
Impact of chronic obstructive pulmonary disease on lung cancer symptom burden: a population-based study in Ontario, Canada
Highlight box
Key findings
• Lung cancer patients with underlying chronic obstructive pulmonary disease (COPD) have worse symptom burden compared to lung cancer patients without COPD.
What is known and what is new?
• COPD and lung cancer have a significant overlap in risk factors and symptoms.
• We found that the lung cancer patients with COPD were more likely to experience any moderate to severe symptom, multiple moderate to severe symptoms and have worse symptom distress scores.
What is the implication, and what should change now?
• Our findings support the need to develop and test interventions that can alleviate symptom burden and also support the consideration of and investigation for COPD among lung cancer patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is very common among patients with lung cancer and is associated with poor prognosis (1). Even though these two diseases commonly co-occur and have a significant overlap in symptoms, little attention has been given as to how the additional disease burden of COPD impacts the severity, number, or type of symptoms that lung cancer patients experience. Prior studies among this population have focused on respiratory symptoms, demonstrating that dyspnea is negatively correlated with quality of life and functional status among lung cancer patients (2) and respiratory symptoms are more common among patients with COPD specifically (3,4). However, the generalizability of these studies is limited due to being conducted in single centres, with small sample sizes, and lack of a comprehensive symptom burden assessment which includes non-respiratory symptoms that are common among patients with COPD or lung cancer such as pain, fatigue, and poor mental health (5-10). Furthermore, little attention has been given to undiagnosed COPD, which can also be symptomatic (11,12). Undiagnosed COPD is very common among individuals at risk for (13,14) or with lung cancer (15,16). To better guide clinical care, it is pertinent to understand the symptom management needs of lung cancer patients with COPD, including those with undiagnosed or newly diagnosed disease. For example, patients with significant symptom burden may benefit from early integration of supportive care and optimization of their COPD-related care.
Understanding the prevalence of symptoms and various combinations thereof, is important to identify the need for symptom management among this population. This can be accomplished through the use of patient-reported outcome measures (PROMs). Cancer centres in Ontario, Canada, routinely assess symptoms via the Edmonton Symptom Assessment Scale (ESAS) which includes nine symptoms (17). Through combining these PROMs with health administrative data, we evaluated symptom burden in a large-scale population-based study. We aimed to determine if underlying COPD—previously diagnosed or newly diagnosed—increases the collective symptom burden in lung cancer patients, through examining the type, severity, number of symptoms, and total symptom distress scores. We hypothesized that lung cancer patients with COPD have more severe symptom burden compared to lung cancer patients without COPD. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-560/rc).
Methods
Study design
We conducted a population-based cross-sectional study in Ontario, Canada, where the population exceeds 14 million people. This project was approved by the University of Toronto Health Sciences Research Ethics Board (protocol #40390) and individual consent for this retrospective analysis was waived. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Population
The cohort included all individuals aged 18 and over in Ontario who were diagnosed with lung cancer between January 1, 2009 and December 31, 2019 (International Classification of Diseases for Oncology (ICD-O) code C34, morphology codes available in Table S1) and had a complete the ESAS questionnaire within 90 days of diagnosis. We excluded individuals who had missing information on cancer stage or covariates, as well as those with stage 0 or occult lung cancer. Individuals were followed from the date of lung cancer diagnosis for up to 90 days to observe completion of the ESAS questionnaire, with a maximum follow-up date of March 31, 2020.
Data sources
Provincial health administrative databases and disease registries that capture information about Ontario residents were used in this study. These datasets were linked using unique encoded identifiers and analyzed at ICES. ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. Detailed information on the databases available and the procedures at ICES can be found here: https://www.ices.on.ca/.
