||
| No | Ref. | |
| Yes | 1.163 (0.705–1.846) | 0.538 |
| Stomatological and ENT diseases | ||
| No | Ref. | |
| Yes | 1.446 (0.775–2.548) | 0.222 |
| Injuries/sequela | ||
| No | Ref. | |
| Yes | 1.627 (0.638–3.660) | 0.268 |
| Congenital disease | ||
| No | Ref. | |
| Yes | 1.055 (0.055–6.585) | 0.962 |
| Arthritis | ||
| No | Ref. | |
| Yes | 1.057 (0.498–2.035) | 0.877 |
| Dermatopathy | ||
| No | Ref. | |
| Yes | 1.761 (0.087–13.832) | 0.625 |
| Abnormal performance | ||
| No | Ref. | |
| Yes | 1.848 (1.077–3.058) | 0.021 |
| Personal history | ||
| No | Ref. | |
| Yes | 0.875 (0.637–1.195) | 0.404 |
| Comorbidities burden | 1.268 (1.180–1.363) | <0.001 |
POP, postoperative pneumonia; OR, odds ratio; CI, confidence interval; Ref., reference; BMI, body mass index; A, adenocarcinoma; S, squamous carcinoma; O, other types of carcinoma; ENT, ear, nose, and throat.
Table 4
| Variables | Multivariable analysis | Factors selected for model | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Gender | |||||
| Female | Ref. | ||||
| Male | 1.496 (0.850–2.684) | 0.169 | |||
| Age (years) | 1.041 (1.016–1.068) | <0.001 | 1.045 (1.025–1.065) | <0.001 | |
| BMI (kg/m2) | 1.047 (0.993–1.106) | 0.091 | |||
| Smoke index (cigarettes-year) | 1.005 (1.004–1.007) | <0.001 | 1.004 (1.003–1.005) | <0.001 | |
| Pathological type | |||||
| A | Ref. | ||||
| O | 0.747 (0.343–1.525) | 0.441 | |||
| S | 0.359 (0.055–1.662) | 0.232 | |||
| Physical function | 0.949 (0.906–0.994) | 0.025 | 0.946 (0.910–0.986) | 0.007 | |
| Role function | 1.024 (0.992–1.057) | 0.145 | |||
| Fatigue | 0.996 (0.972–1.020) | 0.723 | |||
| Insomnia | 0.997 (0.993–1.002) | 0.266 | |||
| Constipation | 1.007 (0.984–1.029) | 0.531 | |||
| Coughing | 1.008 (0.988–1.026) | 0.412 | |||
| Dyspnea | 1.004 (0.985–1.023) | 0.680 | |||
| Respiratory system disease | |||||
| No | Ref. | Ref. | |||
| Yes | 5.841 (3.587–9.632) | <0.001 | 5.918 (4.030–8.811) | <0.001 | |
| Vascular disease | |||||
| No | Ref. | ||||
| Yes | 0.863 (0.554–1.334) | 0.510 | |||
| Nervous system disease | |||||
| No | Ref. | Ref. | |||
| Yes | 2.545 (1.173–5.370) | 0.016 | 2.233 (1.066–4.541) | 0.029 | |
| Diabetes | |||||
| No | Ref. | ||||
| Yes | 1.623 (0.940–2.762) | 0.077 | |||
| Digestive and abdominal diseases | |||||
| No | Ref. | ||||
| Yes | 1.639 (0.808–3.221) | 0.160 | |||
| Abnormal performance | |||||
| No | Ref. | ||||
| Yes | 1.804 (0.865–3.622) | 0.105 | |||
| Comorbidities burden | 1.027 (0.889–1.184) | 0.713 | |||
OR, odds ratio; CI, confidence interval; BMI, body mass index; A, adenocarcinoma; S, squamous carcinoma; O, other types of carcinoma.
Model development and validation
Based on Akaike information criteria obtained by stepwise adverse selection, BMI and diabetes were further included in the final model. Age, BMI, smoke index, physical function, respiratory diseases, diabetes, and neurological diseases were selected to construct a model to predict the probability of having POP among those with lung cancer (Table 4). A nomogram was established based on the model (Figure 2).
