
Safety and efficacy of thoracic radiotherapy combined with chemo-immunotherapy in patients with extensive-stage small cell lung cancer: a multicenter retrospective analysis
Highlight box
Key findings
• Thoracic radiotherapy is an effective and safe treatment option for many patients with extensive-stage small cell lung cancer (ES-SCLC) who are receiving chemo-immunotherapy.
What is known and what is new?
• Current evidence suggests a possible synergistic effect between immunotherapy and radiotherapy.
• Our retrospective analysis of 78 ES-SCLC patients who received thoracic radiotherapy (TRT) and chemo-immunotherapy determined treatment safety and efficacy, and is the first study to identify a subgroup of patients who benefit most from this regimen.
What is the implication, and what should change now?
• TRT is a suitable therapeutic option for many ES-SCLC patients who are receiving chemo-immunotherapy.
• Large prospective studies are needed to confirm our findings and identify biomarkers that predict the efficacy and safety of TRT in patients with ES-SCLC.
Introduction
Small cell lung cancer (SCLC), which is characterized by a high rate of proliferation, a high propensity for early metastasis, and poor patient prognosis, accounts for about 15% of all lung cancers (1). Approximately 60 to 70% of patients with SCLC have extensive-stage (ES) disease at diagnosis, defined by the presence of distant metastasis or a tumor that extends beyond a single radiation port (2).
Before the introduction of immunotherapy, etoposide plus platinum (EP)-based chemotherapy was the main first-line treatment for ES-SCLC. Although ES-SCLC tends to be initially sensitive to chemotherapy, local recurrence or distant metastasis inevitably occurs (3). Because of the limited effectiveness subsequent therapies, these patients have a poor prognosis, with a 5-year survival rate less than 7% (4).
The recent introduction of immune checkpoint inhibitors (ICIs), which target programmed cell death protein 1 (PD-1) or programmed cell death protein ligand 1 (PD-L1), has dramatically changed the treatment algorithms for ES-SCLC, which changed very little during the previous three decades. The IMpower133 (5) and CASPIAN (6) trials documented the efficacy of anti-PD-L1 agents for treatment of ES-SCLC. In particular, there was a significantly prolonged median overall survival (mOS) time when atezolizumab or durvalumab was combined with EP-based chemotherapy compared to chemotherapy alone. The results of the CAPSTONE-1 trial (7), which evaluated adebrelimab plus chemotherapy for ES-SCLC, also demonstrated that anti-PD-L1 agents provided a benefit for ES-SCLC patients. The KEYNOTE-604 trial (8) demonstrated that pembrolizumab plus platinum-EP failed to improve mOS, but did help to control disease. More notably, a randomized, double-blind, international multicenter phase III trial (ASTRUM-005) of serplulimab combined with EP-carboplatin showed that anti-PD-1 agents significantly improved the mOS of patients with ES-SCLC (9). Based on these many studies, the addition of an ICI to platinum-based chemotherapy has become a standard first-line treatment for ES-SCLC because this regimen prolongs survival and has acceptable safety risk level.
Despite this, many obstacles must be overcome to improve the prognosis of patients with ES-SCLC. Evidence is mounting that thoracic radiotherapy (TRT) combined with chemo-immunotherapy is a potential option for these patients. Robust preclinical research of the synergistic effects of radiotherapy and immunotherapy established a basis for the use of radio-immunotherapy treatments (10). Although no large randomized controlled clinical trials have examined this combined regimen for ES-SCLC, some extrapolations can be made in the context of non-small cell lung cancer (NSCLC). In particular, the PACIFIC trial (11) and the PEMBRO-RT trial (12) confirmed the safety and efficacy of a TRT-immunotherapy combination regimen for NSCLC.
To date, clinical trials on the application of immuno-chemoradiotherapy in ES-SCLC are few and limited to early phase and small sample, but the results suggested this combined regimen was tolerable and had promising efficacy (13,14). Hence, the aim of our multi-center retrospective study of patients in routine clinical practice was to determine the effect of TRT in ES-SCLC patients receiving first-line immunotherapy plus chemotherapy and to identify the subgroup of patients who benefit most from this regimen. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-294/rc).
Methods
Objective and patients
Our multicenter, retrospective study followed the Declaration of Helsinki (as revised in 2013) and was approved by the Institution Review Broad of Jinling Hospital (No. 2018NZKY-031-03). Jiangsu Cancer Hospital was also informed and agreed the study. Considering the retrospective characteristic of our study in design, the need for informed consent from the involved patients was waived. This multicenter retrospective cohort study evaluated the effect of TRT for patients with ES-SCLC, with a focus on disease control, survival, and tolerability. Seventy-eight consecutive patients who were diagnosed with ES-SCLC according to Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology (2) and were treated at one of two hospitals in Nanjing (Jinling Hospital, n=36; Jiangsu Cancer Hospital, n=42) from January 2019 to January 2023 were included. The inclusion criteria were: (I) age 18 years or older; (II) cytological or pathological diagnosis of SCLC; (III) confirmation of ES-SCLC due to the presence of distant metastasis or thoracic extension of disease beyond a single radiation port; (IV) receipt of first-line combination of EP-based chemotherapy and immunotherapy; (V) receipt of TRT; and (VI) availability of detailed clinical follow-up data. The exclusion criteria were: (I) limited-stage SCLC; (II) mixed histological types of cancer cells; (III) receipt of chemotherapy with an agent other than platinum and EP; (IV) receipt of immunotherapy only as a maintenance therapy (instead of first-line induction therapy) or receipt of TRT after second-line or later systemic treatment.
Data records and response evaluation
The baseline data included gender, age, smoking status, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), and the specific characteristics of the tumor [stage, size of primary lesion, baseline mediastinal lymph node status, brain and liver metastasis, number of initial distant metastases, pleural and pericardial effusion, and superior vena cava syndrome (SVCS)]. The precise chemo-immunotherapy and radiotherapy regimens were also recorded. These regimens were determined by the treating clinicians based on the guidelines of the National Comprehensive Cancer Network or the Chinese Society of Clinical Oncology.
