
Clinical outcomes of KRAS-mutant non-small cell lung cancer under untargeted therapeutic regimes in the real world: a retrospective observational study
Highlight box
Key findings
• G12C performed as the primary isotype of Kirsten rat sarcoma viral oncogene homolog (KRAS)-mutant non-small-cell lung cancers (NSCLCs).
• Immune checkpoint inhibitors performed seemingly beneficial to chemotherapy and anti-angiogenesis as the first-line intervention in this restricted population.
• Therapeutic drugs and lines barely correlated to long-term outcomes in KRAS-mutant isotypes of NSCLC patients.
What is known and what is new?
• KRAS mutation seemingly suffered less effective therapeutic schedules in the absence of widely-accepted targeted drugs compared with other mutation types in NSCLC.
• Existing therapeutic regimes drive no long-term benefits for KRAS-mutant NSCLCs regardless of isotypes, which needed to be targeted as a priority.
What is the implication, and what should change now?
• KRAS-mutant NSCLC may benefit from accurate genetic identification and targeted strategies in an extending population, setting other potential regimes as the secondary alternatives.
Introduction
Lung cancer is the second most common cancer worldwide and carries the highest mortality rate (1). Accounting for 85% of lung cancer, the majority of non-small cell lung cancer (NSCLC) patients feature local or advanced stage at the time of diagnosis, especially a subset named adenocarcinoma. Genetically, about 25% of lung adenocarcinoma patients carry Kirsten rat sarcoma viral oncogene homolog (KRAS) gene mutation, and present shorter survival than those with KRAS wildtype, which is related to the poor prognosis (2). Isotypes of KRAS mutations varies with the most common a single guanine-to-thymine substitution resulting in a glycine-to-cysteine substitution (KRAS G12C), which occurs in approximately 13% of NSCLC (3). Fortunately, tyrosine-kinase inhibitors (TKI), including sotorasib (4) and adagrasib (5) have been developed for this type of mutation. However, personal financial burden and lack of sufficient evidences in KRAS isotypes weakened their clinical utilization, which still needed further attention to alternatives for KRAS-mutant advanced NSCLC besides targeted drugs instead.
Of note, optimal strategies for KRAS mutant NSCLC seemingly failed to reach a consensus before the emergency of specific targeted therapy. It has been highly appreciated that chemotherapy performs as a fundamental strategy in combination with other interventional approaches or just as a single agent. Another study has reported that immunotherapy seems to be beneficial to KRAS patients (6), while the results of increasing real-world clinical observation studies showed no difference between immune combined chemotherapy and chemotherapy alone (7,8), leading to an uncertainty whether this benefit was different from the overall population of NSCLC patients without gene mutation.
Considering these confusions, we enrolled KRAS-mutant advanced NSCLC patients in our own center, and retrospectively analyzed their corresponding therapeutic regimes and long-term outcomes, in order to explicit the alternatives to extend their life expectancy in the absence of targeted options. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-449/rc).
Methods
Study cohort enrollment
We retrospectively screened out advanced lung cancer in-patients who visited at the Department of Pulmonary and Critical Care Medicine, Xijing Hospital from November 2018 and December 2020. All selected patients have undergone pathological and genetic examinations to confirm the presence of KRAS mutation after the suspected malignant lesions according to computer tomographic scanning. Exclusive criterion was shown in Figure 1. The clinical data of the biological features of participants were collected, including age, sex, smoking status, clinical stage, Eastern Cooperative Oncology Group (ECOG) performance status at diagnosis, histology and molecular status, programmed death ligand 1 (PD-L1) TPS score (<1%, 1–49%, ≥50%), presence of brain metastases (BMS) at diagnosis and at any time following initial diagnosis, systemic therapy including start and stop dates, date of diagnosis of metastatic or recurrent disease, date of death, and date of last follow-up. The first line of therapy was defined as the initiation of systemic therapy drug. Several treatment-related characteristics were also collected, including the starting time of each first-line treatment, the time of each systemic evaluation of progress, the specific organ of progress, and the drug combination of different treatment regimens. End points were confirmed at the time of death. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of the Fourth Military Medical University (No. KY20232309-C-1) and conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Detection of KRAS mutations
All patients were routinely undergoing lung cancer tissue gene detection by real-time quantitative PCR using the AmoyDx Multi-Gene Mutations Detection Kit. For this analysis, NSCLC patients who had available KRAS mutation test results and did not have an EGFR, ALK, or ROS1 gene aberration were included.
