
Residual tumor descriptors proposed by the International Association for the Study of Lung Cancer may not be applicable to stage I and ground-glass opacity-featured non-small cell lung cancer
Highlight box
Key findings
• Residual tumor descriptors proposed by the International Association for the Study of Lung Cancer (IASLC) may not be applicable to stage I and ground-glass opacity (GGO)-featured NSCLC.
What is known and what is new?
• Many studies have validated the prognostic value of the R descriptors proposed by IASLC.
• However, GGO was recently considered to be a special clinical subtype with excellent survival when compared to solid nodules, which was not considered or studied as a special subgroup in IASLC R proposal and subsequent validation study of proposed R descriptors.
What is the implication, and what should change now?
• Our study provided an external validation for new residual tumor descriptors for NSCLC proposed by IASLC. Proposed residual tumor descriptors were applicable in radiologic solid NSCLC and stage II–III NSCLC, but were ineffective for GGO-featured or stage I NSCLC.
Introduction
Background
Surgical resection remains a crucial part of the treatments for most malignancies, and the completeness of surgical resection is an important variable in evaluating the therapeutic effect. In 1987, the Union for International Cancer Control (UICC) proposed the R classification: R0 (no residual tumor), R1 (microscopic tumor residual), and R2 (macroscopic tumor residual) (1). However, the clinical scenarios might be so complex that the above classification system could miss some important information. Therefore, the R classification was revised by the International Association for the Study of Lung Cancer (IASLC) in 2005 (2).
Rationale and knowledge gap
Previous studies have reported the prognostic effect of R classification from IASLC (3-7). Nevertheless, these studies did not include radiologic appearance, which is an emerging prognostic factor in lung cancer (8,9). In addition, most studies lack sub-group analyses to investigate the prognostic role of R classification. Thus, it is necessary to conduct a study in a large-scale cohort involving radiologic appearance and restrict its scope of clinical application.
Objective
This study verified the prognostic role of the uncertain resection [R(un)] classification proposed by IASLC and specified its scope of clinical application, which shed light on the stratification and treatments for patients with non-small cell lung cancer (NSCLC). We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-497/rc).
Methods
Patient cohort
The study was conducted by searching patients who underwent surgical resection from January 2010 to May 2019 in the Department of Thoracic Surgery, Fudan University Shanghai Cancer Center. Only patients with NSCLC were finally enrolled. Patients with the diagnoses of adenocarcinoma in situ (AIS)/minimally invasive adenocarcinoma (MIA) or receiving sublobar resection were excluded.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Institutional Review Board of Fudan University Shanghai Cancer Center (2008223-9). Due to the retrospective nature of this study, informed consent was waived.
Radiological and pathological evaluation
Thin-section computed tomography (CT) scans were reviewed by two senior radiologists to distinguish between the ground-glass opacity (GGO) groups (patients with GGO) and solid groups (patients with solid nodule) independently. Mostly solid with a small peripheral GGO component was classified as GGO group. GGO was defined as a nonspecific radiologic finding showing a hazy opacity without blocking underlying pulmonary vessels or bronchial structures, according to the Fleischner Society (10). For the cases with different opinions from radiologists, the agreement was reached through discussion. Intraobserver and interobserver agreements were reported in our previous studies (9,11). Postoperative pathologic reviews including diagnosis and staging were based on the IASLC/American Thoracic Society/European Respiratory Society classification and 8th tumor-node-metastasis (TNM) classification (12,13).
Re-classification of residual tumors descriptors
Residual tumor classification (R descriptors) was based on the 2005 IASLC proposal (2). Complete resection (R0 resection) was defined as follows: (I) the resection margin is negative under the microscope; (II) systematic nodal dissection (SND) or lobe-specific SND (LSND) is performed; (III) the tumor does not invade the tissues around the lymph nodes (LNs), and lung specimens; and (IV) the highest LN resected is negative. Incomplete resection (microscopic residual R1 resection and macroscopic residual R2 resection) was defined as follows: (I) the tumor involves the resection margin; (II) the tumor has extracapsular invasion; (III) there are known positive LNs but have not been resected; and (IV) pleural effusion cytology is negative. R(un) (resection) was defined as those with no tumor involvement at the resection margin under the microscope, but one of the following conditions: (I) there is no extensive SND or LSND; (II) the highest LN resected is positive; (III) the bronchial resection margin shows carcinoma in situ (CIS); and (IV) pleural lavage fluid is positive.
