
Identifying factors causing failure of nodal staging by endobronchial ultrasound-guided transbronchial needle aspiration in non-small cell lung cancer
Highlight box
Key findings
• Despite the addition of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), nodal staging in non-small cell lung cancer failed in 21 of 264 patients (8.0%).
• Failures categorized as punctured and not-punctured, with the latter further divided into: (I) difficult-to-reach; (II) omission due to false-positive rapid on-site cytologic evaluation (ROSE) results; and (III) non-significant EBUS findings.
• Adenocarcinoma patients with driver oncogenes had a significantly higher failure rate (16.1% vs. 3.3%, P=0.026).
What is known and what is new?
• EBUS-TBNA is recommended for nodal staging, but poorly investigated regarding failure causes.
• Three main causes for “not-punctured group”: (I) difficult-to-reach; (II) omission due to false-positive ROSE results; and (III) non-significant EBUS findings.
• Novel finding: significantly higher failure rate in adenocarcinoma with driver oncogenes.
What is the implication, and what should change now?
• Identifying failure causes and improvements enhance diagnostic accuracy in nodal staging.
• Exercise caution in interpreting nodal staging results in adenocarcinoma patients with driver oncogenes.
Introduction
Lung cancer is the leading cause of cancer-related deaths, with an estimated 2.2 million new cases and 1.8 million deaths worldwide in 2020, among which 85% of the deaths were due to non-small cell lung cancer (NSCLC) (1).
Accurate nodal staging is crucial for the prognosis and treatment of NSCLC (2-5) without distant metastases. However, nodal staging by contrast-enhanced computed tomography (CT) and 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) is insufficient (2,3). Therefore, invasive nodal staging involving the collection of pathologic specimens from lymph nodes (LNs) is recommended for patients suspicious of mediastinal LN metastasis (N2 or higher) and is also considered in the presence of the following: primary lesion >3 cm, primary lesion centrally located, or N1 suspected on imaging (6-8).
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive and standardized technique for sampling of lesions contacting the central airway, especially hilar and mediastinal LNs. It has a high diagnostic value in nodal staging, with sensitivities and specificities of 80–96% and 100%, respectively (2,3,9-11). Thus, when utilized as the initial examination, it is safer, more cost-effective, and equally as efficient as surgical nodal staging (11-17).
Nevertheless, the occasional failure of nodal staging by EBUS-TBNA can lead to delayed diagnosis and inappropriate treatment strategies. Notably, clinical stage III treatment plans vary based on nodal staging results. When EBUS-TBNA reveals mediastinal involvement, further confirmatory tests are generally unnecessary due to the exceedingly low likelihood of false-positive results (18). The primary concern arises from false-negative EBUS-TBNA outcomes, where patients are potentially downstaged to a clinical stage N2, resulting in incorrect surgical indications. While current guidelines recommend that cases of negative EBUS-TBNA with strong suspicion of LN metastases should undergo additional surgical staging, such as cervical mediastinoscopy (8,19), a recent randomized controlled trial has reported the validity of omitting it (20).
Furthermore, the landscape of early-NSCLC treatment is evolving with the introduction of neoadjuvant therapies, including immune checkpoint inhibitor combinations for non-oncogene-driven NSCLC and molecular targeted therapies for oncogene-driven NSCLC, as indicated by several ongoing phase III trials (21). In the future, there is anticipated to be an increase in the number of cases that are currently deemed inoperable but have the potential to be downstaged through preoperative treatments and thus to become eligible for surgical resection.
Hence, while EBUS-TBNA offers high reliability in nodal staging, it may not be infallible, prompting the need for an in-depth investigation of factors contributing to the diagnostic failures. In the quest for enhanced diagnostic precision and the ever-evolving landscape of precision medicine, this study aimed to identify factors that may contribute to nodal staging failures via EBUS-TBNA in NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-264/rc).
Methods
Patients
Consecutive patients who underwent EBUS-TBNA at the National Cancer Center Hospital between January 2017 and December 2020 were reviewed. Of them, cases wherein the purpose was systematic nodal staging were included. Note that we perform systematic nodal staging based on imaging, usually for cases of suspected mediastinal LN metastasis, and often also for cases of centrally located primary tumors and/or N1. We do not cover cases in which extensive mediastinal LN metastases are evident, nor do we establish criteria based on primary tumor size. Cases for (I) re-staging purposes after any treatment; (II) those found to be stage IV; and (III) those diagnosed with other than NSCLC were excluded; and the remaining cases were eligible for analyses.