Procedures
At the time of each patient’s initial diagnosis and treatment, we analyzed the initial diagnosis, the stage of the tumor, the site of metastasis, and each first-line treatment regimen. At each point of systemic progression, the time of progression in tissues and organs were assessed. Changes in treatment were also assessed, until the time of last follow up or the patient died.
Statistical analyses
Data are presented as means ± standard deviation (SD) for normally distributed data and as medians with interquartile ranges in parentheses for skewed data. Kaplan-Meier curves were used for univariate survival analyses. The Cox proportional hazards model was used to complete univariate and multivariate survival analyses with the hazard ratio (HR) and corresponding 95% confidence internal. Progression-free survival (PFS) was defined as the time from the date of initial diagnosis or treatment to the date of systemic progression or censored at the date of the last follow-up. Statistical analysis of the data was performed using SPSS version 21.0 (SPSS Inc., IBM Corp., Chicago, IL, USA), and two-sided P values <0.05 were statistically significant.
Results
Patient enrollment and clinical characteristics
Between November 2018 and December 2020, a total of 638 patients with advanced NSCLC searched for medical consultation in our department. After rigorous screening based on genetic test, 69 (10.8%) of them were diagnosed bearing KRAS mutation, 66 of which were enrolled into this study eventually (Figure 1). Detailed information of NSCLC patients with KRAS mutation was shown in Table 1. Of the 66 NSCLC with KRAS mutation patients, 18 were female (27.3%) and 48 were male (72.7%). The median age of all patients was 62 years old, with 40.9% <60 years old and 59.1% ≥60 years old. As for clinical characteristics, 87.9% of patients were classified as stage IV, and 58 cases were categorized into adenocarcinoma according to histological examinations, accounting for 87.9%. The expression level of PD-L1 was detected in 22 of 66 patients, excluding 44 unknowns, of which 8 cases (36.4%) were <1%, 6 cases (27.3%) were 1–49%, and 8 cases (36.4%) were ≥50%. BMS were seen in 12 cases, accounting for 18.2%.
Table 1
| Factors | Number (%) or median [range] |
|---|---|
| Gender | |
| Female | 18 (27.3) |
| Male | 48 (72.7) |
| Age, years | 62 [32–91] |
| <60 | 27 (40.9) |
| ≥60 | 39 (59.1) |
| Smoking history | |
| Yes | 45 (68.2) |
| No | 21 (31.8) |
| Stage | |
| IIIB | 8 (12.1) |
| IV | 58 (87.9) |
| Performance score | |
| 1 | 57 (86.4) |
| 2 | 5 (7.6) |
| Unknown | 4 (6.1) |
| Histology | |
| Adenocarcinoma | 58 (87.9) |
| Non-adenocarcinoma | 8 (12.1) |
| PD-L1 expression | |
| <1% | 8 (12.1) |
| 1−49% | 6 (9.1) |
| ≥50% | 8 (12.1) |
| Unknown | 44 (66.7) |
| Brain metastasis | |
| Yes | 12 (18.2) |
| No | 54 (81.8) |
KRAS, Kirsten rat sarcoma viral oncogene homolog; PD-L1, programmed death ligand 1.