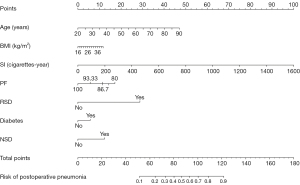
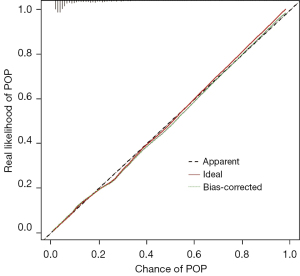
Receiver operating characteristic (ROC) curves were drawn to evaluate the performance of the prediction model (Figure 3). The AUC, sensitivity, and specificity of the model were 0.851 (95% CI: 0.821–0.881), 80.1%, and 74.4%, respectively. The Brier score of the model was 0.091 (95% CI: 7.9–10.2). The calibration curve (Figure 4) showed a convincing agreement between the predicted and the actual results. The results showed that the model has good discrimination and correction ability and provides a convenient tool for managing patients at risk of POP (Figure 5).


Discussion
The diagnostic basis and manifestations of comorbidity mainly come from clinical manifestations and laboratory tests. To avoid predictor variable interaction and collinearity, we only used demographic characteristics, comorbidity diagnosis information, and lung resection proportion as predictors. For instance, poor lung function before surgery was associated with a higher rate of postoperative respiratory complications. Although we initially included indicators of preoperative pulmonary function, we recognized the potential collinearity with preoperative respiratory comorbidities. To focus on preoperative comorbidity and to save time and cost, we omitted the collection of pulmonary function indicators.
While there are various postoperative complications that can occur in patients with lung cancer, POP is often considered a significant and relevant outcome in assessment. POP is one of the most common complications from lung cancer surgery. Its high occurrence rate makes it an important consideration when evaluating postoperative outcomes. POP can lead to significant morbidity rates. It can prolong hospital stays, increase healthcare costs, and potentially result in respiratory failure or even death (17-21), making it a crucial outcome to monitor. While not all complications can be prevented, there are various strategies available to reduce the risk of POP. By focusing on preventing this specific complication, healthcare providers can potentially improve overall postoperative outcomes. POP is often linked with other postoperative complications, such as atelectasis or respiratory failure. By monitoring and managing POP, healthcare providers can indirectly address and potentially prevent other related complications.
After screening the predictive variables of the enrolled participants, it was determined that respiratory diseases, diabetes, and nervous system diseases, age, BMI, smoking index, physiological function, were independent risk factors for POP in patients with lung cancer undergoing thoracoscopic surgery. Dutkowska et al. (22), in their review of the comorbidity characteristics of patients with lung cancer, pointed out that age, chronic obstructive pulmonary disease (COPD), cardiovascular diseases, cerebrovascular diseases, and diabetes are all negative prognostic factors for lung cancer, which are consistent with the findings of this study.
Respiratory diseases, especially various lower respiratory tract diseases, are important risk factors for POP in patients with lung cancer. COPD is the most common comorbidity of lung cancer (23). Many studies have confirmed that COPD is an independent risk factor for perioperative lung cancer (24-27). Lin et al. (28) compared 24,109 surgical patients who had asthma before surgery to 24,109 non-asthmatic patients who underwent major surgery. Their results showed a significant increase in postoperative complications and mortality in patients with asthma. Nowadays, exacerbation of postoperative interstitial pneumonia in patients with interstitial lung disease and lung cancer has become a serious problem (29). Carr et al. (30) also confirmed that acute exacerbation of idiopathic interstitial pneumonia and POP are important postoperative complications in thoracic and nonthoracic surgery groups through their perioperative study of patients with idiopathic interstitial pneumonia. Hata et al. (31) performed a chart review of 250 patients with lung cancer who underwent lung resection to study the efficacy of radical surgery for lung cancer combined pulmonary fibrosis and emphysema (CPFE). Their results showed that the prognosis of CPFE patients identified on computed tomography scans was worse than that of patients with emphysema or normal lungs. When patients with lung cancer have CPFE before the surgery, radical surgery should be carefully selected because of the associated poor prognosis.
Many studies have reported that diabetes significantly affects the survival of non-small cell lung cancer (NSCLC) patients (22,32). However, the pathogenesis of POP of lung cancer caused by diabetes remains unclear. It may be related to the complex complications of diabetes.