LN dissection
In our study, LN stations 10–14 were considered as N1, whereas stations 2–9 as N2 (14,15). LSND was performed according to tumor locations: (I) right upper and right middle lobes: LN stations of 2R, 4R, and 7; (II) right lower lobe: LN stations of 4R, 7, 8, and 9; (III) upper left lobe: LN stations of 5, 6, and 7; and (IV) lower left lobe: LN stations of 7, 8, and 9. In addition, at least three dissected LNs were required for both the N1 station and N2 station (16).
Follow-up strategy for patients
The follow-up protocol was made according to guidelines (17). The postoperative follow-up of the patient started from the completion of the operation, including chest CT scans, ultrasonography of abdominal and cervical/supraclavicular regions, head magnetic resonance imaging (MRI)/CT, and bone scans. Overall survival (OS) was defined as the interval from surgery to death from any cause. Recurrence-free survival (RFS) was defined as the time between surgery and recurrence of lung cancer or death. The sites of first recurrence were classified as “thorax”, “abdomen”, “neck”, “brain”, and “bone” according to the follow-up protocols.
Statistical analysis
The statistical analysis of this study was performed in R software (version 4.0.3, R Foundation, Vienna, Austria). Survival analysis was conducted using the “survival” and “surviminer” packages in the R software, and the visualization was performed using the “ggplot2” package (18). Two categoric variables’ correlations were analyzed using Fisher exact test or Pearson’s chi-squared test. For the comparison of continuous variables between the two groups, the student’s t-test was used. Kaplan-Meier plots were used for survival analysis (19), and the log-rank test was used to explore the differences between groups in univariate analysis. Multivariate Cox regression models were used to identify independent prognostic factors. Variables with a P value less than 0.1 in univariable analysis were used in the multivariable survival analysis. All tests were two-tailed and P<0.05 was defined as statistical significance.
Results
Patient characteristics
A total of 5,200 patients were included in our study (Figure 1). Average follow-up time was 40.1 months. The clinicopathological characteristics are shown in Table 1 and Table S1. Solid nodules were found in 3,591 patients (69.1%), and the majority of this cohort had adenocarcinoma (75.6%). Stage I, stage II, and stage III disease accounted for 60.1%, 14.8%, and 25.1%, respectively (Table 1). Forty-five cases of other type of lung cancer included 28 adenosquamous carcinomas and 17 large cell carcinomas. There were significant differences in sex, age, smoking status, pathology types, pathological T (pT) stage, pathological N stage, number of resected LN, pathological TNM (pTNM) stage, and proposed R descriptors between GGO and solid nodule groups (P<0.001) (Table S1).