G12C performed as the primary isotype of KRAS mutation in NSCLC
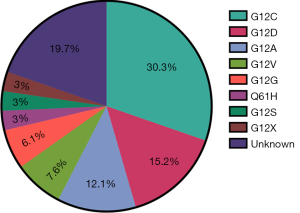
Previous study (9) reported that diverse isotypes of KRAS-mutation exist, which may contribute to various molecular biological characteristics, and in turn, distinctive clinical outcomes to the same treatment. Thus, we analyzed the detectable isotypes of KRAS-mutation at different sites among enrolled group, and further compared their corresponding incidence proportionally. Consequently, we found that the most common KRAS mutation type was G12C (30.3%), followed by G12D (15.2%), and the remainder comprising other mutation types including G12A (12.1%), G12V (7.6%), G12G (6.1%), G12X (3%), G12S (3%), and Q61H (3%) (Figure 2). These findings were in line with that from other studies, illustrating that G12C performed as the primary isotype and there was also a necessity to execute precise molecular diagnosis in KRAS-mutant NSCLC before initial treatment.
Therapeutic drugs and lines barely correlated to long-term outcomes in KRAS-mutant isotypes of NSCLC patients
Due to the lack of targeted therapy, it seemed a priority to select appropriate plans to KRAS-mutant NSCLC, aiming to improve curable efficacy and in turn extend life expectancy to some extent. Thus, we collected the existing therapeutic schemes to KRAS mutation without specific targeted agents, and further illustrated several optimal approaches to this special mutation in NSCLC. Generally speaking (Table 2), in first-line chemotherapy, 8 patients (12.1%) received paclitaxel, 48 patients (72.7%) received pemetrexed, 3 patients (4.5%) received gemcitabine, and 7 patients (10.6%) received no treatment. In first-line treatment, 28 patients (42.4%) received combined antiangiogenic therapy (bevacizumab or anlotinib) and 10 patients (15.2%) received combined immune checkpoint inhibitor (pembrolizumab, nivolumab, tislelizumab, camrelizumab or sintilimab), while 37 patients received second-line treatment, and 9 received second-line treatment combined with anti-angiogenic drugs and immune checkpoint inhibitors. Third-line treatment was received by 17 patients, while 8 patients received fourth-line treatment and three patients received fifth-line treatment. Among the 28 patients with posterior line therapy, 8 (28.6%) had chemotherapy alone, 5 (17.9%) had anti-angiogenic therapy alone, 5 (17.9%) had chemotherapy combined with anti-angiogenic therapy, 2 (7.1%) had chemotherapy combined with immunotherapy, and 2 (7.1%) received anti-angiogenic intervention combined with immunotherapy. TKI was used in 2 cases (7.1%), and 1 patient (3.6%) had chemotherapy combined with TKI. These results partially indicated that it failed to reach a consensus to KRAS-mutant NSCLC with the absence of targeted drugs.
Table 2
| Factors | Number (%) |
|---|---|
| 1st-line chemotherapy | |
| Taxanes-based | 8 (12.1) |
| Pemetrexed-based | 48 (72.7) |
| Gemcitabine-based | 3 (4.5) |
| No | 7 (10.6) |
| 1st-line angiogenesis inhibitors | |
| Yes | 28 (42.4) |
| No | 38 (57.6) |
| 1st-line immune therapy | |
| Yes | 10 (15.2) |
| No | 56 (84.8) |
| 2nd-line therapy | |
| Yes | 37 (56.1) |
| No | 27 (40.9) |
| Unknown | 2 (3.0) |
| 2nd-line therapy | |
| Taxanes-based | 20 (30.3) |
| Pemetrexed-based | 8 (12.1) |
| Gemcitabine-based | 3 (4.5) |
| Other | 6 (9.1) |
| No | 29 (43.9) |
| 2nd-line angiogenesis inhibitors | |
| Yes | 9 (13.6) |
| No | 28 (42.4) |
| 2nd-line immune therapy | |
| Yes | 9 (13.6) |
| No | 28 (42.4) |
| 3rd-line therapy | |
| Yes | 17 (25.8) |
| No | 49 (74.2) |
| 4th-line therapy | |
| Yes | 8 (12.1) |
| No | 58 (87.9) |
| 5th-line therapy | |
| Yes | 3 (4.5) |
| No | 63 (95.5) |
| Posterior line therapy | 28 (100.0) |
| Chemotherapy | 8 (28.6) |
| Angiogenesis inhibitors | 5 (17.9) |
| Chemotherapy + angiogenesis inhibitors | 5 (17.9) |
| Chemotherapy + immune therapy | 2 (7.1) |
| Angiogenesis inhibitors + immune therapy | 5 (17.9) |
| TKI | 2 (7.1) |
| Chemotherapy + TKI | 1 (3.6) |
Posterior line therapy: 3rd-line, 4th-line and 5th-line therapy. KRAS, Kirsten rat sarcoma viral oncogene homolog; TKI, tyrosine-kinase inhibitor.