This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the National Cancer Center Institutional Review Board (No. 2018-090). The requirement for informed consent was waived due to the retrospective nature of this study. However, the information used in this study was entered into a database independent of the electronic medical record, and patient identification was controlled by a separately assigned study number so that third parties cannot identify them.
Equipment and procedures
Either of the two linear ultrasound bronchoscopes (UC260FW or UC290F; Olympus, Tokyo, Japan) was used, and almost all cases involved the latter after its introduction. Various needle types, including 22-gauge needles (NA-201SX-4022/NA-U401SX-4022, Olympus; ExpectTM Pulmonary, Boston Scientific, Natick, MA, USA; EchoTip Ultra®/ProCore®, Cook Medical, Bloomington, IN, USA) or 25-gauge needles (NA-U401SX-4025N, Olympus; ExpectTM Pulmonary, Boston Scientific; EchoTip Ultra®/ProCore®, Cook Medical), were used, depending on the timing of the examination and the discretion of the bronchoscopist.
Moderate-to-deep sedation with a combination of intravenous narcotics (fentanyl or pethidine) and sedatives (propofol or midazolam) was administered. All procedures were performed under the supervision of bronchoscopy experts with more than 10 years of experience. The bronchoscope was inserted orally after topical application of aerosolized 8% lidocaine; intratracheal local anesthesia was administered using 2% lidocaine injection.
Visual LN survey through EBUS was performed in the order of the primary lesion (if observable), N1, N2, and N3 stations. The possibility of metastasis to each LN was systematically assessed with a combination of ultrasound modes. First, the following sonographic features on conventional B-mode were observed according to the previous report: short-axis size, shape, margin, echogenicity, and presences of central hilar structure and coagulation necrosis sign (22). Second, the vascularity was evaluated using color/power Doppler mode (23). Third, elastography mode was used when available to assess the relative stiffness of tissues (24). These findings were identified and recorded during real-time evaluation.
Subsequently, targeted lesions with sonographic findings suggestive of malignancy (22,25) or at locations critical to determine the treatment protocols were punctured in the order of the N3, N2, N1 stations, and primary lesion (if approachable) to avoid contamination from higher LNs. EBUS-TBNA was performed using a negative pressure syringe (10 or 20 mL) with around 30–50 agitations per puncture. If there was considerable blood backflow, lower negative pressure or a slow-pull method was applied to the subsequent passes. Whenever possible, the puncture was repeated at least twice to ensure the sample adequacy, while referring to the results of rapid on-site cytologic evaluation (ROSE). Although not performed routinely, transesophageal endoscopic ultrasound with bronchoscope-guided fine needle aspiration (EUS-B-FNA) was also aggressively conducted for cases with LNs with difficult transbronchial access, such as #5 and #8.
Definition of nodal stage
Nodal stages were investigated separately at diagnosis and treatment. The former was categorized as “unconfirmed”, relying on the results of EBUS-TBNA in addition to imaging modalities such as CT and FDG-PET. In contrast, the “confirmed” nodal stages were determined based on the pathological stages of patients who underwent surgery. For patients who did not undergo surgery, the confirmed stages were based on the consensus results of the multidisciplinary team.
Since the prognoses have been reported to differ between patients with single- and multiple-station N2 (N2a and N2b, respectively) (26), and the difference has a significant impact on the indication for surgery, they were investigated separately.
Variables
We defined cases where the unconfirmed and confirmed stages matched as “nodal staging successes”, while those that did not match were defined as “failures”. In this study, we focused on the failure cases and explored factors associated with them. Specifically, since there is an essential difference between (I) cases that were punctured but resulted in failures and (II) cases that were not punctured and resulted in failures, these two groups, “punctured” and “not-punctured” were investigated separately.
The following patient data were collected: age, sex, smoking history, side and location of the primary lesion, lesion size, nodal stage by CT/FDG-PET, and histological type. The smoking history was classified as “never/light” and “heavy”, with the Brinkman index bordering 400. The location was determined by dividing the distance from the pulmonary hilum into three equal parts referring to the previous report: the central two-thirds was defined as “central” and the rest was defined as “peripheral” (27). The lesion size was measured as the short diameter on axial CT images. The histological type was divided into adenocarcinoma and non-adenocarcinoma.