The population-based Ontario Cancer Registry (OCR) contains information on all cancers diagnosed in Ontario, including information on cancer stage and histological subtype. The Canadian Institute of Health Information (CIHI), Discharge Abstract Database (DAD), the National Ambulatory Care Reporting System (NACRS) Database, and the Ontario Health Insurance Plan (OHIP) Physician Claims database cover virtually all hospitalizations, emergency department, or outpatient physician visits in Ontario, Canada. The Registered Persons Database (RPDB) captures demographic information. The Immigration, Refugees and Citizenship Canada’s (IRCC) permanent resident database captures immigration to Canada since 1985. The Symptom Management Reporting Database (SMRD) contains scores for the ESAS questionnaire, which is used in clinical practice at cancer centres in Ontario, Canada to measure symptom severity and distress in cancer patients. The Ontario Drug Benefit (ODB) database contains information on prescription medications for residents aged 65 or over.
Main exposure: COPD
A previously validated case definition was used to identify individuals with physician-diagnosed COPD using health administrative data. This case definition of at least one ambulatory claim or hospitalization for COPD based on ICD-9 codes 491, 492 or 496 and ICD-10 codes J41, J42, J43 or J44 (18) has been shown to have 85% sensitivity and 78% specificity compared to a clinical reference standard (18) and has been used previously to study COPD in population-based studies (19,20). People diagnosed with COPD >90 days prior to lung cancer were considered to have ‘previously diagnosed COPD’. Individuals diagnosed with COPD within 90 days prior to, on the day of, or up to 90 days after lung cancer diagnosis, were considered to have ‘newly diagnosed COPD’.
Outcomes
The ESAS (21-23) is self-administered via an electronic device. Patients rate the severity of nine symptoms; anxiety, depression, drowsiness, lack of appetite, nausea, pain, shortness of breath, tiredness, and wellbeing. The numerical rating scale ranges from 0 (none) to 10 (worst possible). If patients completed the ESAS more than once during the 90-day window, the earliest ESAS completion was used. A score of ≥4 was indicative of a moderate to severe rating (24). A total symptom distress score (out of 90) was also calculated by summing the individual ratings of the nine symptoms.
The primary outcome was the prevalence of moderate to severe symptoms. Secondary outcomes included the number of moderate to severe symptoms and the total symptom distress score.
Covariates
Demographic variables included: age, sex, residing in a rural area (<10,000 residents), neighbourhood income quintile for urban areas, immigration status [recent immigrants to Canada (≤10 years), long-term residents (>10 years) or non-immigrants since 1985], region of Ontario [five regions based on the Local Health Integration Networks (LHINs): Toronto, Central, East, North, and West]. The type of lung cancer [non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), or unspecified], and stage of lung cancer (early: I/II, advanced: III/IV). Stage of lung cancer was ascertained using the ‘Best stage’ variable in the OCR, which uses a collaborative method consistent with the tumour, lymph node, metastasis (TNM) international staging system (25). Comorbidities were ascertained from the John Hopkins ACG System® Version 10.0, using the Aggregated Diagnostic Groups (ADGs). Individuals were categorized as having high (≥10 ADGs) or low comorbidity (<10 ADGs). Additionally, validated algorithms were used to identify individuals with asthma (26) and dementia (since April 1996) (27), and congestive heart failure (28) and diabetes (29) (since April 1991).
Statistical analysis
An overall analysis that considered all stages of lung cancer was followed by stratified analyses for individuals with early (I/II) or advanced (III/IV) stage. For the primary outcome, first descriptive statistics were used to compare the prevalence of moderate to severe symptoms among the disease groups. A standardized mean difference (SMD) >0.1 was considered statistically significant. Second, unadjusted and multivariable modified Poisson regression models were used to assess the impact of COPD (previously diagnosed and newly diagnosed) on the relative risk (RR) of reporting any moderate to severe symptom. Multivariable regression analyses were adjusted for the following covariates; sex, age, immigration status, rurality and urban income quintile, and comorbidities using the Hopkins ADGs.
In secondary analysis, unadjusted and multivariable negative binomial regression models were used to assess the impact of COPD on the relative rate of number of moderate to severe symptoms, and unadjusted and multivariable linear regression models were used to assess the impact of COPD on the total symptom distress score.