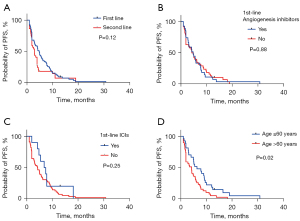
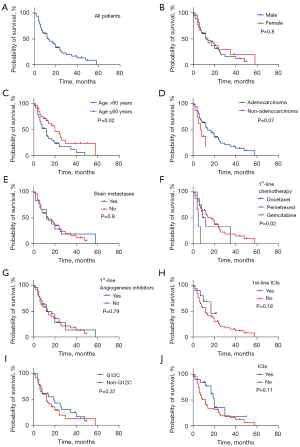
Another, we further explored the clinical outcomes of KRAS-mutant NSCLC with different therapeutic regimes. Consequently, the median PFS (mPFS) reached 4.8 months for first-line treatment and 3 months for second-line treatment, respectively (Figure 3A). The mPFS of first-line combined immunotherapy was 7.1 months and that of first-line combined antiangiogenesis therapy was 4.8 months (Figure 3B,3C). Additionally, the first-line mPFS was 6.3 months in patients ≤60 years old and 4.2 months in patients >60 years old (P=0.02) (Figure 3D). As for overall survival (OS), the median OS (mOS) of all NSCLC patients with KRAS mutations was 14 months (Figure 4A). In subgroup analysis, gender-associated orientation was not the dominant factor in KRAS-mutant NSCLC (Figure 4B), but age under 60 seemed to an evaluation factor for poor prognosis in this cohort (Figure 4C). It still deserved attention that long-term outcomes of KRAS-mutant NSCLC barely correlated with pathological types with 14 months for adenocarcinoma and 7.5 months for non-adenocarcinoma (P=0.07) (Figure 4D), similar to that within BMS subgroup (mOS, with or without BMS for 12 and 14 months, respectively, P=0.8) (Figure 4E). Next, we further evaluated that whether the long-term survival of KRAS-mutant NSCLC could be affected by potential first-line therapies. What exceeded expectations was that the first-line utilization of anti-angiogenesis, chemotherapeutic drugs, and even immune checkpoint inhibitors failed to benefit sufferers totally (Figure 4F-4H), no matter which isotypes of KRAS were characterized or whether ICIs were performed (Figure 4I,4J). These results showed that taking chemotherapy as a priority in first-line treatment mattered most in benefiting KRAS-mutant NSCLC to a great extent, while other regimes failed to be advantageous of angiogenesis and immune checkpoint inhibitors instead.