If biomarker tests for driver oncogenes had been performed, the results were also extracted. Since the types and methods of those tests varied from time to time, cases in which at least epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) were examined were included.
Statistical analyses
Descriptive statistics are presented as numerical values and frequencies (percentages) or medians (ranges). Univariable analyses were performed using Fisher’s exact test. All statistical analyses were performed using EZR version 1.55 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (28). All P values were two-sided, and those less than 0.05 were considered statistically significant.
Results
During the study period, we performed EBUS-TBNA for 1,287 patients, and 392 cases were intended for the systematic nodal staging. As per the inclusion criteria, 264 cases were included in the analyses (Figure 1).

The baseline patients’ characteristics and details of the nodal staging are summarized in Table 1. The median [range] age was 70 [31–90] years, and 185 patients (70.1%) were male. The primary lesions were located mainly on the right side [175 cases (66.3%)] and at the peripheral location [209 cases (79.2%)]. Of the 724 lesions punctured in total, the most frequent site was #4R [145 lesions (20.0%)] followed by #7 [122 lesions (16.9%)] and #4L [104 lesions (14.4%)]. The histologic type was mostly adenocarcinoma in 154 (58.3%), followed by squamous cell carcinoma in 77 (29.2%), NSCLC, not otherwise specified (NOS) in 13 (4.9%), and others in 20 (7.6%).
Table 1
| Characteristic | Value |
|---|---|
| Age (years), median [range] | 70 [31–90] |
| Sex, n (%) | |
| Male | 185 (70.1) |
| Female | 79 (29.9) |
| Smoking history, n (%) | |
| Never/light | 192 (72.7) |
| Heavy | 72 (27.3) |
| Primary lesion side, n (%) | |
| Right | 175 (66.3) |
| Left | 89 (33.7) |
| Primary lesion location, n (%) | |
| Peripheral | 209 (79.2) |
| Central | 55 (20.8) |
| Punctured lesion (n=724), n (%) | |
| #1R | 6 (0.8) |
| #2L† | 7/1 (1.1) |
| #2R | 66 (9.1) |
| #3p | 16 (2.2) |
| #4L† | 97/7 (14.4) |
| #4R | 145 (20.0) |
| #5† | 4/11 (2.1) |
| #7 | 122 (16.9) |
| #8† | 11/6 (2.3) |
| #10L | 8 (1.1) |
| #10R | 15 (2.1) |
| #11L | 43 (5.9) |
| #11R | 83 (11.5) |
| #12L | 12 (1.7) |
| #12R | 7 (1.0) |
| #13L | 1 (0.1) |
| #14R | 1 (0.1) |
| Primary lesion† | 50/5 (7.6) |
| Histological type, n (%) | |
| Adenocarcinoma | 154 (58.3) |
| Squamous cell carcinoma | 77 (29.2) |
| NSCLC, NOS | 13 (4.9) |
| Others | 20 (7.6) |
| Driver oncogene, n (%) | |
| Positive | 65 (24.6) |
| Negative | 105 (39.8) |
| Not evaluated | 94 (35.6) |
†, marked lesions include those in which EUS-B-FNA was performed. The left side of the slash indicates the number of cases sampled by EBUS-TBNA and the right side indicates that by EUS-B-FNA, respectively. EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; NSCLC, non-small cell lung cancer; NOS, not otherwise specified; EUS-B-FNA, transesophageal endoscopic ultrasound with bronchoscope-guided fine needle aspiration.
All but 11 cases were evaluated with FDG-PET in conjunction with CT. However, 189 cases (71.6%) were correctly staged without EBUS-TBNA, whereas with the addition of EBUS-TBNA, 243 cases (92.0%) were correctly staged (Table S1). There were 21 cases (7.9%) that were upstaged and 33 cases (12.5%) that were downstaged. In other words, despite the addition of EBUS-TBNA, 21 cases (8.0%) failed the nodal staging. Although we analyzed the association between the failure cases and the clinical factors, there were no significant differences (Table 2).