The dose size and fractionation scheme of the radiotherapy regimen was based on the radiobiological responses of cancerous and normal tissues. Conventional radiotherapy was most commonly given as 1.8 to 2.2 Gy single fractions per day, 5 days per week for 3 to 9 weeks, with a total maximum dose between 60 and 90 Gy. Hyperfractionation refers to smaller doses of 0.5 to 1.8 Gy with multiple fractions per day for 2 to 4 weeks, and hypofractionation refers to a single daily fraction of 3 to 20 Gy with a small number of fractions, usually over 1 week (15). The response to induction treatment when TRT was introduced and the best response to immuno-chemoradiotherapy were measured with the guidance of Response Evaluation Criteria in Solid Tumor (RECIST) version1.1 (16). The objective response rate (ORR) was defined as the percentage of patients who showed a complete or partial response (PR), and the disease control rate (DCR) was defined as the percentage of patients who had a complete response, PR, or stable disease (SD). Analysis of adverse events (AEs) focused on the incidence of pneumonitis caused by the combination of ICIs and TRT. The severity of AEs was classified as Grade 1 to 5, according to the Common Terminology Criteria for Adverse Events (CTCAE) (17), and a Grade 3 or higher AE was defined as a “high-grade” AE.
Statistical analysis
SPSS version 26 (IBM SPSS Inc., Chicago, USA) was used for statistical analyses. Considering the exploratory nature of this study, no formal sample size calculation was performed in advance. Clinical and demographic categorical variables were presented as frequencies and percentages. The OS time was calculated as the date from the initial first-line induction treatment to death, and progression-free survival (PFS) as the date from treatment to any relapse or progression. Local PFS (lPFS) referred to progression of the primary thoracic lesion after treatment, and distant PFS (dPFS) referred progression of a primary metastasis or the presence of a new distant metastasis. Survival time was estimated using the Kaplan-Meier method, and all 95% confidence intervals (CIs) were two-tailed. The Cox proportional hazard algorithm was used for univariate and multivariate analysis of variables associated with OS and PFS. All P values were based on a two-sided hypothesis, and a P value below 0.05 was considered significant.
Results
Patient characteristics
After implementing the inclusion and exclusion criteria, we retrospectively examined 78 ES-SCLC patients who were admitted to two institutions in Nanjing from January 2019 to January 2023 (Table 1). Thirty-six patients were from Jinling Hospital, and 42 patients were from Jiangsu Cancer Hospital. At the cut-off date, the median follow-up time was 31.9 months. The median age was 41 years old and the age range was from 38 to 79 years old. Most patients were younger than 65 years old. Sixty-six patients (84.6%) were male and 52 patients (66.7%) were current or former smokers. Almost all patients presented with an ECOG-PS of 0 or 1, although three patients had ECOG-PS of 2. Unfortunately, reliable determination of the size of the primary thoracic lesion could not be retrieved from medical records in 26 patients; 28 patients had primary lesions of 5 cm or more and the other 24 patients had primary lesions less than 5 cm. Nearly 68% of the patients had 0 to 3 initial distant metastases, and nearly 80% had mediastinal lymph node metastasis. Twelve patients had primary brain metastasis, and 11 patients had primary liver metastasis. Twenty-nine patients (37.2%) had pleural effusion and 14 patients (17.9%) had pericardial effusion. Notably, 12 patients (15.4%) suffered from SVCS.
Table 1
| Characteristic | N (%) |
|---|---|
| Age | |
| <65 years | 50 (64.1) |
| ≥65 years | 28 (35.9) |
| Gender | |
| Male | 66 (84.6) |
| Female | 12 (15.4) |
| Smoking status | |
| Never smoker | 26 (33.3) |
| Current or former smoker | 52 (66.7) |
| ECOG-PS | |
| 0–1 | 75 (96.2) |
| 2 | 3 (3.8) |
| Size of primary lesion | |
| NA | 26 (33.3) |
| <5 cm | 24 (30.8) |
| ≥5 cm | 28 (35.9) |
| Metastasis | |
| Mediastinal lymph node metastasis | 62 (79.5) |
| Primary brain metastasis | 12 (15.4) |
| Primary liver metastasis | 11 (14.1) |
| Number of initial distant metastases | |
| 0–3 | 53 (67.9) |
| >3 | 25 (32.1) |
| Pleural effusion | 29 (37.2) |
| Pericardial effusion | 14 (17.9) |
| Superior vena cava syndrome | 12 (15.4) |
ES-SCLC, extensive-stage small cell lung cancer; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; NA, not available.
Chemo-immunotherapy and radiotherapy regimens
All 78 patients received between 2 and 6 cycles of platinum-based chemotherapy and immunotherapy as a first-line systemic treatment (Table S1). Thirty-two patients (41.0%) received an anti-PD-1 agent, and 44 (56.4%) received an anti-PD-L1 agent. Two patients switched from an anti-PD-1 agent to an anti-PD-L1 agent. Nearly half the patients also received immune maintenance therapy, 14 with an anti-PD-1 agent and 27 with an anti-PD-L1 agent. Among those who did not receive immune maintenance therapy, some patients who received a second-line therapy experienced disease progression during the combination of first-line chemo-immunotherapy with radiotherapy. Some other patients refused immune maintenance therapy due to poor health or financial constraints, and opted for regular follow-up or other maintenance therapies, such as etoposide capsules or anlotinib.