Discussion
The KRAS gene is an important driving gene in the occurrence and development of NSCLC, and its mutation rate is up to 20–30% in Western populations and 10–15% in Asian populations (10,11). Similar to the results of studies mentioned above, the incidence of KRAS gene mutation in NSCLC patients in this study was 10.8%, with a median age of 62 years, which mainly occurred in lung adenocarcinoma patients. Smokers accounted for a relatively high proportion, and 18.2% of patients had BMS. However, different from the results of individual studies, the proportion of male NSCLC patients with KRAS mutation in this study was higher than that of females (8). Mechanically, mutations at sites 12, 13, or 61 of the KRAS gene lead to amino acid substitution, mainly occurring at codon 12 and 13 of exon 2, among which G12C point mutations are the most common, followed by G12V, G12D, and G12A point mutations (12-14), being in accordance with that in this study. Consecutive activated KRAS and switched on the downstream RAS signaling cascade, which may further interact with multiple effectors including mitogen-activated protein kinase, phosphoinositol 3-kinase, signal transduction, and transcription cascade activators that lose their normal function of regulating the cell cycle, leading to the development of tumors (15). In practice, KRAS-mutant NSCLCs have shorter OS and PFS in first-line chemo- or immune-therapies than those with KRAS wild-type (13,16), which was in accordance with our results. Due the limitations of detective approaches and restricted enrolled population, the long-term benefiting impacts from KRAS co-mutation could not be discussed in this study, which still deserved additional attentions.
Immunotherapy, which was identified to get great achievements in multiple cancer types, may facilitate the long-term survival within KRAS-mutant NSCLC. To fill the gap in the application of ICI therapy in NSCLC patients with positive KRAS mutations, we retrospectively analyzed the OS rate within those diagnosed in this cohort, indicating that the positive expression rate of PD-L1 followed by KRAS mutation accounted for 63.6%, which may be attributed to KRAS mutants associated neoantigens that may trigger an anti-tumor immune response (17). Tumor cells inclined to compensate for high immune visibility through abnormal activation of immune checkpoints to offset the cytotoxic effect of an early immune response (18). Correspondingly, immune checkpoint inhibitors seemed more beneficial for NSCLS patients with KRAS mutations compared with those received platinum-based chemotherapy in clinic.
Up to now, large prospective clinical trials have been rarely conducted to investigate the efficacy of PD-1/PD-L1 inhibitors in patients with advanced KRAS-mutant NSCLCs. In a retrospective study of immunotherapy for advanced NSCLC, patients with KRAS mutations who received PD-1 inhibitors showed significant benefits in OS and PFS compared with wild-type patients (6). A real-world study also showed that the clinical outcome of the first-line ICIs was similar with that in advanced NSCLC, regardless of KRAS alteration (19). Among the KRAS-mutated NSCLC patients, G12C-positive tumors showed increased immunogenicity and potential sensitivity to ICIs (20). In addition, a subgroup analysis of the KEYNOTE 042 study indicated that compared with KRAS wild-type patients, pembrolizumab not only resulted in a higher ORR in patients with KRAS mutations (56.6% vs. 26.1%), but also significantly reduced the risk of tumor-oriented death (21). A meta-analysis of anti-PD-(L)1 for advanced KRAS-mutant NSCLC showed that anti-PD-(L)1 combined with or without chemotherapy to advanced KRAS-mutant NSCLC appeared to obtain longer OS and PFS than chemotherapy alone, with greater OS benefit than that with wild-type ones (22). However, a real-world retrospective study analysis of first-line treatment of KRAS mutations with pembrolizumab monotherapy showed similar survival rates to those of patients with KRAS wild-type stage IV lung adenocarcinoma, suggesting KRAS had no prognostic value with respect to treatment with pembrolizumab (8).
