
Osimertinib versus comparator first-generation epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in patients with advanced EGFR-mutated non-small cell lung cancer: a Chinese, multicenter, real-world cohort study
Highlight box
Key findings
• This multicenter, real-world study revealed that progression-free survival (PFS) and overall survival (OS) for EGFR mutant non-small cell lung cancer (NSCLC) patients receiving osimertinib as first-line therapy were 19.4 and 40.5 months, respectively, both significantly longer than those receiving first-generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs). Besides, osimertinib demonstrated a similar safety profile to comparator EGFR-TKIs.
What is known and what is new?
• In FLAURA, osimertinib showed a positive benefit for the first-line strategy of patients with advanced EGFR-mutant NSCLC compared with first-generation EGFR-TKIs.
• In real-world settings, osimertinib also demonstrated longer PFS, OS, and a similar safety profile to comparator first-generation EGFR-TKIs when given as first-line strategy to Chinese NSCLC patients.
What is the implication, and what should change now?
• Osimertinib is safe and effective in real world populations. Studies with more patients are needed to confirm these results across diverse geographies and ethnicities.
Introduction
Lung cancer is a major global health concern, responsible for the highest number of cancer-related deaths worldwide (1). The majority of lung cancer cases (more than 85%) fall under the category of non-small cell lung cancer (NSCLC). Among the various driver genes in NSCLC, epidermal growth factor receptor (EGFR) mutation is the most significant, and the detection of EGFR mutations is crucial for the determination of personalized targeted therapy. The most common sensitizing driver mutations in NSCLC patients are exon 19 deletions and L858R substitutions within exon 21. These mutations have a higher prevalence in Asian patients (around 50%) compared to Caucasian patients (approximately 10%) (2,3).
Numerous global clinical trials involving patients with EGFR-mutated advanced NSCLC have demonstrated the safety and efficacy of first- or second-generation tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, icotinib, or afatinib, in the first-line setting. These TKIs have been shown to extend median progression-free survival (mPFS) to approximately 10 months compared to chemotherapy (4-11). Several guidelines recommend EGFR-TKIs as the standard first-line treatment for advanced NSCLC patients with a sensitive EGFR mutation (12,13).
Despite the progression-free survival (PFS) advantage, there is no significant overall survival (OS) improvement for patients receiving first-generation EGFR-TKIs. Furthermore, patients may develop acquired resistance to first-generation TKIs, with a predominant mechanism being exon 20 T790M, after an average of 10–14 months. Osimertinib was firstly developed as a 2nd-line agent for patients with this acquired resistance. Fortunately, further studies showed that osimertinib also had positive effects as a first-line strategy for patients with advanced EGFR-mutant NSCLC, including exon 19 deletion and exon 21 L858R substitution (14,15). Besides, FLAURA trial demonstrated a considerably prolonged OS for patients in the osimertinib group compared to those in the reference group (38.6 vs. 31.8 months; P=0.046) (16). Additional study results from Asian and Chinese populations confirmed the effectiveness and safety of osimertinib as a first-line therapy for patients with advanced EGFR-mutated NSCLC (17-19). Based on the results of clinical trials, osimertinib was approved as a first-line treatment for patients with EGFR-mutant advanced NSCLC by the Food and Drug Administration (FDA) (20).
However, although multiple studies demonstrated the PFS benefits associated with first-line osimertinib compared with first-generation EGFR-TKIs, whether first-line osimertinib produced an OS benefit yielded varying results in different studies (16-22). Furthermore, the sample sizes of current real-world studies were relatively small. Therefore, we conducted a retrospective study using the data extracted from a real-world, multicenter, prospective observational cohort in China to assess the effectiveness and safety of osimertinib as a first-line strategy for patients with EGFR-mutated stage IIIc–IV NSCLC, compared against first-generation EGFR-TKIs. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-577/rc).
Methods
Patients
The CAPTRA-Lung study (NCT03334864), collecting real-world data of patients with advanced or metastatic NSCLC, is a prospective, multicenter, observational study underway throughout China (23). As of October 31, 2022, the CAPTRA-Lung study encompassed the participation of 36 research centers and gathered data from 10,156 patients diagnosed with advanced or metastatic NSCLC. We conducted a retrospective cohort study using the data extracted from database of CAPTRA-Lung study. Patients were eligible for inclusion for our study according to the following standards: (I) with pathologically confirmed stage IIIc–IV NSCLC [according to the 8th tumor-node-metastasis (TNM) staging by the American Joint Committee on Cancer (AJCC)] in the CAPTRA-Lung database between January 1, 2010, and October 31, 2022; (II) having EGFR mutations and receiving either osimertinib or a first-generation EGFR-TKI as their first-line treatment; (III) with complete information regarding diagnosis, first-line treatment, and survival.
Patients were excluded from our study according to the following criteria: (I) with pathologically confirmed small cell lung cancer; (II) with TNM staging earlier than IIIc or receiving a first-generation EGFR-TKI for postoperative adjuvant therapy; (III) receiving a second-generation EGFR-TKI as their first-line treatment; (IV) with incomplete or unknown important clinical information. The observation period for all patients included in our study started from the initiation of EGFR-TKI treatment. Subsequently, all enrolled patients underwent regular follow-up assessments every three months until April 8, 2023, or death or loss to follow-up. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Peking Union Medical College Hospital on 24 December 2022 (Ethics Approval Number: I-22PJ1112) and the requirement for individual consent for this retrospective analysis was waived.
