
Pathological and radiological T descriptors in invasive lung adenocarcinoma: from correlations to prognostic significance
Highlight box
Key findings
• Ground-glass opacity (GGO) component rather than lepidic component was an independent factor for recurrence-free survival (RFS).
What is known and what is new?
• The eighth T classification suggested that pathological and clinical T-stage should be determined by the maximum pathological invasive size (PIS) and radiological solid size (RSS) respectively, excluding the lepidic component and GGO component.
• The correlation between PIS and RSS in solid nodule was stronger than that in part-solid nodule. Some pathological invasive components except solid component were featured as GGO component. GGO ratio was an independent prognostic factor for RFS in T1 invasive lung adenocarcinoma, whereas lepidic ratio was not.
What is the implication, and what should change now?
• GGO component rather than lepidic component should be considered as an additional T descriptor.
Introduction
Approximately ten years ago, radiological solid size (RSS) was demonstrated to have superior prognostic value than whole tumor size of lung cancers on computed tomography (CT) scan (1,2). Similarly, pathological invasive size (PIS) was reported to be a better predictor of survival than total tumor size in lung adenocarcinoma (3,4). PIS and RSS were supposed to be relevant (5) but there was an absence of consensus (6,7). The eighth edition of tumor node metastasis (TNM) classification suggested that pathological and clinical T-stage should be determined by the maximum PIS and RSS respectively, excluding the lepidic component and ground-glass opacity (GGO) component (8,9).
Lepidic component tended to be corresponded with GGO component (10,11). However, the weak correlation of lepidic ratio and GGO ratio was reported lately (12). Concerns about the correlations between pathological and radiological features are remained. Recently, studies have demonstrated that the presence of GGO component was an independent prognostic factor, regardless of RSS (13-17) and that adenocarcinoma with lepidic component had better survival (9,15,16). Moreover, lepidic present lung adenocarcinoma had excellent survival regardless of pT-stage (18). These findings have provoked controversies about whether lepidic component and GGO component should still be considered in pathological (p)T and clinical (c)T classification.
To adequately address these concerns and controversies, we comprehensively evaluated the correlations between pathological and radiological T descriptors in invasive lung adenocarcinoma and analyzed the prognostic significance of these T descriptors (lepidic component, GGO component, PIS and RSS) on recurrence-free survival (RFS). We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-457/rc).
Methods
Patients and clinicopathological characteristics
We reviewed patients with completely resected lung adenocarcinoma at the Department of Thoracic Surgery, Fudan University Shanghai Cancer Center from January 2017 to December 2019. Patients who received neoadjuvant chemotherapy, patients with pathologically diagnosed adenocarcinoma in situ, minimally invasive adenocarcinoma or adenocarcinoma with mucinous component, patients with previous cancer history or multiple synchronous lung nodules were excluded. Cases without available pathological slide and cases with unclear or missed CT images were not included (Figure S1).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center (IRB2008223-9) and individual consent for this retrospective analysis was waived.
Age at diagnosis, gender, smoking status, surgery approach, lymphovascular invasion (LVI), histological features, CT appearance, pT-stage, cT-stage and N-stage according to the eighth edition TNM classification were collected.
Radiological evaluation
All CT scans were conducted with a 64- or 40-slice multidetector scanner (Siemens Somatom Sensation, Berlin, Germany). The scanning parameters were as follows: pitch, 1.2; section thickness and interval, 5.0 and 5.0 mm, respectively; reconstruction section width and interval, 1.0 and 1.0 mm, respectively; field of view, 375 mm; voltage, 120 kV; and electric charge, 270 mAs.
CT scans were independently reviewed by two experienced radiologists. Consolidation tumor ratio (CTR) was defined as the ratio of the maximum diameter of consolidation to the maximum radiological tumor diameter. GGO ratio was calculated as 1-CTR. Radiological features were categorized into pure GGO (CTR =0), part-solid (0< CTR <1) and solid (CTR =1) according to the last CT scan before surgery. Any discrepancies were re-evaluated and resolved through consensus.