Lung cancer surgery in patients with neurological comorbidities is a major challenge in anesthesia and postoperative management, as cerebrovascular disease increases POP and mortality compared to patients with lung cancer without such comorbidities. Aging is a very adverse prognostic factor for thoracic surgery for elderly lung cancer patients. According to Dominguez-Ventura et al. (33), the risk of death increased 4-fold in patients aged ≥80 years with a history of stroke.
Although there are few studies specifically investigating the correlation between demographic factors and POP in lung cancer patients, we can indirectly understand the association between age and POP in lung cancer patients from a large body of literature discussing age and postoperative complications in this population.
Wang et al. (34) evaluated the use of first-line guideline-recommended therapy in 20,511 veterans aged ≥65 years with NSCLC. More predictors, including age and comorbidities, are needed for individualized decision-making to target the treatment to benefit older patients with lung cancer. However, the results of Pei et al.’s (35) yielded promising results regarding the occurrence of postoperative complications in elderly patients with NSCLC after undergoing lung resection. Pneumonectomy procedures and longer surgical durations were found to be positively associated with an increased likelihood of developing postoperative complications, while thoracoscopic minimally invasive surgery for NSCLC in elderly patients was linked to fewer complications and shorter hospital stays compared to traditional thoracotomy approaches.
BMI has different effects on short- and long-term prognosis after lung cancer surgery. Benker et al. (36) recruited 1,219 patients who underwent NSCLC resection between 2000 and 2015. They concluded that advanced age, low BMI, and low forced expiratory volume in 1 second (FEV1) could predict greater complication risk and shorter survival. However, Li et al. and Nitsche et al. (37,38) came up with opposite results, showing that obesity has beneficial effects on in-hospital outcomes and the long-term survival of surgical patients with lung cancer. The “obesity paradox” can potentially exist in lung cancer surgery. Launer et al. (39) and Kaw et al. (40) had another conclusion that obese patients have an increased risk for postoperative pulmonary complications.
The impact of smoking on lung cancer is widely recognized in the medical field. Not only is smoking being considered as the primary causative factor for lung cancer, but it is also closely associated with respiratory comorbidities and serves as a significant risk factor for POP (41). The detrimental effects of smoking extend beyond lung cancer. It is a major risk factor for various respiratory diseases, including COPD, emphysema, and bronchitis. Additionally, smoking has been linked to an increased risk of cardiovascular diseases, such as heart attacks and strokes. Moreover, it negatively impacts the overall respiratory function, leading to decreased lung capacity and impaired gas exchange. This viewpoint has been incorporated into major diagnosis and treatment guidelines (10,11,21,42) and is also deeply ingrained among physicians.
Russotto et al. (6) pointed out that poor functional status, defined as a decline in the ability to perform activities of daily living, is a recognized factor that increases morbidity and mortality and has been reported as a risk factor for pneumonia in previously developed models. In addition, patients with poorer functional status are at an increased risk for aspiration pneumonia. In addition, many studies have confirmed the impact on quality of life of postoperative lung cancer (43,44) and proposed that optimizing preoperative functional status can improve postoperative status (45,46).
There are several limitations in this study. Due to the limited sample size, the dataset was not divided into a training set and a validation set; only internal validation was performed. This is a main limitation of this study. On the other hand, the influence of preoperative factors on intraoperative conditions, such as operation time and intraoperative bleeding, was not discussed in this paper. In addition, we did not explicitly discuss the comparison with the cited study’s mortality rate in our text. However, we will consider addressing this point in future research or in the limitations section of our study. Finally, given the constraints of the established research framework, it proves challenging to deconstruct respiratory diseases with the currently available data. However, the model developed in this study exhibited an acceptable level of accuracy in predicting the incidence of POP, thus serving as a valuable tool for assessing the risk of POP in patients undergoing thoracoscopic lung resection for lung cancer. A numerical risk stratification system can be employed to calculate the likelihood of developing POP, wherein each factor is assigned a specific point value and cumulative points are tallied.
Conclusions
This study identifies several independent variables associated with POP in lung cancer patients undergoing thoracoscopic surgery. This knowledge can be applied to clinical practice. By using this model, clinicians can anticipate the risk of POP and implement planned interventions and rehabilitation treatments to reduce complications and improve patient outcomes.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-512/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-512/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-512/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-512/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Tianjin Medical University General Hospital (No. IRB2021-YX-242-01) and Affiliated Hospital of Chifeng University was informed and agreed on this study. Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moltó A, Dougados M. Comorbidity indices. Clin Exp Rheumatol 2014;32:S-131-4.