There were large variations in the purpose, timing, and dose-fractionation of the TRT in our cohort (Table 2). TRT was given to 58 patients who responded to systemic treatment as consolidation therapy; it was given sequentially with chemo-immunotherapy in 45 of these patients and concurrently with chemo-immunotherapy in the other 13 patients. Eight of the 13 patients who received TRT concurrently with chemo-immunotherapy had their immunotherapy halted during the TRT. There were also 11 patients who received palliative radiotherapy for SVCS and tumor compression, and 9 patients who received salvage therapy for disease progression. Almost 56% of our cohort received TRT after 3 cycles of systemic induction which we named after late TRT and the other 34 patients received TRT within the 3 cycles of systemic induction which were classified as early TRT. Sixteen patients lacked information about TRT dose. Among the other 62 patients, hypofractionation was used in 35 patients (44.9%), hyperfractionation was used in 15 patients (19.2%), and conventional fractionation was used in 12 patients (15.4%).
Table 2
| Characteristic | N (%) |
|---|---|
| Purpose | |
| Consolidative radiotherapy | 58 (74.3) |
| Sequential | 45 (57.7) |
| Concurrent with chemo-immunotherapy | 13 (16.6) |
| Palliative radiotherapy for SVCS and oncothlipsis | 11 (14.1) |
| Salvage radiotherapy after disease progression | 9 (11.5) |
| Timing | |
| Early TRT (≤3 cycles of induction therapy) | 34 (43.6) |
| Late TRT (>3 cycles of induction therapy) | 44 (56.4) |
| Dose | |
| NA | 16 (20.5) |
| Conventional fractionation | 12 (15.4) |
| Hyperfractionation | 15 (19.2) |
| Hypofractionation | 35 (44.9) |
| Prior response to induction treatment | |
| NA | 13 (16.7) |
| PR | 17 (21.8) |
| SD | 39 (50.0) |
| PD | 9 (11.5) |
| Extrathoracic radiotherapy | 28 (35.9) |
| PCI | 3 (3.8) |
SVCS, superior vena cava syndrome; TRT, thoracic radiotherapy; NA, not available; PR, partial response; SD, stable disease; PD, progressive disease; PCI, prophylactic cranial irradiation.
Efficacy
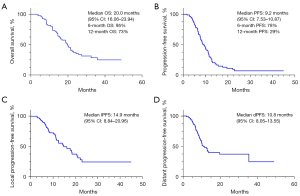
According to RECIST criteria, 46 patients (59%) achieved PR, 24 patients (30.8%) had SD, and 8 patients (10.3%) had progressive disease (PD) as the best overall response to chemo-immunotherapy combined with TRT. The ORR was 59% and the DCR was 89.8%. At the median follow-up time of 31.9 months, the median OS (mOS) was 20 months (95% CI: 16.06–23.94) (Figure 1A). The estimated 6-month OS was 95% and 12-month OS was 73%. The median PFS (mPFS) was 9.2 months (95% CI: 7.53–10.87) (Figure 1B), the 6-month PFS was 78%, and the 12-month PFS was 29%. The median lPFS (mlPFS) was 14.9 months (95% CI: 8.84–20.96) (Figure 1C) and the median dPFS (mdPFS) was 10.8 months (95% CI: 8.05–13.55) (Figure 1D). None of the patients who received prophylactic cranial irradiation (PCI) experienced new brain metastasis, and 11 patients experienced intracranial failure with no associated neurological symptoms.
We performed univariate and multivariate analysis to identify predictors of PFS (Table 3). The presence of primary liver metastasis, pleural effusion, receipt of an anti-PD-L1 agent and receipt of consolidative TRT were significant variables in the univariate analysis and further tested in multivariate analysis. The presence of primary liver metastasis [hazard ratio (HR): 2.82; 95% CI: 1.38–5.77; P<0.05] and pleural effusion (HR: 1.99; 95% CI: 1.18–3.33; P<0.05) were negatively associated with PFS, and receipt of consolidative TRT (HR: 0.44; 95% CI: 0.25–0.79; P<0.05) was positively associated with PFS. Receipt of an anti-PD-L1 agent did not have a significant effect in the multivariate analysis.
Table 3
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≥65 vs. <65 years) | 0.61 (0.36–1.03) | 0.056 | – | – | |
| Gender (female vs. male) | 0.90 (0.46–1.77) | 0.767 | – | – | |
| Smoking status (yes vs. no) | 1.16 (0.70–1.94) | 0.563 | – | – | |
| ECOG-PS (>1 vs. ≤1) | 0.71 (0.17–2.91) | 0.633 | – | – | |
| Size of primary lesion (≥5 vs. <5 cm) | 1.51 (0.83–2.76) | 0.179 | – | – | |
| Mediastinal lymph node metastasis (yes vs. no) | 1.51 (0.80–2.84) | 0.202 | – | – | |
| Primary brain metastasis (yes vs. no) | 1.17 (0.62–2.20) | 0.630 | – | – | |
| Primary liver metastasis (yes vs. no) | 3.06 (1.58–5.93) | 0.001 | 2.82 (1.38–5.77) | 0.005 | |
| Number of initial distant metastases (≥3 vs. <3) | 1.54 (0.92–2.59) | 0.099 | – | – | |
| Superior vena cava syndrome (yes vs. no) | 1.44 (0.73–2.85) | 0.297 | – | – | |
| Pleural effusion (yes vs. no) | 1.96 (1.18–3.24) | 0.009 | 1.99 (1.18–3.33) | 0.010 | |
| Pericardial effusion (yes vs. no) | 1.35 (0.72–2.54) | 0.348 | – | – | |
| Immune checkpoint inhibitor | |||||
| Anti-PD-1 drug (yes vs. no) | 1.65 (1.00–2.71) | 0.050 | – | – | |
| Anti-PD-L1 drug (yes vs. no) | 0.55 (0.33–0.90) | 0.017 | – | 0.050 | |
| PCI (yes vs. no) | 0.50 (0.12–2.05) | 0.334 | – | – | |
| Extrathoracic radiotherapy (yes vs. no) | 1.12 (0.68–1.85) | 0.655 | – | – | |
| Consolidative TRT (yes vs. no) | 0.41 (0.23–0.73) | 0.002 | 0.44 (0.25–0.79) | 0.006 | |
| Timing of TRT (late vs. early) | 0.78 (0.48–1.28) | 0.325 | – | – | |
| Dose of TRT | |||||
| Conventional radiotherapy (yes vs. no) | 0.90 (0.45–1.81) | 0.767 | – | – | |
| Hyperfractionated radiotherapy (yes vs. no) | 0.63 (0.32–1.24) | 0.632 | – | – | |
| Hypofractionated radiotherapy (yes vs. no) | 1.52 (0.87–2.66) | 0.144 | – | – | |
| Response to systemic therapy before TRT (CR/PR vs. SD/PD) | 1.08 (0.63–1.85) | 0.773 | – | – | |
ES-SCLC, extensive-stage small cell lung cancer; HR, hazard ratio; CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group performance status; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PCI, prophylactic cranial irradiation; TRT, thoracic radiotherapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
We used the same statistical methods as above for identification of variables associated with lPFS and dPFS (Table S2). The results indicated that locoregional thoracic lesion progression was more likely to occur in those with primary liver metastasis (HR: 2.74; 95% CI: 1.10–6.83; P<0.05) and less likely to occur when consolidative TRT was administered as a first-line treatment (HR: 0.23; 95% CI: 0.11–0.48; P<0.05). Analysis of distant metastasis demonstrated that more than 3 initial distant metastases was a negative predictor (HR: 1.92; 95% CI: 1.05–3.50; P<0.05).