Table 2
| Variable | Success (n=243) | Failure (n=21) | P value |
|---|---|---|---|
| Age (years), n (%) | >0.99 | ||
| <70 | 127 (92.0) | 11 (8.0) | |
| >70 | 116 (92.0) | 10 (8.0) | |
| Sex, n (%) | >0.99 | ||
| Male | 170 (91.9) | 15 (8.1) | |
| Female | 73 (92.4) | 6 (7.6) | |
| Smoking history, n (%) | 0.610 | ||
| Never/light | 178 (92.7) | 14 (7.3) | |
| Heavy | 65 (90.3) | 7 (9.7) | |
| Primary lesion side, n (%) | 0.639 | ||
| Right | 162 (92.6) | 13 (7.4) | |
| Left | 81 (91.0) | 8 (9.0) | |
| Primary lesion location, n (%) | 0.264 | ||
| Peripheral | 190 (90.9) | 19 (9.1) | |
| Central | 53 (96.4) | 2 (3.6) | |
| Primary lesion size (mm), n (%) | 0.646 | ||
| <30 | 146 (91.2) | 14 (8.8) | |
| >30 | 97 (93.3) | 7 (6.7) | |
| Suspected nodal stage on imaging, n (%) | >0.99 | ||
| >1 | 216 (91.9) | 19 (8.1) | |
| 0 | 27 (93.1) | 2 (6.9) | |
| Histological type, n (%) | 0.820 | ||
| Adenocarcinoma | 141 (91.6) | 13 (8.4) | |
| Non-adenocarcinoma | 102 (92.7) | 8 (7.3) | |
Next, all 21 patients who failed the nodal staging are summarized (Table 3): 10 were in the punctured group and 11 were in the not-punctured group. The latter, when reviewed in detail down to the causes that prevented punctures, were subdivided into the following three categories: (I) two of difficult-to-reach LNs; (II) two of omission at lower level LNs due to false-positive ROSE results at the higher level; and (III) seven of LNs with non-significant EBUS findings (i.e., <5 mm in the short diameter, not corresponding to the puncture target).
Table 3
| Group | Age (years) | Sex | Primary lesion site | Failed lesion | Histological type | Driver oncogene | Failure cause |
|---|---|---|---|---|---|---|---|
| P | 68 | Male | Left hilum | #4R | Sq | Negative | False negative |
| P | 81 | Female | RUL | #7 | Sq | Negative | False negative |
| P | 65 | Male | RLL | #4R | AdSq | Negative | False negative |
| P | 64 | Male | RUL | #2R, #4R | Sq | Not evaluated | False negative |
| P | 75 | Female | LLL | #4L | Sq | Negative | False negative |
| P | 65 | Male | RLL | #7 | Pleomorphic | Not evaluated | False negative |
| P | 74 | Male | RLL | #4R | Ad | EGFR | False negative |
| P | 67 | Male | LUL | #5 | Ad | Negative | False negative |
| P | 65 | Male | LUL | #5 | Ad | Not evaluated | False negative |
| P | 57 | Male | ML | #1R | Ad | RET | False negative |
| NP | 82 | Male | RLL | #14R | Sq | Not evaluated | Difficult-to-reach |
| NP | 66 | Male | LLL | #10L | Sq | Negative | Difficult-to-reach |
| NP | 54 | Male | LLL | #5 | Ad | Not evaluated | Omission due to false-positive ROSE results |
| NP | 75 | Male | LUL | #10L, #12L, #13L | Ad | Negative | Omission due to false-positive ROSE results |
| NP | 79 | Female | LLL | #4L, #5, #7, #8, #11L | Ad | HER2 | Non-significant EBUS findings |
| NP | 53 | Female | RLL | #7, #11R, #12R | Ad | ALK | Non-significant EBUS findings |
| NP | 77 | Male | RUL | #2R | Ad | MET | Non-significant EBUS findings |
| NP | 71 | Female | RLL | #7 | Ad | EGFR | Non-significant EBUS findings |
| NP | 69 | Female | RUL | #2R | Ad | ALK | Non-significant EBUS findings |
| NP | 73 | Male | RLL | #4R | Ad | EGFR | Non-significant EBUS findings |
| NP | 85 | Male | RUL | #2R | Ad | EGFR | Non-significant EBUS findings |
P, punctured; Sq, squamous cell carcinoma; RUL, right upper lobe; RLL, right lower lobe; AdSq, adenosquamous carcinoma; LLL, left lower lobe; Ad, adenocarcinoma; EGFR, epidermal growth factor receptor; LUL, left upper lobe; ML, middle lobe; RET, rearranged during transfection; NP, not-punctured; ROSE, rapid on-site cytologic evaluation; HER2, human epidermal growth factor receptor 2; EBUS, endobronchial ultrasound; ALK, anaplastic lymphoma kinase; MET, mesenchymal-epithelial transition.