Data collection
Baseline information including gender, age, Eastern Cooperative Oncology Group (ECOG) performance status score, smoking history, family history of tumors, pathology, EGFR mutations, and central nervous system (CNS) metastases was all collected from the CAPTRA-Lung database. A family history of tumor was defined as a self-reported history of cancer in first-degree or second-degree relatives. First-degree relatives included parents, siblings, or children, while second-degree relatives included nieces, nephews, aunts, uncles, or grandparents. In addition, data about the efficacy of EGFR-TKIs (treatment response, PFS, OS) and treatment-related adverse events (AEs) was also gathered.
Evaluation of efficacy and safety
The objective response rate (ORR) and disease control rate (DCR) were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) standard (24). PFS and OS were calculated from the initiation of EGFR-TKIs until tumor progression or death, respectively. The severity of AEs was graded based on the Common Terminology Criteria for Adverse Events (CTCAE) 5.0 (25).
Statistical analysis
To adjust for differences in baseline characteristics between the osimertinib and reference groups, one-to-two propensity score matching (PSM) was performed using nearest neighbor matching (26). Variables that could influence the outcomes of treatment were used to generate a propensity score, including gender, age, ECOG, smoking history, pathology, EGFR mutations, and CNS metastases. Given that many studies have indicated a connection between a family history of malignancies and the prognosis of lung cancer patients, we also integrated “family history of tumor” into our propensity model (27-29). The standardized mean differences (SMD) before and after PSM were calculated to measure balance between groups. To minimize immortal time bias, only patients receiving osimertinib or first-generation EGFR-TKIs for the first time were included in this study and the observation period started from the initiation of EGFR-TKI treatment.
Statistical analysis was carried out with the software SPSS 22.0 (IBM Corp., Armonk, NY, USA) and R software (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria). The χ2 test or Fisher’s exact test were used to compare categorical variables. Kaplan-Meier survival analysis was applied to evaluate mPFS and median overall survival (mOS), and the log-rank test was operated to determine the statistical difference. Cox regression models were carried out to evaluate the factors influencing survival. All statistical tests were 2-tailed and P<0.05 was considered as being statistically significant. Figures were generated using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA) and R software (version 4.1.1, R Foundation for Statistical Computing).
Results
Baseline characteristics
From January 1, 2010 to October 31, 2022, 1,556 patients with EGFR mutant stage IIIc–IV NSCLC were included in the CAPTRA-Lung database, all of whom were Asian population. Among them, 202 patients received first-line osimertinib, and 1,354 patients received first-generation EGFR-TKIs (Figure 1). Before matching, baseline characteristics including gender, age, ECOG performance status, smoking history, family history of tumors, and pathology were similar between two groups (Table 1).
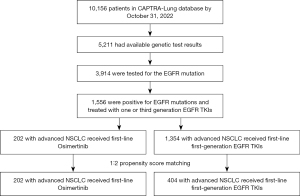
Table 1
| Characteristics | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| First-line osimertinib (n=202), n (%) | First-line first-generation EGFR-TKIs (n=1,354), n (%) | P value | SMD | First-line osimertinib (n=202), n (%) | First-line first-generation EGFR-TKIs (n=404), n (%) | P value | SMD | ||
| Gender | 0.554 | 0.045 | 0.320 | 0.041 | |||||
| Male | 76 (37.6) | 539 (39.8) | 76 (37.6) | 169 (41.8) | |||||
| Female | 126 (62.4) | 815 (60.2) | 126 (62.4) | 235 (58.2) | |||||
| Age (years) | 0.084 | 0.129 | 0.374 | 0.025 | |||||
| ≤60 | 81 (40.1) | 459 (33.9) | 81 (40.1) | 147 (36.4) | |||||
| >60 | 121 (59.9) | 895 (66.1) | 121 (59.9) | 257 (63.6) | |||||
| ECOG performance status | 0.813 | 0.018 | 0.084 | 0.097 | |||||
| 0–1 | 173 (85.6) | 1,151 (85.0) | 173 (85.6) | 365 (90.3) | |||||
| ≥2 | 29 (14.4) | 203 (15.0) | 29 (14.4) | 39 (9.7) | |||||
| Smoking history | 0.326 | 0.075 | 0.305 | 0.011 | |||||
| No | 151 (74.8) | 967 (71.4) | 151 (74.8) | 286 (70.8) | |||||
| Yes | 51 (25.2) | 387 (28.6) | 51 (25.2) | 118 (29.2) | |||||
| Family history of tumor | 0.579 | 0.043 | 0.397 | 0.008 | |||||
| No | 178 (88.1) | 1,174 (86.7) | 178 (88.1) | 365 (90.3) | |||||
| Yes | 24 (11.9) | 180 (13.3) | 24 (11.9) | 39 (9.7) | |||||
| Pathology | 0.607 | 0.040 | 0.312 | 0.064 | |||||
| Adenocarcinoma | 196 (97.0) | 1,304 (96.3) | 196 (97.0) | 385 (95.3) | |||||
| Others† | 6 (3.0) | 50 (3.7) | 6 (3.0) | 19 (4.7) | |||||
| EGFR mutations | <0.001 | 0.440 | 0.188 | 0.025 | |||||
| Exon 19 deletion | 87 (43.1) | 471 (34.8) | 87 (43.1) | 143 (35.4) | |||||
| 21L858R | 80 (39.6) | 474 (35.0) | 80 (39.6) | 180 (44.6) | |||||
| Uncommon mutations | 13 (6.4) | 42 (3.1) | 13 (6.4) | 21 (5.2) | |||||
| Detail unknown | 22 (10.9) | 367 (27.1) | 22 (10.9) | 60 (14.9) | |||||
| CNS metastases | 0.001 | 0.241 | 0.377 | 0.005 | |||||
| No | 138 (68.3) | 1,068 (78.9) | 138 (68.3) | 290 (71.8) | |||||
| Yes | 64 (31.7) | 286 (21.1) | 64 (31.7) | 114 (28.2) | |||||
| Type of first-generation TKIs | |||||||||
| Gefitinib | N/A | 638 (47.1) | N/A | 171 (42.3) | |||||
| Icotinib | N/A | 589 (43.5) | N/A | 188 (46.5) | |||||
| Erlotinib | N/A | 127 (9.4) | N/A | 45 (11.1) | |||||
†, others include unclassified, squamous cell carcinoma and adenosquamous carcinoma. PSM, propensity score matching; SMD, standardized mean differences; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; CNS, central nervous system; TKI, tyrosine kinase inhibitor; N/A, not applicable.