Table 1
| Variables | All (n=5,200) | R0 (n=3,228) | R(un) (n=1,727) | R1 (n=151) | R2 (n=94) | P value |
|---|---|---|---|---|---|---|
| Sex | <0.001 | |||||
| Male | 2,885 (55.5) | 1,808 (56.0) | 897 (51.9) | 114 (75.5) | 66 (55.5) | |
| Female | 2,315 (44.5) | 1,420 (44.0) | 830 (48.1) | 37 (24.5) | 28 (44.5) | |
| Age | 0.481 | |||||
| <60 years | 2,478 (47.7) | 1,519 (47.1) | 842 (48.8) | 68 (45.0) | 49 (47.7) | |
| ≥60 years | 2,722 (52.3) | 1,709 (52.9) | 885 (51.2) | 83 (55.0) | 45 (52.3) | |
| Smoking | <0.001 | |||||
| Never | 3,204 (61.6) | 1,999 (61.9) | 1093 (63.3) | 64 (42.4) | 48 (61.6) | |
| Former/current | 1,996 (38.4) | 1,229 (38.1) | 634 (36.7) | 87 (57.6) | 46 (38.4) | |
| CT appearance | <0.001 | |||||
| GGO | 1,609 (30.9) | 1,052 (32.6) | 531 (30.7) | 17 (11.3) | 9 (30.9) | |
| Solid | 3,591 (69.1) | 2,176 (67.4) | 1,196 (69.3) | 134 (88.7) | 85 (69.1) | |
| Pathology types | <0.001 | |||||
| IAC | 3,932 (75.6) | 2,425 (75.1) | 1,406 (81.4) | 51 (33.8) | 50 (75.6) | |
| SCC | 1,223 (23.5) | 771 (23.9) | 311 (18) | 99 (65.6) | 42 (23.5) | |
| Others | 45 (0.9) | 32 (1.0) | 10 (0.6) | 1 (0.7) | 2 (0.9) | |
| pT stage | <0.001 | |||||
| 1a | 377 (7.3) | 234 (7.2) | 134 (7.8) | 6 (4.0) | 3 (7.3) | |
| 1b | 1,637 (31.5) | 1,072 (33.2) | 530 (30.7) | 23 (15.2) | 12 (31.5) | |
| 1c | 1,147 (22.1) | 738 (22.9) | 367 (21.3) | 32 (21.2) | 10 (22.1) | |
| 2a | 1,052 (20.2) | 605 (18.7) | 380 (22.0) | 44 (29.1) | 23 (20.2) | |
| 2b | 379 (7.3) | 216 (6.7) | 129 (7.5) | 24 (15.9) | 10 (7.3) | |
| 3 | 366 (7.0) | 218 (6.8) | 111 (6.4) | 16 (10.6) | 21 (7.0) | |
| 4 | 242 (4.7) | 145 (4.5) | 76 (4.4) | 6 (4.0) | 15 (4.7) | |
| pN stage | <0.001 | |||||
| 0 | 3,639 (70.0) | 2,600 (80.5) | 956 (55.4) | 48 (31.8) | 35 (70) | |
| 1 | 487 (9.4) | 366 (11.3) | 73 (4.2) | 30 (19.9) | 18 (9.4) | |
| 2 | 1,066 (20.5) | 260 (8.1) | 693 (40.1) | 72 (47.7) | 41 (20.5) | |
| 3 | 8 (0.2) | 2 (0.1) | 5 (0.3) | 1 (0.7) | 0 (0.2) | |
| Number of LN resected | 18.0±32.3 | 20.3±40.2 | 13.7±9.0 | 18.0±8.2 | 18.2±9.0 | <0.001 |
| pTNM stage | <0.001 | |||||
| Stage I | 3,127 (60.1) | 2,215 (68.6) | 856 (49.6) | 36 (23.8) | 20 (60.1) | |
| Stage II | 769 (14.8) | 588 (18.2) | 123 (7.1) | 37 (24.5) | 21 (14.8) | |
| Stage III | 1,304 (25.1) | 425 (13.2) | 748 (43.3) | 78 (51.7) | 53 (25.1) |
Data are presented as n (%) or mean ± SD. R(un), uncertain resection; CT, computed tomography; GGO, ground-glass opacity; IAC, invasive adenocarcinoma; SCC, squamous cell carcinoma; pT, pathological tumor; pN pathological node; LN, lymph node; pTNM, pathological tumor-node-metastasis; SD, standard deviation.
According to criteria released by UICC, 4,964 cases (95.5%) had R0 resection, 142 cases (2.7%) had R1 resection, and 94 cases (1.8%) had R2 resection. Furthermore, survival analyses demonstrated that the R0 group had significantly longer OS and RFS than R1 (OS, P<0.001; RFS, 1,380/4,964, P<0.001) (Figure S1).