- Rubio FJ, Alvares D, Redondo-Sanchez D, et al. Bayesian variable selection and survival modeling: assessing the Most important comorbidities that impact lung and colorectal cancer survival in Spain. BMC Med Res Methodol 2022;22:95. [Crossref] [PubMed]
- Fowler H, Belot A, Ellis L, et al. Comorbidity prevalence among cancer patients: a population-based cohort study of four cancers. BMC Cancer 2020;20:2. [Crossref] [PubMed]
- Anwar SL, Cahyono R, Prabowo D, et al. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer 2021;21:590. [Crossref] [PubMed]
- Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2015;26:1091-101. [Crossref] [PubMed]
- Russotto V, Sabaté S, Canet J, et al. Development of a prediction model for postoperative pneumonia: A multicentre prospective observational study. Eur J Anaesthesiol 2019;36:93-104. [Crossref] [PubMed]
- Islam KM, Jiang X, Anggondowati T, et al. Comorbidity and Survival in Lung Cancer Patients. Cancer Epidemiol Biomarkers Prev 2015;24:1079-85. [Crossref] [PubMed]
- Charlson ME, Carrozzino D, Guidi J, et al. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother Psychosom 2022;91:8-35. [Crossref] [PubMed]
- Mehta HB, Sura SD, Adhikari D, et al. Adapting the Elixhauser comorbidity index for cancer patients. Cancer 2018;124:2018-25. [Crossref] [PubMed]
- British Thoracic Society. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Prommik P, Tootsi K, Saluse T, et al. Simple Excel and ICD-10 based dataset calculator for the Charlson and Elixhauser comorbidity indices. BMC Med Res Methodol 2022;22:4. [Crossref] [PubMed]
- Sun JW, Rogers JR, Her Q, et al. Adaptation and Validation of the Combined Comorbidity Score for ICD-10-CM. Med Care 2017;55:1046-51. [Crossref] [PubMed]
- Abbott TEF, Fowler AJ, Pelosi P, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth 2018;120:1066-79. [Crossref] [PubMed]
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. [Crossref] [PubMed]
- Abdel Jalil R, Abou Chaar MK, Al-Qudah O, et al. Early surgical and oncological outcomes during adoption of a single port VATS lung resection in a tertiary cancer center: a retrospective analysis. J Cardiothorac Surg 2022;17:26. [Crossref] [PubMed]
- Chen TF, Xie CY, Rao BY, et al. Surgical treatment to multiple primary lung cancer patients: a systematic review and meta-analysis. BMC Surg 2019;19:185. [Crossref] [PubMed]
- Ely S, Jiang SF, Dominguez DA, et al. Effect of thoracic surgery regionalization on long-term survival after lung cancer resection. J Thorac Cardiovasc Surg 2022;163:769-77. [Crossref] [PubMed]
- Powell HA, Tata LJ, Baldwin DR, et al. Early mortality after surgical resection for lung cancer: an analysis of the English National Lung cancer audit. Thorax 2013;68:826-34. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Dutkowska AE, Antczak A. Comorbidities in lung cancer. Pneumonol Alergol Pol 2016;84:186-92. [Crossref] [PubMed]
- Grose D, Milroy R. Chronic obstructive pulmonary disease: a complex comorbidity of lung cancer. J Comorb 2011;1:45-50. [Crossref] [PubMed]
- Gupta H, Ramanan B, Gupta PK, et al. Impact of COPD on postoperative outcomes: results from a national database. Chest 2013;143:1599-606. [Crossref] [PubMed]
- Matsuyama W, Suetsugu T, Kawabata T, et al. Successful lobectomy in 3 lung cancer cases with severe COPD after treatment with tiotropium bromide. Nihon Kokyuki Gakkai Zasshi 2007;45:194-7.