Last, focusing on biomarker tests, 65 of the 170 patients who were checked for driver oncogenes were positive. Stratified analysis was performed separately for adenocarcinoma and non-adenocarcinoma, considering the difference in driver oncogene positive rates; the association between histology type and nodal staging failure is expressed in Table 4. In cases of adenocarcinoma, the nodal staging failure rate was significantly higher in cases positive for driver oncogenes than in negative cases [9/56 (16.1%) vs. 2/60 (3.3%), P=0.026]. Note that all cases categorized as non-significant EBUS findings in the not-punctured group involved various driver oncogenes (Table 3).
Table 4
| Histological type | Driver oncogene | Nodal staging | P value | ||
|---|---|---|---|---|---|
| Success | Failure | Total | |||
| Adenocarcinoma | Positive | 47 | 9 | 56 | 0.026 |
| Negative | 58 | 2 | 60 | ||
| Total | 105 | 11 | 116 | ||
| Non-adenocarcinoma | Positive | 9 | 0 | 9 | 0.576 |
| Negative | 40 | 5 | 45 | ||
| Total | 49 | 5 | 54 | ||
Discussion
This study demonstrated the nodal staging failure cases by EBUS-TBNA in NSCLC and its associated factors. There were 21 cases (8.0%) of nodal staging failure: 10 (3.8%) in the punctured group and 11 (4.2%) in the not-punctured group. The not-punctured group was subdivided into the following three categories: (I) difficult-to-reach; (II) omission due to false-positive ROSE results; and (III) non-significant EBUS findings. Focusing on biomarker tests, the nodal staging failure rate was significantly higher in adenocarcinoma cases that were positive for driver oncogenes than in negative cases [9/56 (16.1%) vs. 2/60 (3.3%), P=0.026]. Note that all cases categorized as having non-significant EBUS findings in the not-punctured group involved various driver oncogenes. To the best of our knowledge, few studies have investigated the factors leading to the failure of nodal staging by EBUS-TBNA and evaluated their relationship to driver oncogenes.
LNs with a short diameter of ≥1 cm on chest CT are generally diagnosed as positive for metastasis, with the sensitivity and specificity of 59% and 78%, respectively (9). Meanwhile, FDG-PET has the sensitivities and specificities of 80–90% and 85–95%, respectively (2,3). The sensitivities of the systematic nodal staging by EBUS-TBNA have been reported to be 86–92% (2,3,9-11). In the present study, the sensitivities before and after EBUS-TBNA were 71.6% and 92.0%, respectively. While FDG-PET was utilized with CT in almost all cases, nodal staging through EBUS-TBNA produced more valuable results and mostly aligned with those reported in the previous literatures.
The punctured failures could have been influenced by a variety of factors, such as the number of times each LN was punctured, needle size used, and bronchoscopist’s experience. However, a systematic review and meta-analysis showed similarly high diagnostic sensitivities of EBUS-TBNA for diagnosing NSCLC regardless of the needle size used (29). Furthermore, it has been reported that the diagnostic yield did not increase after three or more punctures (9), nor did experienced physicians perform the punctures (30). Thus, false negatives can be considered a limitation in the diagnostic performance of EBUS-TBNA itself.
On the other hand, in the not-punctured group, one of the causes was difficult-to-reach LNs, including #10L behind the left pulmonary artery trunk and #14R. Approaches to these LNs require overcoming the challenges of limited mobility due to the combination of ultrasound bronchoscopes and needles. Some LNs were difficult to approach with the conventional ultrasound bronchoscope, but since the advent of the newer bronchoscope, the miniaturization of the ultrasound tip and the modified puncture angle have improved this situation considerably (31,32). Nevertheless, although it is currently almost impossible to approach the intrapulmonary LNs, a prototype ultrasound bronchoscope with an even thinner tip has been reported (33), which should make the approach feasible in the future. In addition, although we have not actively performed it for staging purposes, the utility of EBUS-guided transvascular sampling for lesions located behind major vessels has been reported (34) and may be considered.