Recategorization using R descriptors released by IASLC
After recategorization according to the R descriptors proposed by IASLC, 1,727 cases of R0 were recategorized as R(un), and 9 cases of R0 were recategorized as R1. As a result, there were 3,228 cases of R0 (62.1%), 1,727 cases of R(un) (33.2%), 151 (2.9%) cases of R1, and 94 (1.8%) cases of R2 using the new R descriptors. Details of recategorization were summarized (Table S2), and the main reasons for recategorization from R0 to R(un) were not performing SND/LSND (1,179 cases in total, 68.3%) and the positive highest LN (663 cases in total, 38.3%). Extracapsular invasion (9 cases, 100%) was the only reason for reclassification from R0 to R1. The details of LN dissection (Table S3) showed that N1 samples <3 & N2 samples <3, site-specific LNs not dissected, and neither standard met accounted for 50.2%, 24.9%, and 24.9% in 1,179 cases who were reclassified to R(un) due to not performing rigorous LSND/SND.
After recategorization according to the IASLC proposed R descriptors, patients were divided into R0, R(un), R1, and R2 groups. Compared with R0 groups and R1 groups, R(un) had superior OS to R1 (P<0.001) and inferior OS to R0 (P<0.001) (Figure 2A). Cox proportion hazard analyses demonstrated that R descriptor was an independent prognostic factor for OS in patients with NSCLC (Table S4). Similarly, R(un) had intermediate RFS, compared to R0 (P<0.001) and R1 (P<0.001) (Figure 2B). R descriptor also was an independent prognostic factor for RFS in patients with NSCLC (Table S5). The recurrence pattern between R0, R(un), R1, and R2 groups are shown in Table S6. First recurrence on thorax is the most frequent among all first recurrence sites in R0, R(un), R1, and R2 groups (57.8%, 48.4%, 58.3%, and 52.9%).
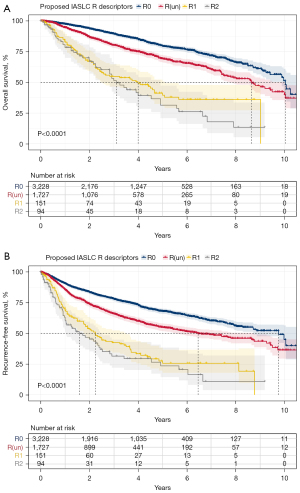
Subgroup analyses and survival analyses
To investigate the prognostic role of R descriptors proposed by IASLC in different groups that was utilized in daily practice, subgroup analyses were further performed (Figure 3). The prognostic effect of R descriptors manifested in most groups, except patients with GGO and stage I (Figure 3). Specifically, in patients with GGO, no statistical difference of OS and RFS between R0 and R(un) was observed [OS, hazard ratio (HR): 1.19, 95% confidential interval (CI): 0.78–1.81, log-rank P=0.4; RFS, HR: 1.19, 95% CI: 0.88–1.62, log-rank P=0.29] (Figure 3 and Figure S2). As for stage I, patients with R(un) had similar OS to R0 patients (HR: 0.88, 95% CI: 0.68–1.14, log-rank P=0.34) (Figure 3 and Figure S3A). Surprisingly, patients with R(un) had better RFS than those with R0 (HR: 0.81, 95% CI: 0.61–0.98, log-rank P=0.024) (Figure 3 and Figure S3B). Number of events for OS and RFS is shown in Figure 3. Specially, in stage I radiographical solid adenocarcinoma, patients with R(un) had similar OS to R0 patients (HR: 1.07, 95% CI: 0.75–1.53, P=0.7), while in stage II/III radiographical solid adenocarcinoma, patients with R(un) had worse OS to R0 patients (HR: 1.46, 95% CI: 1.18–1.80, P<0.001) (Figure S4).