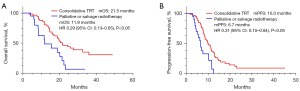
Univariate and multivariate analysis of factors associated with OS demonstrated that primary liver metastasis was a predictor of poor OS (HR: 4.12; 95% CI: 1.89–8.99; P<0.05) and consolidative TRT had a protective effect (HR: 0.43; 95% CI: 0.23–0.80; P<0.05; Table S3). Patients who received consolidative TRT had a longer PFS (HR: 0.31; 95% CI: 0.15–0.64; P<0.05) and OS (HR: 0.29; 95% CI: 0.13–0.65; P<0.05) than those who were received palliative TRT for SVCS and tumor compression or salvage radiotherapy after intrathoracic disease progression (Figure 2).
Safety
At the cut-off date, no treatment-related deaths were observed in our cohort (Table 4). The major AE was bone marrow suppression (n=50, 64.1%), and this AE was Grade 3 or 4 in 23 cases (29.5%). All 23 of these patients experienced significant improvement after prompt symptomatic treatment. Eighteen patients (23.1%) had clinically significant treatment-related pneumonitis, 16 with Grade 1 or 2. Two patients experienced high-grade pneumonitis that deteriorated into respiratory failure, but they recovered after timely and appropriate treatment and remained alive at the cut-off date. Four patients had Grade 1 or 2 radiation esophagitis. Notably, 1 of these 4 patients who received TRT with concurrent chemo-immunotherapy and who continued immunotherapy during radiotherapy developed radiation pneumonitis and radiation esophagitis.
Table 4
| Adverse event | Incidence, n (%) | |
|---|---|---|
| All-grade | High-grade* | |
| Treatment-related pneumonitis | 18 (23.1) | 2 (2.6) |
| Radiation esophagitis | 4 (5.1) | 0 |
| Rash | 2 (2.6) | 0 |
| Renal insufficiency | 2 (2.6) | 0 |
| Immune-related thyroiditis | 1 (1.3) | 0 |
| Immune-related diabetes | 1 (1.3) | 0 |
| Abnormal liver function | 3 (3.8) | 0 |
| Marrow suppression | 50 (64.1) | 23 (29.5) |
*, high-grade: Grade 3 or higher. ES-SCLC, extensive-stage small cell lung cancer.
Discussion
SCLC is a highly aggressive tumor with a devastating prognosis (18), particularly when it has progressed to the extensive stage, with spread to regions including the bone and brain (19). Prior to the use of immunotherapy, TRT was not considered an important part of the treatment for ES-SCLC until the pivotal studies of Slotman et al. (20) and Jeremic et al. (21), which found that TRT conferred survival benefits when combined with chemotherapy. The results of the IMpower133, CASPIAN, and CAPSTONE-1 trials (5-7), led to the standardized use of immunotherapy plus EP-based chemotherapy for ES-SCLC. Given this background, it is worth reconsidering the role of TRT with immunotherapy for treatment of ES-SCLC. To our knowledge, the current retrospective analysis is the largest study of patients with ES-SCLC who received TRT and chemo-immunotherapy and the first study to examine the subgroups of patients who benefit most from this treatment.
Our multi-institutional case series of patients with ES-SCLC showed the mOS time was 20 months, with 6-month OS of 95% and 12-month OS of 73% and the mPFS time was 9.2 months, with a mlPFS time of 14.9 months, and a mdPFS time of 10.8 months. These results are consistent with those from a multicenter study in the USA. that examined 20 patients who received first-line chemo-immunotherapy followed by consolidative TRT (22), which reported a median OS time of 16 months and a median PFS time of 6.7 months. This combination regimen in our study led to an ORR of 59% and a DCR of 89.8%. Two retrospective analyses conducted in different regions also found that survival outcome was better when chemo-immunotherapy was combined with TRT (23,24). The largest of these two studies examined 63 patients using a National Hospital-Based Registry (23); after propensity score matching, there was a trend for improved median OS time (from 9 to 11 months) and the 2-year OS rate was 18.1% with the addition of TRT. The results were similar in an Israeli real-world retrospective study that examined 25 patients who received TRT following chemotherapy with atezolizumab or durvalumab (24), which found that the mOS and mPFS times were significantly longer for patients who received TRT. A comparison of our results with those of other landmark modern clinical trials that examined the effect of immunotherapy for ES-SCLC indicated that TRT combined with chemo-immunotherapy led to significant survival benefits for these patients (Table 5).