Another cause was that lower-level LNs, necessary for the true staging, were not punctured due to the false-positive ROSE results at the higher level. ROSE during EBUS-TBNA has been reported to contribute to improved diagnostic outcomes, reduced puncture attempts, and decreased complications (35). Although only a few articles have described the concordance rate of ROSE in EBUS-TBNA as compared to the final cytology, the accuracy has been reported to range from 89% to 98% (35-37). It is generally rare to encounter false-positive results with EBUS-TBNA, but it is possible with ROSE (35,38). In particular, reactive bronchial epithelial cells have the potential to mimic neoplastic cells and may pose a challenge for less experienced cytopathologists when interpreting the results. It should be noted that false-positive results with ROSE have been reported not only in cases of lung cancer, but also in cases of granulomatous diseases (39). Therefore, it is crucial to be aware of these potential challenges and maintain accuracy control when using ROSE in conjunction with nodal staging.
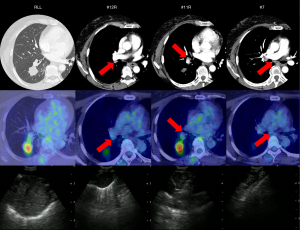
The remaining cause was that the EBUS findings were deemed non-significant and thus were not considered for punctures. The LNs to be sampled for staging are mainly three mediastinal LNs, including #4R, #4L, and #7, usually with the short diameter of 5 mm or greater (40). However, the LNs in this category were visualized as tiny lesions, did not meet this size criterion, and thus resulted in false negatives. Interestingly, all these patients had adenocarcinoma with various driver oncogenes. In an additional stratified analysis, adenocarcinomas with positive driver oncogenes were significantly more likely to fail the staging than those without. According to Marulli et al., LN metastasis was more common in patients with adenocarcinoma when the clinical stage was the same, and the relative risk of unexpected postoperative LN metastasis was about seven times higher than that in patients with squamous cell carcinoma (41). Furthermore, Seto et al. reported an increased incidence of subclinical LN metastasis in EGFR mutation-positive and ALK translocation-positive adenocarcinomas (42). Based on the above, adenocarcinoma patients with driver oncogenes are considered to be at high risk for LN metastasis, even if the image findings are obscure, which would have contributed to the staging failures. We present a representative case of nodal staging failure in a 53-year-old female with ALK translocation-positive adenocarcinoma (Figure 2).
Recently, neoadjuvant therapies have attracted attention as an alternative to conventional adjuvant therapies for perioperative treatment. Based on the results of the CheckMate 816 trial, neoadjuvant nivolumab plus chemotherapy has already been introduced into clinical practice for NSCLC (43). However, EGFR mutation- or ALK translocation-positive patients were excluded in the trial. The KEYNOTE-671 trial also reported the efficacy of neoadjuvant pembrolizumab plus chemotherapy followed by surgery and adjuvant pembrolizumab for NSCLC (44). However, it is argued that very few patients with EGFR-mutation or ALK-translocation were enrolled in this trial, limiting insights into the efficacy of this combination therapy for these subgroups. On the other hand, following the ADAURA trial of adjuvant osimertinib for EGFR mutation-positive NSCLC, which showed longer overall survival in patients who received osimertinib (45), another trial of osimertinib in the neoadjuvant setting is underway (ClinicalTrials.gov identifier: NCT04351555, JapicCTI-205325). Moreover, an umbrella trial investigating neoadjuvant therapies for various driver oncogenes is in progress, and it is becoming increasingly important to examine these genetic statuses preoperatively even in resectable cases (46). Thus, our results suggest the need for caution when interpreting nodal staging in patients who are preoperatively determined to be driver oncogene-positive.
Our study has several limitations. First, this was a retrospective, single-center study, and the type of the ultrasound bronchoscope and needles used depended on the timing of the examination and/or the discretion of each bronchoscopist. Second, in confirming the final nodal stages, not all cases were determined by their postoperative pathological stages. For non-surgical cases, the confirmed stages were determined based on the consensus results of the multidisciplinary team. Although the confirmed stages may be somewhat inaccurate, this study included patients who were upstaged after EBUS-TBNA, thus avoiding unnecessary surgeries. The presence of these cases is one of the important indicators of the efficacy of nodal staging as well, and their inclusion would be reasonable to reflect actual clinical practice. Third, not all patients underwent biomarker tests for driver oncogenes, and the types and methods used were subject to the time period. Nevertheless, the fact that nodal staging was prone to failure in adenocarcinomas that were positive for driver oncogenes raises significant attention.