Histopathological assessment
Pathological slides were independently reviewed by two professional pathologists. Histological subtypes were classified as lepidic predominant adenocarcinoma (LPA), acinar predominant adenocarcinoma (APA), papillary predominant adenocarcinoma (PPA), micropapillary predominant adenocarcinoma (MPA) and solid predominant adenocarcinoma (SPA) (19). Predominant pattern was defined when a type of histological component was over 50%. Following this histological classification, included patients were categorized into LPA, lepidic-present ADC (invasive adenocarcinomas with lepidic component) and lepidic-absent ADC (invasive adenocarcinomas without lepidic component) groups.
The ratio of each histological component was recorded in 5% increments. Lepidic ratio was defined as the percentage area of lepidic component to the total area. PIS was defined as the maximum diameter of invasive component. For samples with multiple invasive foci, PIS was calculated as pathological total size multiplied by the ratio of invasive components, following previous studies and 2015 World Health Organization classification (4,19,20).
LVI and lymph node metastasis were confirmed by postoperative pathological slides. Any discrepancies were re-reviewed using microscope and resolved through consensus.
Follow-up strategy
Patients were followed up every 6 months for the first 3 years after the operation, every 8 months for the next 2 years, and every 12 months thereafter. At each follow-up, we routinely conducted chest CT, brain CT or magnetic resonance imaging, bone scanning, and ultrasonography of the abdominal and supraclavicular regions to detect any evidence of local or distant recurrence. Survival information was recorded from the follow-up visits and supplemented by telephone. The last telephone follow-up for all patients in this cohort was performed in August 2022. RFS was defined as the time from the date of surgery to the date of first recurrence and death or last negative follow-up.
Statistical analysis
R Statistical Language (version 3.6.1) were used to analyze these data. Clinicopathological characteristics were compared among groups by Chi-squared test or Fisher’s exact test. Correlation analysis between pathological and radiological features was examined by Chi-squared test. RFS was estimated by Kaplan-Meier method and log-rank test is used to compare the survival curves. Cox regression was used to assess independent prognostic factors for RFS. Variables with P value less than 0.1 in univariable analysis were included in multivariable analysis. All the tests were two-tailed with statistical significance set at P<0.05.
Results
Baseline and clinicopathological characteristics
This study identified 1,490 patients the mean age of 60.4±9.2 years. There were 64 (4.3%) LPA, 488 (32.8%) lepidic-present ADC and 938 (62.9%) lepidic-absent ADC patients. There were 55 (3.7%) pure GGO, 600 (40.3%) part-solid nodules and 835 (56.0%) solid nodules (Table 1).
Table 1
| Variables | Total (n=1,490) | LPA (n=64) | Lepidic-present ADC (n=488) | Lepidic-absent ADC (n=938) | P value |
|---|---|---|---|---|---|
| Age, year | 60.4±9.2 (54.0–67.0) | 60.3±9.5 (55.0–66.8) | 60.6±9.3 (54.0–67.0) | 60.3±9.2 (54.0–67.0) | 0.837 |
| Sex | 0.013* | ||||