Table 5
| Outcome | Chemo-immunotherapy with TRT (our study) | Chemo-immunotherapy alone | |||
|---|---|---|---|---|---|
| IMpower133 (5) | CASPIAN (6) | KEYNOTE-604 (8) | ASTRUM-005 (9) | ||
| mOS, months | 20.0 | 12.3 | 12.9 | – | 15.4 |
| mPFS, months | 9.2 | 5.2 | 5.1 | 4.8 | 5.8 |
| mlPFS, months | 14.9 | – | – | – | – |
| mdPFS, months | 10.8 | – | – | – | – |
| ORR, % | 59.0 | 60.2 | 67.9 | 70.6 | 68.9 |
| DCR, % | 89.8 | 81.1 | – | 88.1 | 93.1 |
ES-SCLC, extensive-stage small cell lung cancer; TRT, thoracic radiotherapy; mOS, median overall survival; mPFS, median progression-free survival; mlPFS, median local progression-free survival; mdPFS, median distant progression-free survival; ORR, objective response rate; DCR, disease control rate.
The efficacy of TRT combined with chemo-immunotherapy may be due to the synergistic interaction of immunotherapy and radiotherapy. In particular, radiotherapy can transform an ineffective immune response into an effective and durable response by increasing antigen presentation and activation of T cells by release of cancer-specific peptides, upregulating major histocompatibility complex class I (MHC-I) expression on tumor cells, activating the transcription of interferon (IFN), and enhancing macrophage phagocytosis (10,25). This response occurs at the irradiated site and also in out-of-field lesions (26), which is referred to as the “abscopal effect”. An alternative possibility is that activation of T-cells by immunotherapy may sensitize tumors to radiation by promoting normalization of tumor vasculature and tissue hypoxia. Previous preclinical studies of NSCLC reported an interaction between radiotherapy and treatment with an anti-PD-1 or anti-PD-L1 antibody (27,28).
There have been worries regarding the possible development of immune-related toxicity when using the combination of an ICI and radiotherapy (29), and pneumonitis is a major concern in patients receiving TRT-immunotherapy (30-32). The incidence of pneumonitis in our study was 23%, only two patients experienced high-grade pneumonitis, and all patients recovered from respiratory failure after prompt treatment. This is comparable to the results of other studies that examined the effect of first-line chemo-immunotherapy in patients with ES-SCLC (5-9,33,34). Welsh et al. (14) evaluated the combination of pembrolizumab with TRT after first-line chemotherapy in ES-SCLC patients and also reported a favorable safety profile, with 15% of patients experiencing pneumonitis and Grade 3 pneumonitis only in patients who received higher doses of radiation. A phase I trial that examined the effect of consolidative ipilimumab and nivolumab with TRT for ES-SCLC (13) showed a high rate of AEs, although the toxicity rate was similar to that of ipilimumab and nivolumab alone, suggesting that TRT was not responsible for any additional toxicity. The results of several small retrospective studies suggested there was no increased toxicity with addition of TRT (35-37). Notably, four of our patients who received concurrent TRT with chemo-immunotherapy continued immunotherapy during radiotherapy, and one of them experienced radiation pneumonitis and radiation esophagitis. This result should be considered when evaluating the relative safety of sequential radiation with TRT during concurrent chemo-immunotherapy. Although data on the safety of combination treatment for ES-SCLC patients are limited, there are some data regarding this treatment in patients with NSCLC. In particular, the PACIFIC trial (11) evaluated the use of consolidation durvalumab in combination with chemoradiotherapy in patients with stage III unresectable NSCLC and reported the incidence of all-grade pneumonitis was 33.9% and the incidence of high-grade pneumonitis was 3.4%. The PEMBRO-RT trial (12) examined the effect of pembrolizumab after stereotactic body radiotherapy in patients with advanced NSCLC and found a higher incidence of pneumonitis than in the control arm, but no statistically significant difference (26% vs. 8%; P=0.06).
To maximize the benefit of a combined TRT and chemo-immunotherapy regimen, it is necessary to identify patients who benefit most so that individualized radiation plans can be used. Our analysis showed that pleural effusion at presentation was negatively related to PFS. A systematic review and meta-analysis which focused on prognostic impact of pleural effusion in patients with malignancy found that pleural effusion was a prognostic factor associated with poor survival for patients with lung cancer (38). Three retrospective studies also found that the presence of malignant pleural effusion and minimal pleural effusion might be associated with SCLC prognosis (39-41). Our study did not further identify the characteristics of the pleural effusion because most involved patients had minimal pleural effusion which are not recommended for thoracentesis. The minimal pleural effusion at the time of diagnosis may indicate early stage of malignant pleural effusion and may have poor prognostic relevance. Patients with primary liver metastasis received limited benefit from TRT in terms of PFS and OS, possibly because liver metastasis diminishes the systemic efficacy of immunotherapy via macrophage-mediated T cell elimination (42,43). Some researchers reported that liver metastasis created an “immune desert” in preclinical models, and that liver-directed radiotherapy eliminated immunosuppressive hepatic T macrophages. This led to the hypothesis that the combination of liver-directed radiotherapy and immunotherapy can improve the prognosis of ES-SCLC patients who have primary liver metastasis (44,45). Otherwise, our study found that oligometastasis, referring to fewer than three distant metastases, was the only independent positive predictor of dPFS, possibly because oligometastasis tends to connote a lower tumor burden. Prior to the use of immunotherapy, clinicians advocated chest radiotherapy for certain ES-SCLC patients. However, this recommendation was not applied routinely because of conflicting data regarding the benefit of TRT in the RTOG 0937 trial and the CREST trial and the absence of clear and definitive guidelines about dose fractionation, dose volume, and treatment models of radiotherapy (20,46). With a lot of researches going on, it has been proven that the use of TRT allows great local control of ES-SCLC and postponement of second-line treatments. Recent progress in immunotherapy has affected the entire field of oncology, including ES-SCLC. Thus, there is a need to develop appropriate individualized radiation treatment plans for patients with ES-SCLC.