To further look into the possible causes behind this phenomenon, we summarized the reasons for reclassification from R0 to R(un) in patients with stage I disease and GGO. Not performing rigorous LSND/SND was the only reason for the re-evaluation from R0 to R(un) in patients with stage I, while the main reason for the re-evaluation from R0 to R(un) in patients with stage II/III was the metastasis of highest LN resected positive [highest LN resected (+)] (Figure 4A). The situation was similar for patients stratified by GGO and solid nodule. Not performing rigorous LSND/SND resulted in the recategorization from R0 to R(un) in patients with GGO, whereas the highest LN resected (+) remained the main reason for recategorization in patients with solid nodules (Figure 4B). Next, we investigated the survival difference between patients not performing rigorous LSND/SND and patients with the highest LN resected (+). R(un) patients with the highest LN resected (+) had a worse OS than those without performing rigorous LSND/SND only (Figure 5).
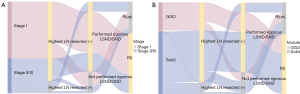

In the different subgroups, multivariable analysis demonstrated the prognosis impact of performing rigorous LSND/SND and highest LN resected (+) (Tables S7,S8). In squamous cell carcinoma group and TNM stage II/III group, performing rigorous LSND/SND was an independent prognostic factor on OS. In all analyzed subgroups, highest LN resected (+) was an independent prognostic factor on OS.
Discussion
As a pan-cancer variable about the residual tumor, the R descriptors proposed by UICC might miss some important information for lung cancer, such as the status of LSND/SND and the metastasis of the highest LN resected. Therefore, in 2005, IASLC proposed a new category of R(un), integrating pathological results of the resection margin, completeness of LN dissection, tumor extracapsular invasion, and status of LN dissection (2). Our study carried out an external validation of proposed R descriptors with a sizable cohort. The main findings of this study included two points. One point was that the complete resection, R(un), and incomplete resection proposed by IASLC were associated with significant differences in survival, which was consistent with previous studies (3-6). The other point was that we firstly demonstrated that proposed residual tumor descriptors were ineffective in GGO-featured NSCLC.
For the R(un) classification advocated by IASLC had not been officially included in the 8th edition of the TNM staging of lung cancer, studies on the R(un) classification proposed by IASLC were sparse. Several previous studies validated the prognostic effect of R(un) (3-6) in OS. However, these studies lacked the information on RFS, and the prognostic effect of R(un) in RFS had not been validated. Some studies investigated incomplete resection as a whole without differentiating between R1 and R2 resection (3,5,6). Nevertheless, with the widespread use of thin-section CT scans, more GGOs were encountered and GGO was recently considered to be a special clinical subtype with excellent survival when compared to solid nodules (11), which was not considered or studied as a special subgroup in IASLC R proposal (2) and subsequent validation study of proposed R descriptors.
After the validation of proposed residual tumor descriptors in OS and RFS, we conducted a subgroup survival analysis to investigate the prognostic impact in various subgroups of NSCLC. The R descriptors proposed by IASLC was found to be applicable to NSCLC as a whole, and to the majority of various subsets of NSCLC, but was ineffective in GGO-featured NSCLC and stage I NSCLC.
The difference in the distribution of the reasons for recategorization, compared with solid nodule NSCLC and pTNM stage II/III NSCLC, may have contributed to the poor prognostic effect of proposed R descriptors in GGO-featured NSCLC and pTNM stage I NSCLC. The reclassification from R0 to R(un) in patients with pTNM stage I, was entirely due to not performing rigorous LSND/SND, whereas in patients with pTNM stage II/III, a large part of the reclassification from R0 to R(un) was due to resected highest LN (+). Compared with highest LN resected (+), not performing rigorous LSND/SND was found to have a smaller effect on survival, which may account for the similar OS between R0 and R(un) in patients with pTNM stage I. Similar findings were made by Edwards and his colleagues: in patients with pN0, OS between R(un) and R0 was nearly identical (4).