| Male | 641 (43.0) | 22 (34.4) | 189 (38.7) | 430 (45.8) | |
| Female | 849 (57.0) | 42 (65.6) | 299 (61.3) | 508 (54.2) | |
| Smoke status | <0.001* | ||||
| Ever | 464 (31.1) | 12 (18.8) | 124 (25.4) | 328 (35.0) | |
| Never | 1,026 (68.9) | 52 (81.3) | 364 (74.6) | 610 (65.0) | |
| Surgery | <0.001* | ||||
| Pneumonectomy | 3 (0.2) | 0 (0) | 0 (0) | 3 (0.3) | |
| Lobectomy | 1,043 (70.0) | 19 (29.7) | 270 (55.3) | 734 (80.4) | |
| Segmentectomy | 159 (10.7) | 11 (17.2) | 85 (17.4) | 63 (6.7) | |
| Wedge | 285 (19.1) | 34 (53.1) | 133 (27.3) | 118 (12.6) | |
| Predominant subtype | <0.001* | ||||
| Lepidic | 64 (4.3) | 64 (100.0) | 0 (0) | 0 (0) | |
| Acinar | 926 (62.1) | 0 (0) | 378 (77.5) | 548 (58.4) | |
| Papillary | 334 (22.4) | 0 (0) | 107 (21.9) | 227 (24.2) | |
| Micropapillary | 44 (3.0) | 0 (0) | 3 (0.6) | 41 (4.4) | |
| Solid | 122 (8.2) | 0 (0) | 0 (0) | 122 (13.0) | |
| Lepidic ratio, % | 9.9±16.6 (0–20.0) | 56.9±8.7 (50.0–60.0) | 22.9±13.5 (10.0–30.0) | 0±0 (0–0) | <0.001* |
| Pathologic total tumor size, mm | 21.2±10.5 (15.0–25.0) | 17.7±4.7 (15.0–20.0) | 17.7±7.3 (13.0–20.0) | 23.3±11.6 (15.0–30.0) | <0.001* |
| Pathologic invasive tumor size, mm | 19.4±11.1 (12.0–25.0) | 7.5±2.2 (6.0–8.0) | 13.7±6.3 (9.1–16.2) | 23.3±11.6 (15.0–30.0) | <0.001* |
| Image | <0.001* | ||||
| Pure GGO | 55 (3.7) | 9 (14.1) | 37 (7.6) | 9 (1.0) | |
| Part-solid | 600 (40.3) | 55 (85.9) | 376 (77.0) | 169 (18.0) | |
| Solid | 835 (56.0) | 0 (0) | 75 (15.4) | 760 (81.0) | |
| GGO ratio, % | 22.2±30.0 (0–43.0) | 68.9±20.3 (57.2–83.5) | 42.3±29.8 (17.0–64.8) | 8.5±2.0 (0–0) | <0.001* |
| Radiologic total tumor size, mm | 22.9±10.9 (15.0–28.0) | 20.2±7.2 (14.0–25.2) | 20.2±8.6 (13.5–25.0) | 24.6±11.9 (16.0–30.0) | 0.032* |
| Radiologic solid size, mm | 18.6±12.7 (9.8–25.0) | 6.1±4.5 (3.0–8.2) | 12.00±8.7 (6.0–16.3) | 22.9±12.8 (14.5–28.7) | <0.001* |
| pT-stage | <0.001* | ||||
| pT1a | 283 (19.0) | 58 (90.6) | 153 (31.3) | 72 (7.7) | |
| pT1b | 680 (45.6) | 6 (9.4) | 271 (55.5) | 403 (43.0) | |
| pT1c | 353 (23.7) | 0 (0) | 55 (11.3) | 298 (31.8) | |
| pT2a | 105 (7.0) | 0 (0) | 6 (1.2) | 99 (10.6) | |
| pT2b | 42 (2.8) | 0 (0) | 2 (0.4) | 40 (4.3) | |
| pT3 | 19 (1.3) | 0 (0) | 1 (0.2) | 18 (1.9) | |
| pT4 | 8 (0.5) | 0 (0) | 0 (0) | 8 (0.9) | |
| cT-stage | <0.001* | ||||
| cTis | 55 (3.7) | 9 (14.7) | 37 (7.6) | 9 (1.0) | |
| cT1mi | 84 (5.6) | 15 (23.4) | 52 (10.7) | 17 (1.8) | |
| cT1a | 282 (18.9) | 30 (46.9) | 161 (33.0) | 90 (9.6) | |
| cT1b | 574 (38.2) | 8 (12.5) | 180 (36.9) | 386 (41.2) | |
| cT1c | 282 (18.9) | 2 (3.1) | 44 (9.0) | 236 (25.2) | |
| cT2a | 117 (7.9) | 0 (0) | 10 (2.0) | 107 (11.4) | |
| cT2b | 65 (4.4) | 0 (0) | 2 (0.4) | 63 (6.7) | |
| cT3 | 26 (1.7) | 0 (0) | 2 (0.4) | 24 (2.6) | |
| cT4 | 6 (0.4) | 0 (0) | 0 (0) | 6 (0.6) | |
| N-stage | <0.001* | ||||
| N0 | 1,259 (84.5) | 64 (100) | 468 (95.9) | 727 (77.5) | |
| N1–2 | 231 (15.5) | 0 (0) | 20 (4.1) | 211 (22.5) | |
| LVI | <0.001* | ||||
| Present | 294 (19.7) | 0 (0) | 31 (6.4) | 263 (28.0) | |
| Absent | 1,196 (80.3) | 64 (100) | 457 (93.6) | 675 (72.0) |
Dara are presented as n (%) or mean ± standard deviation (interquartile range); *, significant difference. LPA, lepidic predominant adenocarcinoma; ADC, adenocarcinoma; GGO, ground-glass opacity; pT, pathologic T; cT, clinic T; LVI, lymphovascular invasion.