Variations in the TRT regimen, in terms of purpose, dose-fractionation, and timing, as well as in patient demographics, could influence its survival benefit when combined with chemo-immunotherapy. Our results showed that patients who received consolidative TRT after chemo-immunotherapy benefited more than those who received TRT as palliative or salvage treatment for SVCS or disease progression. A previous study reported that SVCS was a negative predictor of clinical survival (47). The Chinese Society of Clinical Oncology (CSCO) 2022 trial recommended TRT combined with chemotherapy as a standard treatment, and showed that concurrent chemoradiotherapy was superior to sequential chemoradiotherapy for limited-stage SCLC (LS-SCLC) (48). A preclinical study found that radiotherapy following immunotherapy may kill the T cells that were activated by an ICI, and that radiation weakened the overall antitumor immunological effect (28). The PACIFIC trial (11) and the GEMSTONE-301 trial (49) reported that concurrent chemoradiotherapy followed by sequential immunotherapy was a promising regimen for patients with advanced NSCLC. However, some researchers maintained that the immune system was more competent before radiotherapy, and therefore more likely to respond to an ICI (50). In contrast, the results of a phase II nonrandomized study of two cohorts with stage III NSCLC (51) suggested a promising antitumor effect and a favorable safety profile from pembrolizumab with concurrent chemoradiation therapy. As to ES-SCLC, when TRT is introduced in, the order of immunotherapy and radiotherapy remains invalid. In our study, most patients received sequential TRT, often between two cycles of chemo-immunotherapy or after completion of the whole course of systemic treatment, so there was no overlap with any of the cycles of chemo-immunotherapy. Thirteen of our patients received TRT with concurrent chemo-immunotherapy, which implied that radiotherapy and chemo-immunotherapy overlapped in time, with caution due to safety concerns and more than half of them discontinued immunotherapy during the radiotherapy regimen. Most patients who received TRT with concurrent chemo-immunotherapy were oligometastatic, had no local symptoms, and all of them achieved good ECOG-PS. In addition, 11 of these patients had metastatic mediastinal lymph nodes.
It is also important to determine the optimal radiation dose when administering TRT. Our analysis showed that different dose fractionation schemes had no significant effect, probably because of incomplete data and small set of samples. Previous studies examined the effect of different TRT doses when it was given without immunotherapy (20,21,46,52). A retrospective analysis of the USA. National Cancer Database that examined 3,280 patients reported that a TRT dose of 45 Gy or more was independently associated with longer survival (53). Another retrospective analysis of 306 patients, 170 of whom received TRT (54), also found that a higher radiation dose was associated with longer survival and improved disease control. Considering that TRT combined with immunotherapy may increase the incidence of AEs, especially pneumonitis, it is necessary to carefully consider the most appropriate dose-fractionation to be used for TRT. Welsh et al. (14) reported that 15 fractions and a total dose of 45 Gy was tolerable when administering TRT concurrent with an ICI. The Canadian Consensus Recommendations propose a total TRT dose of 30 Gy in 10 fractions when using immunotherapy (55). However, a higher radiation dose may provide better local control and prolong survival in patients with good ECOG-PS who had an excellent response to concurrent chemo-immunotherapy. On the other hand, dose restrictions to the organ risk and consequent toxicity may limit the actual received dose-fractionation in practice.
The optimal timing of TRT during chemo-immunotherapy is an important issue that also warrants further exploration. Our retrospective analysis found no significant difference between early TRT (≤3 cycles of chemo-immunotherapy) and late TRT (>3 cycles) in analyses of PFS and OS. Similarly, the RTOG 0937 trial and a retrospective Chinese study also showed no significant difference in survival for patients who received early or late TRT (46). Han et al. (56) suggested that receipt of TRT within 6 cycles of chemotherapy may provide better local control. In other words, a systemic response to chemo-immunotherapy before TRT is likely to lead to more favorable outcome. Although response to systemic treatment had no significant effect in our analyses, patients who received TRT as palliative or salvage treatment tended to have poor locoregional control. Interestingly, an additional analysis from CREST trial found no benefit of TRT in patients who had complete intrathoracic responses (57). Taken together, we speculate that TRT should be considered in selected ES-SCLC patients who had a good response or PR after chemo-immunotherapy and residual disease in the thorax.
Previous studies of NSCLC (11,12) provided a rationale for using the combination of TRT and chemo-immunotherapy for ES-SCLC patients. In this context, we have every reason to believe that TRT has its place in ES-SCLC in the era of immunotherapy. Given the development of persistent radiation-induced immunosuppression, the risk of radiation-induced toxicities, and the difficulty in identifying new biomarkers, we suggest that further prospective randomized studies should assess the efficacy and safety adding TRT to first-line chemo-immunotherapy in an appropriate population of ES-SCLC patients. An ongoing multicenter phase II trial named after TREASURE (NCT04462276) is going to investigate the efficacy and feasibility of combining TRT with the IMpower133 regimen as an upfront treatment for ES-SCLC patients. The ongoing RAPTOR trial (NRG-LU007, NCT04402788) randomized patients to two groups (standard chemotherapy with atezolizumab followed by atezolizumab maintenance vs. atezolizumab maintenance with consolidative radiation at up to five thoracic and/or extrathoracic sites) and will evaluate PFS, OS, and safety. Similarly, the ongoing phase III TRIPLEX study (NCT05223647) is assessing the benefit of adding TRT to durvalumab plus EP-based chemotherapy. The results of these trials will help clarify the use of TRT in treatment of patients with ES-SCLC who receive chemo-immunotherapy as first-line treatment.