However, there was a significant difference in the distribution of EGFR mutation types between the osimertinib and comparator arms (P<0.001) as noted below. In the osimertinib group, 87 patients (43.1%) had exon 19 deletion, 80 patients (39.6%) had exon 21 L858R point mutation, 13 patients (6.4%) had uncommon mutations (any EGFR mutation other than common mutations), and data regarding specific EGFR mutation types were unknown for 22 patients (10.9%). In the comparator group, the distribution was 471 patients (34.8%), 474 patients (35.0%), 42 patients (3.1%), and 367 patients (27.1%) for exon 19 deletion, exon 21 L858R point mutation, uncommon mutations, and data unknown, respectively. Additionally, 31.7% of patients receiving first-line osimertinib exhibited CNS metastases at baseline, significantly higher than 21.1% in patients who received first-line first-generation EGFR-TKIs (P=0.001).
To adjust for imbalance in EGFR mutations and CNS metastases rates between the osimertinib and comparator groups, a 1:2 PSM was performed. After performing PSM, 202 patients in the osimertinib group and 404 patients in the comparator group were ultimately included in the matched cohorts (Table 1). Among 202 patients in the osimertinib group, 64 individuals (31.7%) presented with baseline CNS metastases, including 29 (14.4%) with stable CNS metastases, 9 (4.5%) with unstable symptomatic CNS metastases, and 26 (12.9%) with an undisclosed status. Among 404 patients in the first-generation EGFR-TKI group, 114 (28.2%) had baseline CNS metastases, including 80 (19.8%) with stable CNS metastases, 17 (4.2%) with unstable symptomatic CNS metastases, and 17 (4.2%) with an unknown status. The distribution of 202 patients in the osimertinib group across the years 2010–2013, 2014–2018, and 2019–2022 was 0 (n=0), 10.0% (n=20), and 90.1% (n=182), respectively. In the first-generation EGFR-TKI group, the distribution of 404 patients was 7.7% (n=31), 69.6% (n=281), and 22.8% (n=92), respectively. Due to economic considerations, some patients with advanced NSCLC carrying EGFR mutations continued to receive first-generation EGFR-TKIs as their initial treatment after osimertinib was approved by the National Medical Products Administration (NMPA) for first-line treatment in 2019. SMD of all variables included in PSM reduced to less than 0.1, demonstrating a good balance between two groups.
Efficacy of osimertinib versus first-generation TKIs
At the cutoff date (April 8, 2023), the median follow-up period among patients in PSM cohort was 20.3 months in the osimertinib arm and 30.0 months in the comparator arm. In the matched cohort, the ORR was 63.4% in the osimertinib arm compared to 48.0% in the comparator arm (P<0.001). The DCR was 95.5% vs. 96.8% in two groups, respectively (P=0.443). None of the patients achieved a complete response in either arm.
Besides, the mPFS was 19.4 months [95% confidence interval (CI): 14.3–24.4] in the osimertinib group and 10.9 months (95% CI: 9.3–12.5) in the comparator group. The hazard ratio (HR) for progression was 0.47 (95% CI: 0.38–0.59), indicating a significantly reduction of risk for disease progression in the osimertinib group (P<0.001) (Figure 2). Furthermore, the osimertinib group exhibited mOS of 40.5 months (95% CI: 27.1–54.0), which was higher than that of 34.3 months (95% CI: 30.6–38.0) in the first-generation EGFR-TKI group (HR 0.76, 95% CI: 0.58–1.00; P=0.045) (Figure 3).


HRs for PFS and OS in subgroups
Subgroup analysis was performed to compare treatment outcomes between two groups among different EGFR mutations. Osimertinib was associated with significantly improved PFS compared with first-generation EGFR-TKIs for patients with either exon 19 deletions or exon 21 L858R substitutions (Figure 4A,4B). Furthermore, exon 19 deletions patients receiving osimertinib at first-line had PFS of 25.5 months (95% CI: 11.3–39.6), longer than 17.6 months (95% CI: 10.1–25.1) for patients with exon 21 L858R substitutions. The median OS for exon 19 deletions patients receiving osimertinib or first-generation EGFR-TKI at first-line was 44.5 months (95% CI: 32.0–57.0) vs. 36.7 (95% CI: 29.9–43.4), respectively (HR 0.85, 95% CI: 0.54–1.35; P=0.497). Besides, the median OS for patients with exon 21 L858R substitutions in two groups was 33.5 months (95% CI: 22.4–44.6) vs. 33.4 (95% CI: 27.7–39.0), respectively (HR 0.75, 95% CI: 0.48–1.18; P=0.214) (Figure 4C,4D).