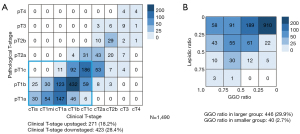
The frequencies of male, ever smoker, lobectomy, N1–2 stage and LVI were all lowest in LPA group and highest in lepidic-absent ADC group. None LPA patient were in p/cT2 stage or higher. Only 9 lepidic-present ADC patients were in pT2a stage or higher and 14 lepidic-present ADC patients were in cT2a stage or higher. There were no LPA, 75 (15.4%) lepidic-present ADCs and 760 (81.0%) lepidic-absent ADCs radiologically featured as solid nodules. Mean GGO ratio was larger than lepidic ratio, especially in lepidic-present ADC group (42.3%±29.8% vs. 22.9%±13.5%) (Table 1).
Correlations between pathological and radiological features
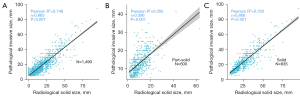
It was obvious that cT-stage was more frequently downstaged than upstaged comparing with the pT-stage (28.4% vs. 18.2%, Figure 1A). We categorized lepidic ratio and GGO ratio into groups by 25% increments and observed that GGO ratio tended to be larger than lepidic ratio (Figure 1B). We then summarized the radiological features of different histological subtypes. All LPA, 47.3% APA, 45.8% PPA, none MPA and none SPA showed GGO component (Table S1). There was a strong correlation between lepidic ratio and GGO ratio (R2=0.748, P<0.001, Figure 2A). The correlation between PIS and RSS in solid nodule was stronger than that in part-solid nodule. (solid: R2=0.750, P<0.001 versus part-solid: R2=0.355, P<0.001, Figure 2B,2C).
Comparative analysis on the prognostic significance of pathological and radiological features
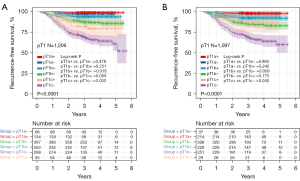
The mean follow-up time was 41.9±10.6 months. We compared the impacts of lepidic component and GGO component on RFS in T1 stage lung adenocarcinoma. LPA and pure GGO patients were excluded because no recurrence events occurred. Patients were divided into “lepidic+ GGO+”, “lepidic− GGO+”, “lepidic+ GGO−” and “lepidic− GGO−” subcategories (+: presence, −: absence). Either in pT1 or cT1, lepidic− GGO+ patients had better RFS than lepidic+ GGO− patients (pT1: P=0.001; cT1: P=0.021). In addition, the survival of lepidic− GGO+ patients were relatively similar to lepidic+ GGO+ patients (pT1: P=0.047; cT1: P=0.065) and lepidic+ GGO− patients did not showed significantly better survival than lepidic− GGO− patients (pT1: P=0.821; cT1: P=0.572) (Figure 3).