Our study was limited by its retrospective design and small sample size. These limitations may have led to selection bias, such as a trend for patients to achieve more favorable responses to systemic therapy and better ECOG-PS. In addition, because not all patients received treatment at the two institutions, we were unable to retrieve some data regarding the dose-fractionation of TRT and the responses to chemo-immunotherapy, and this prevented formulation of a comprehensive TRT regimen and selection of the most appropriate patients. The results of our multivariate analyses, even though they considered possible confounding factors, should also be interpreted with caution. Nevertheless, the results of this study suggest that TRT combined with chemo-immunotherapy is a safe and potentially effective treatment for patients with ES-SCLC, and that further study of this regimen is warranted.
Conclusions
Our results and those of other recent studies (22-24,58,59) suggest that TRT could constitute a feasible therapeutic option in selected ES-SCLC patients who received chemo-immunotherapy. The patients most likely to benefit from this treatment are those who received consolidative TRT after chemo-immunotherapy without primary liver metastasis and pleural effusion at the time of diagnosis. Importantly, consolidative TRT was associated with a longer time to locoregional recurrence. A longer follow-up time is needed to determine whether this treatment improves OS. In addition, large prospective studies are needed to confirm our results and to identify biomarkers that predict the efficacy and safety of TRT in patients with ES-SCLC who received chemo-immunotherapy.
Acknowledgments
We thank all the reviewers for their important contributions.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-294/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-294/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-294/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-294/coif). TL serves as an unpaid editorial board member of Translational Lung Cancer Research from December 2022 to November 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institution Review Broad of Jinling Hospital (No. 2018NZKY-031-03) and Jiangsu Cancer Hospital was also informed and agreed the study. The need for patient consent was waived due to the retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021;7:3. [Crossref] [PubMed]
- Ganti AKP, Loo BW, Bassetti M, et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:1441-64. [Crossref] [PubMed]
- Jett JR, Schild SE, Kesler KA, et al. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e400S-19S.
- Demedts IK, Vermaelen KY, van Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J 2010;35:202-15. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:739-47. [Crossref] [PubMed]
- Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol 2020;38:2369-79. [Crossref] [PubMed]
- Cheng Y, Han L, Wu L, et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022;328:1223-32. [Crossref] [PubMed]
- Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin 2017;67:65-85. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. [Crossref] [PubMed]
- Perez BA, Kim S, Wang M, et al. Prospective Single-Arm Phase 1 and 2 Study: Ipilimumab and Nivolumab With Thoracic Radiation Therapy After Platinum Chemotherapy in Extensive-Stage Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2021;109:425-35. [Crossref] [PubMed]
- Welsh JW, Heymach JV, Chen D, et al. Phase I Trial of Pembrolizumab and Radiation Therapy after Induction Chemotherapy for Extensive-Stage Small Cell Lung Cancer. J Thorac Oncol 2020;15:266-73. [Crossref] [PubMed]
- Makinde AY, Eke I, Aryankalayil MJ, et al. Exploiting Gene Expression Kinetics in Conventional Radiotherapy, Hyperfractionation, and Hypofractionation for Targeted Therapy. Semin Radiat Oncol 2016;26:254-60. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Published: November 27. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute.
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [Crossref] [PubMed]
- Zugazagoitia J, Paz-Ares L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J Clin Oncol 2022;40:671-80. [Crossref] [PubMed]
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36-42. [Crossref] [PubMed]
- Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol 1999;17:2092-9. [Crossref] [PubMed]
- Diamond BH, Verma N, Shukla UC, et al. Consolidative Thoracic Radiation Therapy After First-Line Chemotherapy and Immunotherapy in Extensive-Stage Small Cell Lung Cancer: A Multi-Institutional Case Series. Adv Radiat Oncol 2022;7:100883. [Crossref] [PubMed]
- Gross AJ, Kharouta MZ, Podder TK, et al. Role of thoracic radiotherapy in extensive stage small cell lung cancer (ES-SCLC) in the immunotherapy era: a national hospital-based registry analysis. Int J Rad Oncol Biol Phys 2021;111:e466.
- Daher S, Allen A, Rottenberg Y, et al. Real-world data of consolidative radiotherapy for extensive stage (ES)-SCLC treated by chemo-immunotherapy (chemo-IO). Abstract #432 (poster). European Lung Cancer Congress. Mar 30 - Apr 2, 2022. Prague, Czechia (2022).
- Wang Y, Liu ZG, Yuan H, et al. The Reciprocity between Radiotherapy and Cancer Immunotherapy. Clin Cancer Res 2019;25:1709-17. [Crossref] [PubMed]
- Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, et al. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol 2018;39:644-55. [Crossref] [PubMed]
- Herter-Sprie GS, Koyama S, Korideck H, et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight 2016;1:e87415. [Crossref] [PubMed]
- Gong X, Li X, Jiang T, et al. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1085-97. [Crossref] [PubMed]
- Hwang WL, Pike LRG, Royce TJ, et al. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol 2018;15:477-94. [Crossref] [PubMed]
- Rapoport BL, Shannon VR, Cooksley T, et al. Pulmonary Toxicities Associated With the Use of Immune Checkpoint Inhibitors: An Update From the Immuno-Oncology Subgroup of the Neutropenia, Infection & Myelosuppression Study Group of the Multinational Association for Supportive Care in Cancer. Front Pharmacol 2021;12:743582. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1607-16. [Crossref] [PubMed]
- Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721-8. [Crossref] [PubMed]
- Zhou F, Zhao W, Gong X, et al. Immune-checkpoint inhibitors plus chemotherapy versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer. J Immunother Cancer 2020;8:e001300. [Crossref] [PubMed]
- Sathiyapalan A, Febbraro M, Pond GR, et al. Chemo-Immunotherapy in First Line Extensive Stage Small Cell Lung Cancer (ES-SCLC): A Systematic Review and Meta-Analysis. Curr Oncol 2022;29:9046-65. [Crossref] [PubMed]
- Galuba JM, Stöver I, Koziorowski A, et al. 1652P Safety of simultaneously performed radiotherapy in patients with small cell lung cancer undergoing atezolizumab treatment. Ann Oncol 2021;32:S1165.