Additionally, we conducted subgroup analyses based on the presence or absence of baseline CNS metastases. Among patients without baseline CNS metastases, osimertinib was found to prolong PFS (18.5 vs 12.4 months; P<0.001) (Figure S1A). However, there were no significant differences in OS between the two groups (Figure S1B). Patients with baseline CNS metastases in the osimertinib group exhibited significantly longer PFS and OS compared to the comparator group, with mPFS being 21.0 vs. 8.7 months (P<0.001) and mOS being 40.5 vs. 25.8 months (P<0.001), respectively (Figure S1C,S1D).
We stratified patients based on the stability of their CNS metastasis status. Among patients with baseline stable CNS metastases, the osimertinib group showed significantly extended PFS compared to the comparator group, with median values of 25.5 months (95% CI: 3.1–47.9) vs. 8.4 months (95% CI: 6.3–10.5), respectively (HR 0.26, 95% CI: 0.13–0.53; P<0.001) (Figure S2A). OS was also significantly improved in the osimertinib group, with a median OS that was “not reached” vs. 25.8 months (95% CI: 22.3–29.3) in the comparator group (HR 0.03, 95% CI: 0–0.39; P<0.001) (Figure S2B). For patients with baseline unstable symptomatic CNS metastases, the median PFS (“not reached” vs. 14.1 months; P=0.190) and OS (33.5 vs. 23.4 months; P=0.320) were longer in the osimertinib group than that in the first-generation EGFR-TKI group, although without statistical significance (Figure S2C,S2D).
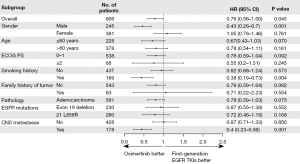
We further performed Cox regression analyses to confirm prognostic factors. Patients receiving osimertinib at first-line demonstrated a longer PFS than those receiving first-generation EGFR-TKIs, regardless of gender, age, ECOG, smoking history, family history of tumor, pathology, EGFR mutations, and CNS metastases (Figure 5). However, only patients who were male, or smokers, or with CNS metastases at baseline exhibited a significantly longer OS when receiving osimertinib at first-line (Figure 6). Besides, results in the subgroup with gefitinib, icotinib, and erlotinib were similar to those for the overall population (Figures S3,S4).

Safety of osimertinib versus first-generation TKIs
The treatment-emergent AEs for osimertinib and first-generation TKIs in matched cohort are summarized in Table 2. AEs of any grade were reported in 36 patients (17.8%) in the osimertinib arm and 64 patients (15.8%) in the comparator arm. Osimertinib resulted in 60 treatment-related adverse reactions, 96.7% of which were categorized as grade 1–2. While first-generation EGFR-TKIs led to 149 treatment-related adverse reactions, 88.6% of which were grade 1–2. Grade 3 AEs were observed in 2 patients (1.0%) and 17 patients (4.2%) in two groups, respectively. No grade 4 AEs and treatment-related deaths were reported in both groups.
Table 2
| Adverse event | Osimertinib (n=202), n (%) | First-generation TKIs (n=404), n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 1 | Grade 2 | Grade 3 | Any grade | Grade 1 | Grade 2 | Grade 3 | ||
| Rash | 25 (12.4) | 22 (10.9) | 3 (1.5) | 0 (0.0) | 116 (28.7) | 89 (22.0) | 25 (6.2) | 2 (0.5) | |
| Oral mucositis | 6 (3.0) | 5 (2.5) | 1 (0.5) | 0 (0.0) | 3 (0.7) | 2 (0.5) | 1 (0.2) | 0 (0.0) | |
| AST/ALT elevation | 4 (2.0) | 2 (1.0) | 0 (0.0) | 2 (1.0) | 55 (13.6) | 29 (7.2) | 18 (4.5) | 8 (2.0) | |
| Anorexia | 3 (1.5) | 3 (1.5) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 1 (0.2) | 1 (0.2) | 0 (0.0) | |
| Paronychia | 2 (1.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |
| Hand-foot syndrome | 2 (1.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |
| Diarrhea | 12 (5.9) | 10 (5.0) | 2 (1.0) | 0 (0.0) | 48 (11.9) | 37 (9.2) | 6 (1.5) | 5 (1.2) | |
| Pruritus | 1 (0.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 6 (1.5) | 6 (1.5) | 0 (0.0) | 0 (0.0) | |
| Fatigue | 1 (0.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 2 (0.5) | 0 (0.0) | 0 (0.0) | |
| QTc prolongation | 1 (0.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hypertension | 1 (0.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Stomatitis | 1 (0.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Leukopenia | 1 (0.5) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 7 (1.7) | 4 (1.0) | 2 (0.5) | 1 (0.2) | |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (2.0) | 7 (1.7) | 1 (0.2) | 0 (0.0) | |
| Hyperbilirubinemia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 1 (0.2) | 1 (0.2) | 0 (0.0) | |
| Anemia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 1 (0.2) | 1 (0.2) | 0 (0.0) | |
| Constipation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 0 (0.0) | 2 (0.5) | 0 (0.0) | |
| Albuminuria | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |
| Pyrexia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |
| Vomiting | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |
| Edema | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |
| Headache | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |
| Dizziness | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |
| Thromboembolism | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |
| Pulmonary interstitial fibrosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.2) | |
| Elevated creatinine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | |
| Low platelets | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | |
TKI, tyrosine kinase inhibitor; AST/ALT, aspartate aminotransferase/alanine aminotransferase.