The similar reasons might lead to similar OS and RFS outcomes between R0 and R(un) in patients with GGO. For GGO-featured NSCLC, the incidence of LN involvement was relatively low. For GGO with consolidation tumor ratio (CTR) of less than 0.5, no LN metastasis was discovered (20), while only 15–34% of patients with CTR from 0.5 to 1 had LN involvement (20,21). Therefore, some surgeons might not choose to conduct rigorous LSND/SND for selected patients with GGO. Nevertheless, these who were considered to have better prognosis were classified as R(un) according to the IASLC R classification system. In addition, patients with GGO had favorable outcomes, which covered the survival effect of R description.
In this study, we excluded the patients with AIS/MIA, because AIS/MIA was reported to have an excellent prognosis (22-24) and LN dissection was not routinely recommended. The main reasons for the recategorization to R(un) were not performing rigorous LSND/SND and positive status in the highest station of LN resected. In fact, there are two reasons why rigorous LSND/SND is not performed. The one reason is that the surgeons were planned to perform LSND/SND, but for some reason did not meet the criteria for rigorous LSND/SND (e.g., it was quite challenging for patients with an inherent LN number of less than three to meet the rigorous LSND/SND standard, even though the surgeons resected certain LN stations entirely). The other reason is that not performing LSND/SND is surgeons’ choice based on the judgement of no LN metastases and a better prognosis in some patients. LN involvement was reported to be one of the most important prognostic factors on survival (25), performing rigorous LSND/SND had potential benefit. On the other hand, considering there was no consensus about the extent of LN dissection, the definition of R(un) needs to be altered in GGO-featured or pTNM stage I patients. According to the definition of TNM stage, R(un) patients with the highest LN resected (+), would be diagnosed as pN1+ or pTNM stage II/III/IV, which caused the difference in the distribution of R(un) between early-stage patients and advanced-stage patients, and may have an impact on the prognostic effect of proposed R descriptors as discussed above.
Therefore, more thought and consideration should be given to the application of proposed R descriptors in patients with GGO and pTNM stage I. As the clinical T descriptors in GGO-featured patients have been modified by the 8th edition of TNM classification, perhaps the definition of R(un) in GGO-featured patients should be changed (26). Performing rigorous SND/LSND, one of the criteria in R(un)’s definition may be modified or relaxed in patients with GGO, because GGO is a significantly different type compared with solid nodules, and GGO was not widely encountered or studied during the period when the concept of R(un) was proposed. These findings reflected the limitation of residual tumor descriptors proposed by IASLC in GGO-featured NSCLC and stage I NSCLC and the necessity of careful application of proposed R descriptors.
There were several limitations in our research. Firstly, pleural lavage cytology is not an established practice in our institution, our data in this study did not include the results of pleural lavage cytology, which may lead to the inadequacy of this study. Secondly, our study was retrospective and was based on a single center. Thirdly, positron emission tomography (PET)/CT was only performed in a small proportion, for PET/CT was not covered in Chinese medical insurance. It would be more meaningful to include PET/CT results in further studies. Fourthly, the number of patients in the R1 and R2 groups was relatively small compared to the R0 and R(un) groups, thus affecting the results of the comparison. At last, follow-up information after 4 to 6 years become really limited and therefore there is still uncertainty around impact on long-term outcome. We are looking forward to a multi-center study to validate our results.
Conclusions
In conclusion, R(un) represented an intermediate type between R0 and R1. Our study provided an external validation for new residual tumor descriptors for NSCLC proposed by IASLC. Proposed residual tumor descriptors was applicable in radiologic solid and stage II–III NSCLC, but was ineffective in GGO-featured NSCLC and stage I NSCLC.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-497/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-497/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-497/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-497/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Institutional Review Board of Fudan University Shanghai Cancer Center (2008223-9). Due to the retrospective nature of this study, informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hermanek P, Scheibe O, Spiessl B, et al. TNM classification of malignant tumors: the new 1987 edition. Rontgenblatter 1987;40:200.