- Wang L, Yu M, Ma Y, et al. Effect of Pulmonary Rehabilitation on Postoperative Clinical Status in Patients with Lung Cancer and Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med 2022;2022:4133237. [Crossref] [PubMed]
- Xu W, Zhu J, Li L, et al. The Prognostic Role of Chronic Obstructive Pulmonary Disease for Lung Cancer After Pulmonary Resection. J Surg Res 2022;275:137-48. [Crossref] [PubMed]
- Lin CS, Chang CC, Yeh CC, et al. Postoperative Adverse Outcomes in Patients With Asthma: A Nationwide Population-based Cohort Study. Medicine (Baltimore) 2016;95:e2548. [Crossref] [PubMed]
- Iyoda A, Azuma Y, Sakamoto S, et al. Surgical treatment for patients with idiopathic pulmonary fibrosis and lung cancer: postoperative acute exacerbation of idiopathic pulmonary fibrosis and outcomes. Surg Today 2022;52:736-44. [Crossref] [PubMed]
- Carr ZJ, Yan L, Chavez-Duarte J, et al. Perioperative Management of Patients with Idiopathic Pulmonary Fibrosis Undergoing Noncardiac Surgery: A Narrative Review. Int J Gen Med 2022;15:2087-100. [Crossref] [PubMed]
- Hata A, Sekine Y, Kota O, et al. Impact of combined pulmonary fibrosis and emphysema on surgical complications and long-term survival in patients undergoing surgery for non-small-cell lung cancer. Int J Chron Obstruct Pulmon Dis 2016;11:1261-8. [Crossref] [PubMed]
- Iachina M, Jakobsen E, Møller H, et al. The effect of different comorbidities on survival of non-small cells lung cancer patients. Lung 2015;193:291-7. [Crossref] [PubMed]
- Dominguez-Ventura A, Allen MS, Cassivi SD, et al. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg 2006;82:1175-9. [Crossref] [PubMed]
- Wang S, Wong ML, Hamilton N, et al. Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans. J Clin Oncol 2012;30:1447-55. [Crossref] [PubMed]
- Pei G, Zhou S, Han Y, et al. Risk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in China. J Thorac Dis 2014;6:1230-8. [Crossref] [PubMed]
- Benker M, Citak N, Neuer T, et al. Impact of preoperative comorbidities on postoperative complication rate and outcome in surgically resected non-small cell lung cancer patients. Gen Thorac Cardiovasc Surg 2022;70:248-56. [Crossref] [PubMed]
- Li S, Wang Z, Huang J, et al. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the 'obesity paradox' really exist? Eur J Cardiothorac Surg 2017;51:817-28. [Crossref] [PubMed]
- Nitsche LJ, Mukherjee S, Cheruvu K, et al. Exploring the Impact of the Obesity Paradox on Lung Cancer and Other Malignancies. Cancers (Basel) 2022;14:1440. [Crossref] [PubMed]
- Launer H, Nguyen DV, Cooke DT. National perioperative outcomes of pulmonary lobectomy for cancer in the obese patient: a propensity score matched analysis. J Thorac Cardiovasc Surg 2013;145:1312-8. [Crossref] [PubMed]
- Kaw RK. Spectrum of postoperative complications in pulmonary hypertension and obesity hypoventilation syndrome. Curr Opin Anaesthesiol 2017;30:140-5. [Crossref] [PubMed]
- Fukui M, Suzuki K, Matsunaga T, et al. Importance of Smoking Cessation on Surgical Outcome in Primary Lung Cancer. Ann Thorac Surg 2019;107:1005-9. [Crossref] [PubMed]
- Yang IA, Brown JL, George J, et al. COPD-X Australian and New Zealand guidelines for the diagnosis and management of chronic obstructive pulmonary disease: 2017 update. Med J Aust 2017;207:436-42. [Crossref] [PubMed]
- Ferguson MK, Parma CM, Celauro AD, et al. Quality of life and mood in older patients after major lung resection. Ann Thorac Surg 2009;87:1007-12; discussion 1012-3. [Crossref] [PubMed]
- Paull DE, Thomas ML, Meade GE, et al. Determinants of quality of life in patients following pulmonary resection for lung cancer. Am J Surg 2006;192:565-71. [Crossref] [PubMed]
- Gagné S, McIsaac DI. Modifiable risk factors for patients undergoing lung cancer surgery and their optimization: a review. J Thorac Dis 2018;10:S3761-72. [Crossref] [PubMed]
- Stokes SM, Wakeam E, Antonoff MB, et al. Optimizing health before elective thoracic surgery: systematic review of modifiable risk factors and opportunities for health services research. J Thorac Dis 2019;11:S537-54. [Crossref] [PubMed]