The most frequent AEs were rash (12.4%), diarrhea (5.9%), and oral mucositis (3%) in the osimertinib arm and rash (28.7%), elevation of aspartate aminotransferase/alanine aminotransferase (AST/ALT; 13.6%), and diarrhea (11.9%) in the comparator arm. Notably, only 1 patient in the comparator arm reported grade 3 pulmonary interstitial fibrosis.
Resistance pattern and follow-up treatment
Until the last follow-up (April 8, 2023), 98 patients (48.5%) in the osimertinib group and 38 patients (9.4%) in the first-generation EGFR-TKI group were still undergoing first-line treatment. Additionally, 22 (10.9%) and 57 patients (14.1%) were dead due to disease progression after first-line treatment within two groups, respectively, and these patients did not have the chance to receive subsequent antineoplastic therapies (Table 3).
Table 3
| Treatment | First-line osimertinib (n=202), n (%) | First-line first generation EGFR-TKIs (n=404), n (%) |
|---|---|---|
| Still receiving first-line therapy | 98 (48.5) | 38 (9.4) |
| No subsequent anticancer therapy (dead) | 22 (10.9) | 57 (14.1) |
| Receiving second-line treatments | 82 (40.6) | 309 (76.5) |
| Detail unknown | 34 (41.5) | 0 |
| Detail known | 48 (58.5) | 309 (100.0) |
| Chemotherapy | 41 (85.4) | 118 (38.2) |
| Immunotherapy | 11 (22.9) | 16 (5.2) |
| Anti-angiogenic therapy | 18 (37.5) | 13 (4.2) |
| Other first- or second-generation EGFR-TKIs | 8 (16.7) | 21 (6.8) |
| Osimertinib | 10 (20.8) | 162 (52.4) |
| MET inhibitors | 1 (2.1) | 0 |
| Receiving third-line treatments | 23 (11.4) | 147 (36.4) |
| Chemotherapy | 15 (65.2) | 81 (59.1) |
| Immunotherapy | 4 (17.4) | 25 (18.2) |
| Anti-angiogenic therapy | 10 (43.5) | 33 (24.1) |
| Other first- or second-generation EGFR-TKIs | 4 (17.4) | 27 (19.7) |
| Osimertinib | 4 (17.4) | 35 (25.5) |
| MET inhibitors | 1 (0.7) | |
| Receiving forth and above-line treatments | 6 (3.0) | 82 (20.3) |
| Chemotherapy | 4 (66.7) | 58 (70.7) |
| Immunotherapy | 1 (16.7) | 21 (25.6) |
| Anti-angiogenic therapy | 1 (16.7) | 45 (54.9) |
| Other first- or second-generation EGFR-TKIs | 1 (16.7) | 28 (34.1) |
| Osimertinib | 0 | 43 (52.4) |
EGFR-TKIs, epidermal growth factor receptor-tyrosine kinase inhibitors; MET, mesenchymal-epithelial transition.
Among 82 patients (40.6%) who received second-line treatment in the osimertinib group, only 17 individuals underwent next-generation sequencing (NGS) genotyping. It is noteworthy that only 1 patient (5.9%) exhibited MET amplification and no patient harbored C797S mutation. Besides, BRAF V600E mutations were observed in 11.8% of the patients.
Information about second-line treatment strategies in the osimertinib group was accessible for 48 out of 82 patients (58.5%). Among 48 patients, 41 patients (85.4%) were administered chemotherapy, 18 patients (37.5%) underwent anti-angiogenic therapy, 11 patients (22.9%) received immunotherapy, 8 patients (16.7%) were prescribed alternative EGFR-TKIs apart from osimertinib, and 1 patient (2.1%) received mesenchymal-epithelial transition (MET) inhibitors. Remarkably, 10 patients (20.8%) continued osimertinib after progression. In these cases, osimertinib was administered in combination with chemotherapy for 6 patients, with anti-angiogenic therapy for 3 patients, and concurrently with both chemotherapy and anti-angiogenic therapy, as well as savolitinib, for one patient. Furthermore, during a median follow-up period of 20.3 months within the osimertinib group, 23 patients (11.4%) received third-line therapy, while 6 patients (3%) underwent fourth-line and subsequent treatments. The median number of lines of therapy was 1 (range, 1–5) (Table 3).
Within 309 patients (76.5%) who underwent second-line treatment in the first-generation EGFR-TKI group, 139 patients (45.0%) were tested positive for the T790M mutation via plasma or tissue-based NGS genotyping. All these individuals subsequently received second-line osimertinib therapy. In addition, 21 patients who were tested negative for the T790M mutation, along with 9 patients whose T790M status remained unknown, also underwent second-line osimertinib treatment. Besides, 118 patients (38.2%) received chemotherapy, 13 patients (4.2%) were administered anti-angiogenic therapy, 16 patients (5.2%) underwent immunotherapy, and 21 patients (6.8%) were treated with alternative EGFR-TKIs. Furthermore, during a median follow-up duration of 30.0 months in the comparator group, 147 patients (36.4%) underwent third-line therapy, and 82 patients (20.3%) received fourth-line and beyond treatments. The median number of therapy lines administered was 2 (range, 1–6).