Conclusions
The present study demonstrated the risk of false positives with ROSE and the involvement of driver oncogenes as factors associated with nodal staging failure in NSCLC by EBUS-TBNA, in addition to limitations of the procedure itself, including sampling performance and reachability. Especially in adenocarcinoma patients with driver oncogenes, the possibility of metastases was significantly high even in LNs with obscure image findings, and their nodal staging results should be interpreted cautiously.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-264/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-264/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-264/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-264/coif). Y.M. receives grants for Medical Research from Japan Society for the Promotion of Science Hitachi, Ltd.; and honoraria for lectures from Olympus, AstraZeneca, NOVARTIS, COOK, AMCO, Thermo Fisher Scientific, Erbe Elektromedizin GmbH, Fujifilm, Chugai, Eli Lilly, Merck, Takeda, and ETHICON. T.I. receives grants from Hitachi High-Tech Corporation; and honoraria for lectures from COOK, Chugai pharma, Eli Lilly, Olympus, Novartis pharma, and Fujifilm; and honoraria for manuscript writing from Thermo Fisher Scientific K.K. K.U. receives grants for Medical Research from Japan Society for the Promotion of Science KAKENHI Grant Number JP22K15698 and KAKENHI Grant Number JP19K16966; honoraria for lectures from Novartis, Thermo Fisher Scientific, AstraZeneca, and Chugai. Y.O. receives grants for Medical Research from AstraZeneca, Eli Lilly, BMS, Dainippon-Sumitomo, Taiho, Takeda, Daiichi-Sankyo, Chugai, ONO, Kyorin, Pfizer, Novartis, Kissei and Janssen; honoraria for lectures from AstraZeneca, Chugai, Eli Lilly, Bayer, MSD, Nippon Kayaku, Eisai, ONO, BMS, Boehringer Ingelheim, Pfizer, Taiho, and Kyowa Hakko Kirin. T.T. receives grants for Medical Research from Japan Agency for Medical Research and Development, Foundation for Promotion Cancer Research, and JSPS KAKENHI Grant; honoraria for Degree Examination Review Committee from Nippon Medical School Foundation; participation on Safety Monitoring Board in Hamamatsu University School of Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the National Cancer Center Institutional Review Board (No. 2018-090). The requirement for informed consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GLOBOCAN. Estimated cancer incidence, mortality and prevalence worldwide in 2020. [Cited 2022 Dec 17]. Available online: https://gco.iarc.fr/
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline®). Non-Small Cell Lung Cancer, Version 2, 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [Crossref] [PubMed]
- Lee PC, Port JL, Korst RJ, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:177-81. [Crossref] [PubMed]
- Dooms C, Tournoy KG, Schuurbiers O, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest 2015;147:209-15. [Crossref] [PubMed]
- Figueiredo VR, Cardoso PFG, Jacomelli M, et al. EBUS-TBNA versus surgical mediastinoscopy for mediastinal lymph node staging in potentially operable non-small cell lung cancer: a systematic review and meta-analysis. J Bras Pneumol 2020;46:e20190221. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Vaidya PJ, Munavvar M, Leuppi JD, et al. Endobronchial ultrasound-guided transbronchial needle aspiration: Safe as it sounds. Respirology 2017;22:1093-101. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [Crossref] [PubMed]
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Harewood GC, Pascual J, Raimondo M, et al. Economic analysis of combined endoscopic and endobronchial ultrasound in the evaluation of patients with suspected non-small cell lung cancer. Lung Cancer 2010;67:366-71. [Crossref] [PubMed]
- Steinfort DP, Liew D, Conron M, et al. Cost-benefit of minimally invasive staging of non-small cell lung cancer: a decision tree sensitivity analysis. J Thorac Oncol 2010;5:1564-70. [Crossref] [PubMed]
- Murthi M, Donna E, Arias S, et al. Diagnostic Accuracy of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA) in Real Life. Front Med (Lausanne) 2020;7:118. [Crossref] [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Bousema JE, Dijkgraaf MGW, van der Heijden EHFM, et al. Endosonography With or Without Confirmatory Mediastinoscopy for Resectable Lung Cancer: A Randomized Clinical Trial. J Clin Oncol 2023;41:3805-15. [Crossref] [PubMed]