Currently, it is unclear whether immunotherapy combined with chemotherapy can significantly benefit patients with KRAS-mutated NSCLC. A retrospective study involving 1,127 patients with advanced non-squamous NSCLC who were strongly positive for PD-L1 showed that in KRAS wild-type patients, the mOS of immunotherapy combined with chemotherapy tended to lengthen long-term outcomes compared with single immunotherapeutic agent. In KRAS-mutant patients, there was no significant difference in mOS between immunotherapy combined with chemotherapy and immunotherapy (23). Although most studies show patients with KRAS mutations may be an effective treatment group for PD-1/PD-L1 inhibitors, not all patients with KRAS mutations can benefit from them, and further discussion is needed to clarify the reasons. One study confirmed KRAS-G12D point mutation as a primary resistance factor to anti-PD-1/PD-L1 immunotherapy in patients with KRAS-mutated NSCLC. The KRAS-G12D mutation inhibited PD-L1 level through the P70S6K/PI3K/AKT axis and decreased CXCL10/CXCL11 levels by down-regulating high mobility histone A2 levels (24). In addition, a clinical trial showed adagrasib had clinical efficacy in patients previously treated with KRAS-G12C mutant NSCLC in the absence of new safety signals (25). Therefore, the development of targeted drugs for KRAS mutation will bring new hope to patients with KRAS mutant NSCLC (26).
There still exist several unavoidable limitations in this study. Firstly, as a single center retrospective study, restricted number of enrollment was less sufficient to illustrate the long-term survival of KRAS-mutant NSCLC to diverse therapeutic plans, which no doubt may weaken the conclusions. In addition, due to the interferences from subjective judgement, the selection of treatment plans tended to be biased, inevitably leading to potential selectivity bias in light of implementations in detailed groupings. Finally, the prevalence of individual variances and tumor heterogeneity also made it susceptible to prognostic disparity, which still required additional large-scale analysis and thorough subtype grouping.
Conclusions
In conclusion, this study analyzed the clinical outcomes of NSCLC patients with KRAS mutation under untargeted therapeutic regimes in the real world, indicating that there was no significant difference in the long-term survival of them, no matter which treatment schemes were performed. Additionally, a large group of people with KRAS-mutant NSCLC benefit from accurate genetic identification and organized treatment strategies, which underscores the need for KRAS-targeted medications.
Acknowledgments
The authors appreciate the great support from Dr. Nicola Normanno (Istituto Nazionale Tumori-IRCCS ‘Fondazione G. Pascale’, Italy), Dr. Satoshi Watanabe (Niigata University Graduate School of Medical and Dental Sciences, Japan), Dr. Kenji Nakahama (Osaka City University Graduate School of Medicine, Japan), Dr. Yuji Uehara (Komagome Hospital, Japan) and Dr. Kozo Kuribayashi (Hyogo Medical University, Japan) in improving the quality of this paper.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-449/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-449/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-449/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-449/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of the Fourth Military Medical University (No. KY20232309-C-1) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of lung cancer. Contemp Oncol (Pozn) 2021;25:45-52. [Crossref] [PubMed]
- Eklund EA, Wiel C, Fagman H, et al. KRAS Mutations Impact Clinical Outcome in Metastatic Non-Small Cell Lung Cancer. Cancers (Basel) 2022;14:2063. [Crossref] [PubMed]
- Nadal E, Beer DG, Ramnath N. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol 2015;10:e9-10. [Crossref] [PubMed]
- Sidaway P. Sotorasib effective in KRAS-mutant NSCLC. Nat Rev Clin Oncol 2021;18:470. [Crossref] [PubMed]