Discussion
In this multicenter, real-world study in China, we observed that first-line osimertinib had better efficacy and similar safety profiles compared with first-generation EGFR-TKIs for EGFR-mutated stage IIIc–IV NSCLC patients, which was consistent with the findings of the FLAURA study. The mPFS was extended by 8.5 months in the osimertinib arm (19.4 months) compared to the comparator arm (10.9 months), with a 53% reduction of risk for disease progression. The Kaplan-Meier curves for PFS clearly showed a separation into two distinct groups at 5 months and remained separated throughout the follow-up period. Moreover, the mOS was 6.2 months longer in the osimertinib group (40.5 months) compared to the comparator group (34.3 months), with a 24% reduction of risk for death. The Kaplan-Meier curves for OS started very close together but diverged at around 20 months, and the gap between two groups increased with longer follow-up. Notably, the follow-up time in the osimertinib group was shorter than that in the first-generation EGFR-TKIs group, which might have an impact on the OS. Subgroup analysis indicated that the PFS advantage of osimertinib over first-generation EGFR-TKIs was consistent across all subgroups. However, only patients who were male, smokers, or had CNS metastases at baseline exhibited a significantly longer OS in the osimertinib group.
To date, numerous studies have affirmed the PFS advantages associated with first-line osimertinib in contrast to first-generation EGFR-TKIs. However, the question of whether first-line osimertinib confers an OS benefit has diverse findings across various investigations. In the FLAURA trial, first-line osimertinib significantly prolonged OS (38.8 vs. 31.8 months; P=0.046) (16). In the FLAURA China subgroup analysis, the osimertinib group exhibited a trend towards prolonged OS, although it did not reach statistical significance (33.1 vs. 25.7 months; P=0.442) (19). Conversely, in the Japanese subgroup, an opposite trend was observed, with the osimertinib group and the comparator group having OS of 39.9 months vs. “not reached” (P=0.215) (30). Furthermore, OS data from other studies exploring the efficacy of first-line osimertinib remained immature (17,31). To the best of our knowledge, our study stood as the first real-world research to support the significant OS extension achieved by osimertinib as observed in the FLAURA trial, further substantiating the superiority of first-line osimertinib over first-generation EGFR-TKIs.
Brain metastasis is a common complication of advanced NSCLC, which could influence treatment outcomes (32). Previous studies have demonstrated that osimertinib has better activity in the CNS than first- or second-generation EGFR-TKIs (33-36). Our study showed that osimertinib in the first-line treatment substantially improved PFS compared with standard EGFR-TKIs regardless of CNS metastases at baseline, consistent with results in the FLAURA study. Additionally, osimertinib also demonstrated a prolonged OS compared to first-generation EGFR-TKIs in patients with CNS metastasis, with a 60% reduction in the risk of death. These findings suggested that first-line osimertinib was particularly suitable for patients with brain metastases at baseline.
Approximately 90% of patients with EGFR-mutated NSCLC have either an exon 19 deletion or an exon 21 L858R substitution (3,37). Patients with exon 19 deletions have longer PFS compared to those with exon 21 L858R mutations after first-line EGFR-TKIs (38,39). In our study, patients with exon 19 deletions in the matched cohort accounting for 43.9% (230 out of 524) and exon 21 L858R substitutions accounting for 49.6% (260 out of 524) of the detected EGFR mutations. Besides, survival outcomes of patients with exon 19 deletion were better than those with exon 21 L858R substitutions, regardless of receiving osimertinib or first-generation EGFR-TKIs.
Despite its remarkable efficacy, the development of resistance to osimertinib is unavoidable. Mechanisms of resistance can be categorized into two groups: on-target EGFR-dependent mechanisms, such as the C797S mutation, and off-target EGFR-independent mechanisms, including MET amplification and small cell transformation (40-43). Recently, potential treatments targeting specific acquired resistance, such as EGFR antibodies, MET inhibitors, and others have been explored (44-47). However, chemotherapy remains the standard therapy for patients who experience progression after first-line osimertinib. In this real-world study, only 20.7% (17 patients) underwent NGS genotyping after progression to first-line osimertinib. The prevalence of MET amplification or EGFR C797S was 5.9% and 0, respectively, lower than that in other researches (43). Most patients in the osimertinib group received chemotherapy as second-line treatment. Moreover, about 20% (10 patients) continued osimertinib after progression.
In EGFR-mutant advanced NSCLC patients, disease progression often occurs after a median of 10–14 months on first-generation EGFR-TKIs (5-9), with approximately half of these patients developing acquired resistance due to the T790M mutation (48,49). The AURA3 trial demonstrated that osimertinib significantly extended PFS of patients acquiring T790M mutation after first-line EGFR-TKIs compared with platinum-pemetrexed chemotherapy (50,51). In our study, among 404 patients in the first-generation EGFR-TKI arm, 309 experienced disease progression, and about half of them acquired the secondary EGFR T790M mutation and received second-line osimertinib.
However, it is essential to emphasize that within 404 patients initially treated with first-generation EGFR-TKIs, 57 patients (14.1%) encountered significant disease deterioration during first-line treatment, resulting in the loss of opportunities for subsequent-line therapies. Similarly, 30% of patients receiving first-line first-generation EGFR-TKIs in the FLAURA trial did not proceed to receive any subsequent therapy after first line of treatment. This underscores that the first-line treatment represented their sole therapeutic opportunity. Therefore, it’s recommended to consider the utilization of osimertinib in the first-line setting (the best first).