- Godoy LA, Chen J, Ma W, et al. Emerging precision neoadjuvant systemic therapy for patients with resectable non-small cell lung cancer: current status and perspectives. Biomark Res 2023;11:7. [Crossref] [PubMed]
- Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010;138:641-7. [Crossref] [PubMed]
- Nakajima T, Anayama T, Shingyoji M, et al. Vascular image patterns of lymph nodes for the prediction of metastatic disease during EBUS-TBNA for mediastinal staging of lung cancer. J Thorac Oncol 2012;7:1009-14. [Crossref] [PubMed]
- Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014;44:956-62. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Yoshino I, Yoshida S, Miyaoka E, et al. Surgical outcome of stage IIIA- cN2/pN2 non-small-cell lung cancer patients in Japanese lung cancer registry study in 2004. J Thorac Oncol 2012;7:850-5. [Crossref] [PubMed]
- Sanz-Santos J, Martínez-Palau M, Jaen À, et al. Geometrical Measurement of Central Tumor Location in cT1N0M0 NSCLC Predicts N1 but Not N2 Upstaging. Ann Thorac Surg 2021;111:1190-7. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Yu Lee-Mateus A, Garcia-Saucedo JC, Abia-Trujillo D, et al. Comparing diagnostic sensitivity of different needle sizes for lymph nodes suspected of lung cancer in endobronchial ultrasound transbronchial needle aspiration: Systematic review and meta-analysis. Clin Respir J 2021;15:1328-36. [Crossref] [PubMed]
- Cornwell LD, Bakaeen FG, Lan CK, et al. Endobronchial ultrasonography-guided transbronchial needle aspiration biopsy for preoperative nodal staging of lung cancer in a veteran population. JAMA Surg 2013;148:1024-9. [Crossref] [PubMed]
- Costagli A, Funke F, Karpf-Wissel R, et al. Does the new generation of Endobronchial Ultrasound Bronchoscopes open new horizons? Eur Respir J 2019;54:PA4770.
- Kalchiem-Dekel O, Hossain S, Gauran C, et al. An evolving role for endobronchial ultrasonography in the intensive care unit. J Thorac Dis 2021;13:5183-94. [Crossref] [PubMed]
- Ishiwata T, Inage T, Gregor A, et al. Preclinical evaluation of thin convex probe endobronchial ultrasound-guided transbronchial needle aspiration for intrapulmonary lesions. Transl Lung Cancer Res 2022;11:1292-301. [Crossref] [PubMed]
- Molina JC, Chaudry F, Menezes V, et al. Transvascular endosonographic-guided needle biopsy of intrathoracic lesions. J Thorac Cardiovasc Surg 2020;159:2057-65. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: a randomized study. Respiration 2013;85:486-92. [Crossref] [PubMed]
- Bonifazi M, Sediari M, Ferretti M, et al. The role of the pulmonologist in rapid on-site cytologic evaluation of transbronchial needle aspiration: a prospective study. Chest 2014;145:60-5. [Crossref] [PubMed]
- Caupena C, Esteban L, Jaen A, et al. Concordance Between Rapid On-Site Evaluation and Final Cytologic Diagnosis in Patients Undergoing Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Non-Small Cell Lung Cancer Staging. Am J Clin Pathol 2020;153:190-7. [Crossref] [PubMed]
- Joseph M, Jones T, Lutterbie Y, et al. Rapid on-site pathologic evaluation does not increase the efficacy of endobronchial ultrasonographic biopsy for mediastinal staging. Ann Thorac Surg 2013;96:403-10. [Crossref] [PubMed]
- Rokadia HK, Mehta A, Culver DA, et al. Rapid On-Site Evaluation in Detection of Granulomas in the Mediastinal Lymph Nodes. Ann Am Thorac Soc 2016;13:850-5. [Crossref] [PubMed]
- Kinsey CM, Arenberg DA. Endobronchial ultrasound-guided transbronchial needle aspiration for non-small cell lung cancer staging. Am J Respir Crit Care Med 2014;189:640-9. [Crossref] [PubMed]
- Marulli G, Verderi E, Comacchio GM, et al. Predictors of unexpected nodal upstaging in patients with cT1-3N0 non-small cell lung cancer (NSCLC) submitted to thoracoscopic lobectomy. J Vis Surg 2018;4:15. [Crossref] [PubMed]
- Seto K, Kuroda H, Yoshida T, et al. Higher frequency of occult lymph node metastasis in clinical N0 pulmonary adenocarcinoma with ALK rearrangement. Cancer Manag Res 2018;10:2117-24. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:491-503. [Crossref] [PubMed]
- Tsuboi M, Herbst RS, John T, et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med 2023;389:137-47. [Crossref] [PubMed]
- Blumenthal GM, Bunn PA Jr, Chaft JE, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J Thorac Oncol 2018;13:1818-31. [Crossref] [PubMed]