- Koga T, Suda K, Fujino T, et al. KRAS Secondary Mutations That Confer Acquired Resistance to KRAS G12C Inhibitors, Sotorasib and Adagrasib, and Overcoming Strategies: Insights From In Vitro Experiments. J Thorac Oncol 2021;16:1321-32. [Crossref] [PubMed]
- Cinausero M, Laprovitera N, De Maglio G, et al. KRAS and ERBB-family genetic alterations affect response to PD-1 inhibitors in metastatic nonsquamous NSCLC. Ther Adv Med Oncol 2019;11:1758835919885540. [Crossref] [PubMed]
- Dudnik E, Moskovitz M, Rottenberg Y, et al. Pembrolizumab as a monotherapy or in combination with platinum-based chemotherapy in advanced non-small cell lung cancer with PD-L1 tumor proportion score (TPS) ≥50%: real-world data. Oncoimmunology 2021;10:1865653. [Crossref] [PubMed]
- Noordhof AL, Damhuis RAM, Hendriks LEL, et al. Prognostic impact of KRAS mutation status for patients with stage IV adenocarcinoma of the lung treated with first-line pembrolizumab monotherapy. Lung Cancer 2021;155:163-9. [Crossref] [PubMed]
- Aredo JV, Padda SK, Kunder CA, et al. Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung Cancer. 2019;133:144-50. [Crossref] [PubMed]
- El Osta B, Behera M, Kim S, et al. Characteristics and Outcomes of Patients With Metastatic KRAS-Mutant Lung Adenocarcinomas: The Lung Cancer Mutation Consortium Experience. J Thorac Oncol 2019;14:876-89. [Crossref] [PubMed]
- Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 2013;24:2371-6. [Crossref] [PubMed]
- Wood K, Hensing T, Malik R, et al. Prognostic and Predictive Value in KRAS in Non-Small-Cell Lung Cancer: A Review. JAMA Oncol 2016;2:805-12. [Crossref] [PubMed]
- Jia Y, Jiang T, Li X, et al. Characterization of distinct types of KRAS mutation and its impact on first-line platinum-based chemotherapy in Chinese patients with advanced non-small cell lung cancer. Oncol Lett 2017;14:6525-32. [Crossref] [PubMed]
- Santarpia M, Ciappina G, Spagnolo CC, et al. Targeted therapies for KRAS-mutant non-small cell lung cancer: from preclinical studies to clinical development—a narrative review. Transl Lung Cancer Res 2023;12:346-68. [Crossref] [PubMed]
- Slebos RJ, Rodenhuis S. The ras gene family in human non-small-cell lung cancer. J Natl Cancer Inst Monogr 1992;23-9.
- Hames ML, Chen H, Iams W, et al. Correlation between KRAS mutation status and response to chemotherapy in patients with advanced non-small cell lung cancer☆. Lung Cancer 2016;92:29-34. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Scheel AH, Ansén S, Schultheis AM, et al. PD-L1 expression in non-small cell lung cancer: Correlations with genetic alterations. Oncoimmunology 2016;5:e1131379. [Crossref] [PubMed]
- Uehara Y, Watanabe K, Hakozaki T, et al. Efficacy of first-line immune checkpoint inhibitors in patients with advanced NSCLC with KRAS, MET, FGFR, RET, BRAF, and HER2 alterations. Thorac Cancer 2022;13:1703-11. [Crossref] [PubMed]
- Tamiya Y, Matsumoto S, Zenke Y, et al. Large-scale clinico-genomic profile of non-small cell lung cancer with KRAS G12C: Results from LC-SCRUM-Asia study. Lung Cancer 2023;176:103-11. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Landre T, Justeau G, Assié JB, et al. Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: a meta-analysis of randomized-controlled trials. Cancer Immunol Immunother 2022;71:719-26. [Crossref] [PubMed]
- Sun L, Hsu M, Cohen RB, et al. Association Between KRAS Variant Status and Outcomes With First-line Immune Checkpoint Inhibitor-Based Therapy in Patients With Advanced Non-Small-Cell Lung Cancer. JAMA Oncol 2021;7:937-9. [Crossref] [PubMed]
- Liu C, Zheng S, Wang Z, et al. KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer. Cancer Commun (Lond) 2022;42:828-47. [Crossref] [PubMed]
- Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRAS(G12C) Mutation. N Engl J Med 2022;387:120-31. [Crossref] [PubMed]
- Spagnuolo A, Maione P, Gridelli C. The treatment of advanced non-small cell lung cancer harboring KRAS mutation: a new class of drugs for an old target—a narrative review. Transl Lung Cancer Res 2022;11:1199-216. [Crossref] [PubMed]