Both osimertinib and first-generation EGFR-TKIs showed tolerable safety profiles in our study. Most AEs were mild, and there were no treatment-related deaths. Notably, no drug-associated pneumonitis was reported in the osimertinib group, though a real-world study from Japan reported a higher incidence of drug-associated pneumonitis (18% of patients with all grades and 4.6% with grade 3 or above) (52). It is important to consider that the occurrence of AEs in our study was lower than that in clinical trials, as AE reporting primarily relied on medical records from various medical centers due to the retrospective nature of this multicenter real-world study, which could potentially result in underreporting or underestimation of AEs. Additionally, patients experiencing severe AEs might seek medical attention at nearby hospitals and subsequently be lost to follow-up. These factors could contribute to a lower incidence of high-grade AEs and severe pneumonia in this study compared to previous researches. Although the occurrence of high-grade adverse reactions is notably low in this study, it remains crucial to maintain careful monitoring during the course of treatment.
There are several limitations in our study. Firstly, due to the real-world nature of the study, the follow-up duration in the osimertinib group was shorter than that of the first-generation EGFR-TKIs, as osimertinib was approved by the NMPA for first-line treatment later than first-generation EGFR-TKIs. This difference in follow-up duration might have influenced the OS outcomes. Secondly, our study did not include patients receiving second-generation EGFR-TKIs at first line due to the limited number of patients. Thirdly, we did not analyze the relationship between PDL1 expression levels and the efficacy of EGFR-TKIs because data on PDL1 expression in the CAPTRA-Lung database were severely lacking.
Conclusions
In the real-world setting, osimertinib demonstrated significantly longer PFS and OS and similar safety profile compared with first-generation EGFR-TKIs as a first-line treatment for patients with advanced EGFR-mutated NSCLC, indicating that osimertinib was an effective and well-tolerated treatment in real world populations. Studies with more patients are needed to confirm these results across diverse geographies and ethnicities.
Acknowledgments
We acknowledge and appreciate the contributions of Fanghua Pan, Yi Wen, He Tong, and Wanying Pei (Medbanks Network Technology Co. Ltd., Beijing, China). Their assistance in managing the data and performing the PSM was invaluable to our study. We also appreciate the great support from Dr. Antonio Passaro (European Institute of Oncology IRCCS, Milan, Italy) in improving the quality of this paper.
Funding: This study is supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-577/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-577/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-577/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-577/coif). A.R. received stock options by IQVIA Holdings Inc. H.H. has received consulting fees from Janssen and Astrazeneca and honoraria from Janssen, Astrazeneca, Neogenomics, and Foundation Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Peking Union Medical College Hospital on 24 December 2022 (Ethics Approval Number: I-22PJ1112) and the requirement for individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Skov BG, Høgdall E, Clementsen P, et al. The prevalence of EGFR mutations in non-small cell lung cancer in an unselected Caucasian population. APMIS 2015;123:108-15. [Crossref] [PubMed]
- Castellanos E, Feld E, Horn L. Driven by Mutations: The Predictive Value of Mutation Subtype in EGFR-Mutated Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:612-23. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol 2017;28:2443-50. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Yang JJ, Zhou Q, Yan HH, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer 2017;116:568-74. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:339-57. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Remon J, Steuer CE, Ramalingam SS, et al. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann Oncol 2018;29:i20-7. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Cho BC, Chewaskulyong B, Lee KH, et al. Osimertinib versus Standard of Care EGFR TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC: FLAURA Asian Subset. J Thorac Oncol 2019;14:99-106. [Crossref] [PubMed]
- Ohe Y, Imamura F, Nogami N, et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol 2019;49:29-36. [Crossref] [PubMed]
- Cheng Y, He Y, Li W, et al. Osimertinib Versus Comparator EGFR TKI as First-Line Treatment for EGFR-Mutated Advanced NSCLC: FLAURA China, A Randomized Study. Target Oncol 2021;16:165-76. [Crossref] [PubMed]
- Yang F, Zhang W, Shang X, et al. Comparison of the efficacy and safety of first-line treatments based on clinicopathological characteristics for patients with advanced epidermal growth factor receptor mutated non-small-cell lung cancer: A systematic review and network meta-analysis. Crit Rev Oncol Hematol 2022;177:103760. [Crossref] [PubMed]
- Lamb YN. Osimertinib: A Review in Previously Untreated, EGFR Mutation-Positive, Advanced NSCLC. Target Oncol 2021;16:687-95. [Crossref] [PubMed]
- Lorenzi M, Ferro A, Cecere F, et al. First-Line Osimertinib in Patients with EGFR-Mutant Advanced Non-Small Cell Lung Cancer: Outcome and Safety in the Real World: FLOWER Study. Oncologist 2022;27:87-e115. [Crossref] [PubMed]
- Xu Y, Zhang L, Fang J, et al. Establishment of a prospective multicenter cohort for advanced non-small cell lung cancer in China (CAPTRA-Lung study). Thorac Cancer 2018;9:1795-800. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- Liang J, Hu Z, Zhan C, et al. Using Propensity Score Matching to Balance the Baseline Characteristics. J Thorac Oncol 2021;16:e45-6. [Crossref] [PubMed]
- Ganti AK, Loberiza FR Jr, Kessinger A. Association of positive family history with survival of patients with lung cancer. Lung Cancer 2009;63:136-9. [Crossref] [PubMed]
- Li N, Shao K, Chen Z, et al. The impact of positive cancer family history on the clinical features and outcome of patients with non-small cell lung cancer. Fam Cancer 2011;10:331-6. [Crossref] [PubMed]
- Lin H, Huang YS, Yan HH, et al. A family history of cancer and lung cancer risk in never-smokers: A clinic-based case-control study. Lung Cancer 2015;89:94-8. [Crossref] [PubMed]
- Nogami N, Ramalingam SS, Imamura F, et al. Osimertinib as first-line therapy for EGFRm advanced NSCLC (FLAURA): final OS in Japanese subset. In: Proceedings of the 60th Annual Meeting of the Japan Lung Cancer Society; 2019.