Then, we analyzed the impact of lepidic component on RFS stratified by pT1 stage and the impact of GGO component on RFS stratified by cT1 stage. LPA and pure GGO patients were also excluded. In overall pT1 adenocarcinoma, lepidic-present ADCs had better RFS than lepidic-absent ADCs (P<0.001). In overall cT1 adenocarcinoma, part-solid nodules had better RFS than solid nodules (P<0.001) (Figure S2). Lepidic component had significant favorable prognostic impact in pT1b (P=0.018) and pT1c stages patients (P=0.033) (Figure 4A, +: lepidic-presence, −: lepidic-absence). Similarly, GGO component had significant favorable prognostic impact in cT1b (P=0.009) and cT1c stages patients (P=0.040). In addition, RFS of cT1b GGO-present nodule was comparable to cT1a solid nodule (P=0.242), and RFS of cT1c GGO-present nodule was similar to cT1b solid nodule (P=0.755) (Figure 4B, +: GGO-presence, −: GGO-absence).
Univariate and multivariate cox regression analysis for RFS
Cox regression analysis for RFS was performed in T1 adenocarcinoma excluding LPA and pure GGO. In the univariate analysis, lepidic component, GGO component, PIS and RSS were all as significant prognostic factors in either pT1 or cT1 adenocarcinoma. We separately tested pathological factors and radiological factors in the multivariate analysis. GGO component, PIS and RSS were independently significant prognostic factors on RFS for either pT1 or cT1 adenocarcinoma, whereas lepidic component was not an independently prognostic factor on RFS for neither pT1 nor cT1 adenocarcinoma (Tables 2,3). Multivariate analysis also demonstrated that PIS and RSS were independently significant prognostic factors for either pT2–4 or cT2–4 adenocarcinoma (Tables S2,S3). The scheme of all the sub-groups in survival analyses was listed in Table S4.
Table 2
| Characteristics | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Pathological factors | Radiological factors | |||||
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||||
| Age | 1.011 (0.994–1.027) | 0.201 | ||||||
| Sex, male | 0.927 (0.690–1.245) | 0.614 | ||||||
| Smoking, ever | 0.816 (0.587–1.134) | 0.225 | ||||||
| Surgery | ||||||||
| Sublobar resection | Reference | |||||||
| Lobectomy | 1.596 (1.121–2.274) | 0.010 | 0.751 (0.515–1.095) | 0.136 | 0.814 (0.562–1.180) | 0.278 | ||
| LVI, presence | 3.975 (2.965–5.330) | <0.001 | 1.674 (1.199–2.336) | 0.002 | 1.716 (1.237–2.382) | 0.001 | ||
| N-stage | ||||||||
| N0 | Reference | |||||||
| N1–2 | 5.456 (4.055–7.341) | <0.001 | 2.596 (1.839–3.664) | <0.001 | 2.496 (1.777–3.506) | <0.001 | ||
| Lepidic ratio | 0.004 (0.001–0.029) | <0.001 | 0.183 (0.024–1.420) | 0.104 | ||||
| Pathological invasive size | 1.134 (1.108–1.161) | <0.001 | 1.100 (1.070–1.131) | <0.001 | ||||
| GGO ratio | 0.015 (0.005–0.045) | <0.001 | 0.091 (0.027–0.300) | <0.001 | ||||
| Radiological solid size | 1.073 (1.060–1.086) | <0.001 | 1.041 (1.024–1.058) | <0.001 | ||||
| Predominant subtype | ||||||||
| Acinar | Reference | |||||||
| Papillary | 0.983 (0.674–1.433) | 0.927 | 0.918 (0.628–1.341) | 0.657 | 0.962 (0.658–1.405) | 0.840 | ||
| Micropapillary | 3.052 (1.681–5.543) | <0.001 | 1.135 (0.614–2.097) | 0.686 | 0.835 (0.448–1.556) | 0.570 | ||
| Solid | 3.118 (2.057–4.726) | <0.001 | 1.443 (0.935–2.227) | 0.098 | 1.235 (0.800–1.906) | 0.341 | ||
CI, confidence interval; LVI, lymphovascular invasion; GGO, ground-glass opacity.