- Bruni A, Bertolini F, D'Angelo E, et al. 147P - Chemo-immunotherapy with or without consolidative radiotherapy in extensive stage small cell lung cancer: An initial report of clinical outcome and safety. Ann Oncol 2022;33:S97-S104.
- Chen H, Ma X, Liu J, et al. Clinical outcomes of atezolizumab in combination with etoposide/platinum for treatment of extensive-stage small-cell lung cancer: A real-world, multicenter, retrospective, controlled study in China. Chin J Cancer Res 2022;34:353-64. [Crossref] [PubMed]
- Yang Y, Du J, Wang YS, et al. Prognostic impact of pleural effusion in patients with malignancy: A systematic review and meta-analysis. Clin Transl Sci 2022;15:1340-54. [Crossref] [PubMed]
- Ryu JS, Lim JH, Lee JM, et al. Minimal Pleural Effusion in Small Cell Lung Cancer: Proportion, Mechanisms, and Prognostic Effect. Radiology 2016;278:593-600. [Crossref] [PubMed]
- Shojaee S, Singh I, Solsky I, et al. Malignant Pleural Effusion at Presentation in Patients with Small-Cell Lung Cancer. Respiration 2019;98:198-202. [Crossref] [PubMed]
- Keidan N, Aujayeb A. Small Cell Lung Cancer and Pleural Effusion: An Analysis from a District General Hospital. Pulm Ther 2023;9:359-65. [Crossref] [PubMed]
- Lindblad KE, Lujambio A. Liver metastases inhibit immunotherapy efficacy. Nat Med 2021;27:25-7. [Crossref] [PubMed]
- O'Leary K. Liver metastases cultivate an immune desert. Nat Rev Cancer 2021;21:143. [Crossref] [PubMed]
- Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021;27:152-64. [Crossref] [PubMed]
- Owonikoko TK, Park K, Govindan R, et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J Clin Oncol 2021;39:1349-59. [Crossref] [PubMed]
- Gore EM, Hu C, Sun AY, et al. Randomized Phase II Study Comparing Prophylactic Cranial Irradiation Alone to Prophylactic Cranial Irradiation and Consolidative Extracranial Irradiation for Extensive-Disease Small Cell Lung Cancer (ED SCLC): NRG Oncology RTOG 0937. J Thorac Oncol 2017;12:1561-70. [Crossref] [PubMed]
- Azizi AH, Shafi I, Shah N, et al. Superior Vena Cava Syndrome. JACC Cardiovasc Interv 2020;13:2896-910. [Crossref] [PubMed]
- Chinese Society of Clinical Oncology Guidelines Working Committee. Chinese Society of Clinical Oncology (CSCO) Guidelines for Diagnosis and treatment of small cell lung cancer, 2022. Beijing: People's Medical Publishing House, 2022.
- Zhou Q, Chen M, Jiang O, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2022;23:209-19. [Crossref] [PubMed]
- Rückert M, Flohr AS, Hecht M, et al. Radiotherapy and the immune system: More than just immune suppression. Stem Cells 2021;39:1155-65. [Crossref] [PubMed]
- Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol 2021;7:1-9. [Crossref] [PubMed]
- Rathod S, Jeremic B, Dubey A, et al. Role of thoracic consolidation radiation in extensive stage small cell lung cancer: A systematic review and meta-analysis of randomised controlled trials. Eur J Cancer 2019;110:110-9. [Crossref] [PubMed]
- Hasan S, Renz P, Turrisi A, et al. Dose escalation and associated predictors of survival with consolidative thoracic radiotherapy in extensive stage small cell lung cancer (SCLC): A National Cancer Database (NCDB) propensity-matched analysis. Lung Cancer 2018;124:283-90. [Crossref] [PubMed]
- Li-Ming X, Zhao LJ, Simone CB 2nd, et al. Receipt of thoracic radiation therapy and radiotherapy dose are correlated with outcomes in a retrospective study of three hundred and six patients with extensive stage small-cell lung cancer. Radiother Oncol 2017;125:331-7. [Crossref] [PubMed]
- Sun A, Abdulkarim B, Blais N, et al. Use of radiation therapy among patients with Extensive-stage Small-cell lung cancer receiving Immunotherapy: Canadian consensus recommendations. Lung Cancer 2023;179:107166. [Crossref] [PubMed]
- Han J, Fu C, Li B. Clinical outcomes of extensive-stage small cell lung cancer patients treated with thoracic radiotherapy at different times and fractionations. Radiat Oncol 2021;16:47. [Crossref] [PubMed]
- Slotman BJ, Faivre-Finn C, van Tinteren H, et al. Which patients with ES-SCLC are most likely to benefit from more aggressive radiotherapy: A secondary analysis of the Phase III CREST trial. Lung Cancer 2017;108:150-3. [Crossref] [PubMed]
- Xie Z, Liu J, Wu M, et al. Real-World Efficacy and Safety of Thoracic Radiotherapy after First-Line Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer. J Clin Med 2023;12:3828. [Crossref] [PubMed]
- Fang M, Wang L, Gu Q, et al. Efficacy and safety of thoracic radiotherapy for extensive stage small cell lung cancer after immunotherapy in real world. Clin Exp Metastasis 2023;40:423-9. [Crossref] [PubMed]