- Zhou J, Zhou J, Zheng J, et al. Real-World Outcomes of First-Line Osimertinib for EGFR Mutated Advanced NSCLC Patients in China: Interim Analysis of FLOURISH Study. 2022ESMO 1123P.
- Wang B, Guo H, Xu H, et al. Research Progress and Challenges in the Treatment of Central Nervous System Metastasis of Non-Small Cell Lung Cancer. Cells 2021;10:2620. [Crossref] [PubMed]
- Zhao Y, Li S, Yang X, et al. Overall survival benefit of osimertinib and clinical value of upfront cranial local therapy in untreated EGFR-mutant nonsmall cell lung cancer with brain metastasis. Int J Cancer 2022;150:1318-28. [Crossref] [PubMed]
- Xie L, Nagpal S, Wakelee HA, et al. Osimertinib for EGFR-Mutant Lung Cancer with Brain Metastases: Results from a Single-Center Retrospective Study. Oncologist 2019;24:836-43. [Crossref] [PubMed]
- Colclough N, Chen K, Johnström P, et al. Preclinical Comparison of the Blood-brain barrier Permeability of Osimertinib with Other EGFR TKIs. Clin Cancer Res 2021;27:189-201. [Crossref] [PubMed]
- Huang YH, Hsu KH, Tseng JS, et al. The Difference in Clinical Outcomes Between Osimertinib and Afatinib for First-Line Treatment in Patients with Advanced and Recurrent EGFR-Mutant Non-Small Cell Lung Cancer in Taiwan. Target Oncol 2022;17:295-306. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Zhou J, Ben S. Comparison of therapeutic effects of EGFR-tyrosine kinase inhibitors on 19Del and L858R mutations in advanced lung adenocarcinoma and effect on cellular immune function. Thorac Cancer 2018;9:228-33. [Crossref] [PubMed]
- Zhang Y, Sheng J, Kang S, et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS One 2014;9:e107161. [Crossref] [PubMed]
- Planchard D, Loriot Y, André F, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol 2015;26:2073-8. [Crossref] [PubMed]
- Shi P, Oh YT, Zhang G, et al. Met gene amplification and protein hyperactivation is a mechanism of resistance to both first and third generation EGFR inhibitors in lung cancer treatment. Cancer Lett 2016;380:494-504. [Crossref] [PubMed]
- Bertoli E, De Carlo E, Del Conte A, et al. Acquired Resistance to Osimertinib in EGFR-Mutated Non-Small Cell Lung Cancer: How Do We Overcome It? Int J Mol Sci 2022;23:6936. [Crossref] [PubMed]
- Chmielecki J, Gray JE, Cheng Y, et al. Candidate mechanisms of acquired resistance to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer. Nat Commun 2023;14:1070. [Crossref] [PubMed]
- Di Noia V, D'Aveni A, D'Argento E, et al. Treating disease progression with osimertinib in EGFR-mutated non-small-cell lung cancer: novel targeted agents and combination strategies. ESMO Open 2021;6:100280. [Crossref] [PubMed]
- Johnson M, Garassino MC, Mok T, et al. Treatment strategies and outcomes for patients with EGFR-mutant non-small cell lung cancer resistant to EGFR tyrosine kinase inhibitors: Focus on novel therapies. Lung Cancer 2022;170:41-51. [Crossref] [PubMed]
- Tian X, Wang R, Gu T, et al. Costunolide is a dual inhibitor of MEK1 and AKT1/2 that overcomes osimertinib resistance in lung cancer. Mol Cancer 2022;21:193. [Crossref] [PubMed]
- Hartmaier RJ, Markovets AA, Ahn MJ, et al. Osimertinib + Savolitinib to Overcome Acquired MET-Mediated Resistance in Epidermal Growth Factor Receptor-Mutated, MET-Amplified Non-Small Cell Lung Cancer: TATTON. Cancer Discov 2023;13:98-113. [Crossref] [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [Crossref] [PubMed]
- Stewart EL, Tan SZ, Liu G, et al. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl Lung Cancer Res 2015;4:67-81. [Crossref] [PubMed]
- Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Papadimitrakopoulou VA, Mok TS, Han JY, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol 2020;31:1536-44. [Crossref] [PubMed]
- Sato Y, Sumikawa H, Shibaki R, et al. Drug-Related Pneumonitis Induced by Osimertinib as First-Line Treatment for Epidermal Growth Factor Receptor Mutation-Positive Non-Small Cell Lung Cancer: A Real-World Setting. Chest 2022;162:1188-98. [Crossref] [PubMed]