Table 3
| Characteristics | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Pathological factors | Radiological factors | |||||
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||||
| Age | 1.015 (0.997–1.032) | 0.105 | ||||||
| Sex, male | 1.003 (0.736–1.367) | 0.985 | ||||||
| Smoking, ever | 0.863 (0.613–1.215) | 0.399 | ||||||
| Surgery | ||||||||
| Sublobar resection | Reference | |||||||
| Lobectomy | 1.332 (0.927–1.914) | 0.121 | ||||||
| LVI, presence | 3.541 (2.589–4.845) | <0.001 | 1.685 (1.173–2.419) | 0.005 | 1.644 (1.150–2.349) | 0.006 | ||
| N-stage | ||||||||
| N0 | Reference | |||||||
| N1–2 | 4.716 (3.424–6.497) | <0.001 | 2.399 (1.643–3.505) | <0.001 | 2.227 (1.525–3.253) | <0.001 | ||
| Lepidic ratio | 0.007 (0.001–0.053) | <0.001 | 0.175 (0.022–1.390) | 0.099 | ||||
| Pathological invasive size | 1.099 (1.077–1.120) | <0.001 | 1.071 (1.047–1.095) | <0.001 | ||||
| GGO ratio | 0.022 (0.007–0.071) | <0.001 | 0.173 (0.048–0.619) | 0.007 | ||||
| Radiological solid size | 1.113 (1.088–1.140) | <0.001 | 1.065 (1.035–1.095) | <0.001 | ||||
| Predominant subtype | ||||||||
| Acinar | Reference | |||||||
| Papillary | 1.064 (0.723–1.566) | 0.753 | 0.991 (0.672–1.463) | 0.964 | 1.093 (0.742–1.609) | 0.654 | ||
| Micropapillary | 2.620 (1.274–5.388) | 0.009 | 1.281 (0.611–2.684) | 0.512 | 1.050 (0.500–2.207) | 0.897 | ||
| Solid | 3.012 (1.940–4.676) | <0.001 | 1.535 (0.974–2.420) | 0.065 | 1.497 (0.949–2.361) | 0.083 | ||
CI, confidence interval; LVI, lymphovascular invasion; GGO, ground-glass opacity.
Discussion
In the eighth edition TNM classification, lepidic component and GGO component are no longer considered in T classification because these two descriptors are considered as non-invasiveness component in pathology and radiology (8). This study aimed to evaluate the correlations between pathological and radiological T descriptors and their prognostic significance in invasive lung adenocarcinoma. Studies have reported the relevance between PIS and RSS. Hsu et al. (6) reported PIS was relatively smaller than RSS because it was hard to prevent alveolar collapse after resection. Yanagawa et al. (7) found CT-measured solid size was larger than that of the actual invasive component. In the study of Lee et al., 3D measured solid size showed a tendency to be larger than invasive size, whereas 2D measurements tended to be similar to invasive size (5). In this study, PIS was a little bit larger than RSS (Table 1), and PIS and RSS were strongly correlated.
There have been few studies investigated the distributions of pT-stage and cT-stage of lung adenocarcinoma. We found that cT-stage tended to be downstaged comparing to pT-stage in T1 lung adenocarcinoma. We also observed GGO ratio tended to be larger than lepidic ratio and nearly half of APA and PPA histological subtype featured as GGOs. This further indicated that GGO component represented not only lepidic component but also other invasive histological subtypes. Reasonably, the correlation between PIS and RSS in solid nodule was stronger than that in part-solid nodule. A rising number of GGOs are detected along with the widely using of low-dose CT screening (21). The explanation for larger PIS and downstaged cT-stage was that we collected a large cohort with 44% GGOs.
Based on these findings, we analyzed the prognostic impacts of lepidic component and GGO component. Either in pT1 or cT1 group, lepidic− GGO+ patients were found to have better RFS than lepidic+ GGO− patients. Notably, RFS of lepidic− GGO+ patients were similar to lepidic+ GGO+ patients, and lepidic+ GGO− patients and lepidic− GGO− patients had comparable RFS. Therefore, GGO component was a more effective prognostic predictor than lepidic component. In this study, lepidic− GGO+ adenocarcinomas were APA or PPA with GGO component (Table 2). It is indicated that GGO-featured invasive adenocarcinoma had better survival regardless of lepidic component.