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Gagliasso M, Migliaretti G, Ardissone F. Assessing the prognostic impact of the International Association for the Study of Lung Cancer proposed definitions of complete, uncertain, and incomplete resection in non-small cell lung cancer surgery. Lung Cancer 2017;111:124-30. [Crossref] [PubMed]
- Edwards JG, Chansky K, Van Schil P, et al. The IASLC Lung Cancer Staging Project: Analysis of Resection Margin Status and Proposals for Residual Tumor Descriptors for Non-Small Cell Lung Cancer. J Thorac Oncol 2020;15:344-59. [Crossref] [PubMed]
- Osarogiagbon RU, Faris NR, Stevens W, et al. Beyond Margin Status: Population-Based Validation of the Proposed International Association for the Study of Lung Cancer Residual Tumor Classification Recategorization. J Thorac Oncol 2020;15:371-82. [Crossref] [PubMed]
- Yun JK, Lee GD, Choi S, et al. A Validation Study of the Recommended Change in Residual Tumor Descriptors Proposed by the International Association for the Study of Lung Cancer for Patients With pN2 NSCLC. J Thorac Oncol 2021;16:817-26. [Crossref] [PubMed]
- Lee J, Lee J, Hong YS, et al. Validation of the IASLC Residual Tumor Classification in Patients with Stage III-N2 Non-small Cell Lung Cancer Undergoing Neoadjuvant Chemoradiotherapy Followed by Surgery. Ann Surg 2022; Epub ahead of print. [Crossref]
- Ye T, Deng L, Xiang J, et al. Predictors of Pathologic Tumor Invasion and Prognosis for Ground Glass Opacity Featured Lung Adenocarcinoma. Ann Thorac Surg 2018;106:1682-90. [Crossref] [PubMed]
- Fu F, Zhang Y, Wen Z, et al. Distinct Prognostic Factors in Patients with Stage I Non-Small Cell Lung Cancer with Radiologic Part-Solid or Solid Lesions. J Thorac Oncol 2019;14:2133-42. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Ye T, Deng L, Wang S, et al. Lung Adenocarcinomas Manifesting as Radiological Part-Solid Nodules Define a Special Clinical Subtype. J Thorac Oncol 2019;14:617-27. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 1.2015. J Natl Compr Canc Netw 2014;12:1738-61. [Crossref] [PubMed]
- Ginestet C. ggplot2: Elegant Graphics for Data Analysis. J R Stat Soc Ser A Stat Soc 2011;174:245-6.
- Efron B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J Am Stat Assoc 1988;83:414-25.
- Zhang Y, Fu F, Wen Z, et al. Segment Location and Ground Glass Opacity Ratio Reliably Predict Node-Negative Status in Lung Cancer. Ann Thorac Surg 2020;109:1061-8. [Crossref] [PubMed]
- Suzuki S, Sakurai H, Yotsukura M, et al. Clinical Features of Ground Glass Opacity-Dominant Lung Cancer Exceeding 3.0 cm in the Whole Tumor Size. Ann Thorac Surg 2018;105:1499-506. [Crossref] [PubMed]
- Behera M, Owonikoko TK, Gal AA, et al. Lung Adenocarcinoma Staging Using the 2011 IASLC/ATS/ERS Classification: A Pooled Analysis of Adenocarcinoma In Situ and Minimally Invasive Adenocarcinoma. Clin Lung Cancer 2016;17:e57-64. [Crossref] [PubMed]
- Borczuk AC. Assessment of invasion in lung adenocarcinoma classification, including adenocarcinoma in situ and minimally invasive adenocarcinoma. Mod Pathol 2012;25:S1-10. [Crossref] [PubMed]
- Li D, Deng C, Wang S, et al. Ten-year follow-up of lung cancer patients with resected adenocarcinoma in situ or minimally invasive adenocarcinoma: Wedge resection is curative. J Thorac Cardiovasc Surg 2022;164:1614-1622.e1. [Crossref] [PubMed]
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.