In the whole pT1 adenocarcinoma cohort, lepidic-present ADCs showed better survival. When stratified by T-stages, lepidic-present ADCs also had significant favorable RFS in pT1b and pT1c, but not in pT1a. Okubo et al. (12) found that lepidic-positive ADCs had significantly better RFS in pT1, in addition, the survival curves of lepidic-positive group still showed superior tendency in pT1b and pT1c, but significance differences disappeared. Similarly, we found part-solid nodules had better RFS than solid nodules in overall cT1 adenocarcinoma and GGO component had significant favorable prognostic impact in cT1b and cT1c stages. Ye et al. (22) demonstrated part-solid tumors had better RFS than solid tumors in same cT-stage (cT1b and cT1c). Fan et al. (23) reported that the prognosis of GGO lesions exceeding 3 cm were better than that of solid lesions in the same cT category.
The prognostic value of GGO component besides solid component leads to concerns about whether lepidic component is also a prognostic factor for survival regardless of invasive component. Zhu et al. (18) reported lepidic presence was a prognostic factor independent from pT-stage. In our study, PIS and RSS were demonstrated to be independent prognostic factors for RFS of invasive lung adenocarcinoma. To note, GGO ratio was demonstrated to be an independent prognostic factor for RFS in T1 adenocarcinoma, but lepidic ratio was not.
Nearly half APA and PPA presenting GGO component indicated that GGO component does not entirely correspond to lepidic component, the superior survival of lepidic-absent invasive adenocarcinoma with GGO component probably blunts the favorable prognostic value of lepidic component. These facts indicate a possibility that the malignancy of invasive histological subtypes featured as GGO is not as severe as those featured as solid. And it might be the reason why the radiological assessment of the GGO component was more valuable for RFS than the pathological assessment of the lepidic component. Extensive resection and timely postoperative follow-up are more needed for adenocarcinoma without GGO component.
Hattori et al. (13) suggested that GGO-present adenocarcinoma should be classified as cT1a stage. We found that RFS of cT1b GGO-present nodule was comparable to cT1a solid nodule and better than cT1b solid nodule, meanwhile, cT1c GGO-present nodule had similar survival to cT1b solid nodule and favorable prognosis than cT1c solid nodule. Hence, we recommend that cT1b and cT1c adenocarcinoma with GGO component should be classified into cT1a and cT1b, respectively. Similarly, pT1b and pT1c adenocarcinoma with lepidic component could be considered to be classified into pT1a and pT1b, respectively.
This study demonstrated that PIS and RSS were two highly correlated parameters and reliable for current T classification, GGO component showed stronger prognostic significance than lepidic component. A relatively large simple size of pathologically invasive adenocarcinoma was collected and those with mucinous component were excluded to get rid of the influence in survival (24,25). Besides, LPA and pure GGO were not involved in survival analysis to reduce bias.
The limitations of this study are that this is a single-center retrospective research and 41.9 months is a relatively short mean follow-up time. Besides, positron emission tomography-computed tomography (PET-CT) is rarely performed because the expensive cost is not covered by fundamental medical insurance in China. Another limitation is the lack of data of PET-CT. Studies on pathological and radiological T descriptors are warranted to offer more evidence for future T classification.
Conclusions
Among T1 invasive lung adenocarcinoma, GGO ratio showed independent prognositic value for RFS, regardless of RSS. Meanwhile, lepidic ratio was not an independent RFS factor. GGO component rather than lepidic component should be considered as an additional T descriptor.
Acknowledgments
The abstract of this study was presented as a poster in 2023 American Association for Thoracic Surgery International Thoracic Surgical Oncology Summit.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-457/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-457/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-457/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-457/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center (IRB2008223-9) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDeri
References
- Maeyashiki T, Suzuki K, Hattori A, et al. The size of consolidation on thin-section computed tomography is a better predictor of survival than the maximum tumour dimension in resectable lung cancer. Eur J Cardiothorac Surg 2013;43:915-8. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: a multicenter study. J Thorac Cardiovasc Surg 2012;143:607-12. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Mimae T, et al. The prognostic role of pathologic invasive component size, excluding lepidic growth, in stage I lung adenocarcinoma. J Thorac Cardiovasc Surg 2013;146:580-5. [Crossref] [PubMed]
- Kameda K, Eguchi T, Lu S, et al. Implications of the Eighth Edition of the TNM Proposal: Invasive Versus Total Tumor Size for the T Descriptor in Pathologic Stage I-IIA Lung Adenocarcinoma. J Thorac Oncol 2018;13:1919-29.
- Lee KH, Goo JM, Park SJ, et al. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol 2014;9:74-82. [Crossref] [PubMed]
- Hsu PK, Huang HC, Hsieh CC, et al. Effect of formalin fixation on tumor size determination in stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:1825-9. [Crossref] [PubMed]
- Yanagawa M, Kusumoto M, Johkoh T, et al. Radiologic-Pathologic Correlation of Solid Portions on Thin-section CT Images in Lung Adenocarcinoma: A Multicenter Study. Clin Lung Cancer 2018;19:e303-12. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- Aokage K, Yoshida J, Ishii G, et al. Identification of early t1b lung adenocarcinoma based on thin-section computed tomography findings. J Thorac Oncol 2013;8:1289-94. [Crossref] [PubMed]
- Okubo Y, Kashima J, Teishikata T, et al. Prognostic Impact of the Histologic Lepidic Component in Pathologic Stage IA Adenocarcinoma. J Thorac Oncol 2022;17:67-75. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Prognostic impact of a ground glass opacity component in the clinical T classification of non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:2102-2110.e1. [Crossref] [PubMed]
- Aokage K, Miyoshi T, Ishii G, et al. Influence of Ground Glass Opacity and the Corresponding Pathological Findings on Survival in Patients with Clinical Stage I Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:533-42. [Crossref] [PubMed]
- Miyoshi T, Aokage K, Katsumata S, et al. Ground-Glass Opacity Is a Strong Prognosticator for Pathologic Stage IA Lung Adenocarcinoma. Ann Thorac Surg 2019;108:249-55. [Crossref] [PubMed]
- Hattori A, Hirayama S, Matsunaga T, et al. Distinct Clinicopathologic Characteristics and Prognosis Based on the Presence of Ground Glass Opacity Component in Clinical Stage IA Lung Adenocarcinoma. J Thorac Oncol 2019;14:265-75. [Crossref] [PubMed]
- Hattori A, Suzuki K, Takamochi K, et al. Prognostic impact of a ground-glass opacity component in clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;161:1469-80. [Crossref] [PubMed]
- Zhu E, Dai C, Xie H, et al. Lepidic component identifies a subgroup of lung adenocarcinoma with a distinctive prognosis: a multicenter propensity-matched analysis. Ther Adv Med Oncol 2020;12:1758835920982845. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- Zhang Y, Fu F, Chen H. Management of Ground-Glass Opacities in the Lung Cancer Spectrum. Ann Thorac Surg 2020;110:1796-804. [Crossref] [PubMed]
- Ye T, Deng L, Wang S, et al. Lung Adenocarcinomas Manifesting as Radiological Part-Solid Nodules Define a Special Clinical Subtype. J Thorac Oncol 2019;14:617-27. [Crossref] [PubMed]
- Fan F, Zhang Y, Fu F, et al. Subsolid Lesions Exceeding 3 Centimeters: The Ground-Glass Opacity Component Still Matters. Ann Thorac Surg 2022;113:984-92. [Crossref] [PubMed]
- Lee HY, Cha MJ, Lee KS, et al. Prognosis in Resected Invasive Mucinous Adenocarcinomas of the Lung: Related Factors and Comparison with Resected Nonmucinous Adenocarcinomas. J Thorac Oncol 2016;11:1064-73. [Crossref] [PubMed]
- Luo J, Wang R, Han B, et al. Analysis of the clinicopathologic characteristics and prognostic of stage I invasive mucinous adenocarcinoma. J Cancer Res Clin Oncol 2016;142:1837-45. [Crossref] [PubMed]