Additional analyses
Among individuals with COPD, we also further stratified by ‘advanced COPD’ and ‘non-advanced COPD’ to address the impact of disease severity on the risk of moderate to severe symptoms. Individuals were determined to have advanced COPD if they met one of the following criteria; one or more hospitalizations within the previous year or after index, with a most-responsible diagnosis for COPD-related respiratory disease (ICD-10 codes J10-18, J20, J22, J40, J42-J44) or receipt of long-term oxygen therapy (30). To account for the potential misclassification of COPD, we performed a sensitivity analysis using a stricter case definition (two or more outpatient claims or at least one hospitalization for COPD within a 2-year period prior to lung cancer diagnosis) which has higher specificity (91.5%) (18). We also assessed the impact of COPD medications on reporting any moderate to severe symptom through a sensitivity analysis of individuals with previously diagnosed COPD who were aged 66 or above. In Ontario, medication data is only available for individuals aged 65 and above and we included a look-back period to capture medications prescribed within one-year of lung cancer diagnosis. Individuals were considered to be taking COPD medications if they had a prescription claim for any of the following classes of medications: long-acting anti-cholinergic (LAAC), long-acting beta agonist (LABA), inhaled corticosteroid (ICS), combination LABA + LAAC, LABA + ICS.
Results
Among 97,797 individuals diagnosed with lung cancer during the study period, 58,899 individuals were excluded (Figure 1). Among those excluded, 153 had stage 0 or occult cancer, 15,068 had missing stage information, 43,599 did not complete the ESAS within 90 days of diagnosis, and 79 had missing covariate information (Figure 1). There were notable differences between individuals who completed the ESAS within 90 days of diagnosis and individuals who did not complete the ESAS within 90 days of diagnosis (Table S2). Individuals who did not completed the ESAS within 90 days of diagnosis were, on average, older, more likely to have early-stage lung cancer or an unspecified type of lung cancer, live in the Toronto region, and have dementia or congestive heart failure. There was no difference in proportions of people by COPD status among those who did and did not complete the ESAS within 90 days of diagnosis.
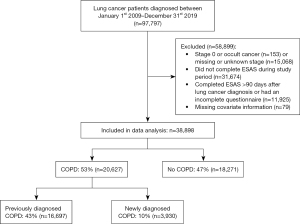
A total of 38,898 lung cancer patients completed the ESAS within 90 days of diagnosis and were included in the study (Figure 1), most of whom were diagnosed with advanced stage lung cancer (77%, n=29,995). The median time from lung cancer diagnosis to completion of ESAS was 28 days [interquartile range (IQR), 16 to 48 days]. Overall, 47% of patients (n=18,271) did not have COPD, 43% (n=16,697) had previously diagnosed COPD, and 10% (n=3,930) had newly diagnosed COPD. On average, the time between COPD and lung cancer diagnosis was 9.7 years (IQR, 4.7 to 16.4 years) for people with previously diagnosed COPD, and +1 day (IQR, −20 to +36 days) for people with newly diagnosed COPD. Table 1 depicts the characteristics of the study cohort.
Table 1
| Characteristics | No COPD (n=18,271) | Previously diagnosed COPD (n=16,697) | Newly diagnosed COPD (n=3,930) | SMD |
|---|---|---|---|---|
| Stage, n (%) | 0.252 | |||
| Early (I/II) | 3,356 (18.40) | 4,791 (28.70) | 756 (19.20) | |
| Advanced (III/IV) | 14,915 (81.60) | 11,906 (71.30) | 3,174 (80.80) | |
| Type of lung cancer, n (%) | ||||
| NSCLC | 15,383 (84.20) | 13,278 (79.50) | 3,207 (81.60) | 0.081 |
| SCLC | 2,164 (11.80) | 2,579 (15.40) | 572 (14.60) | 0.07 |
| Unspecified | 724 (4.00) | 840 (5.00) | 151 (3.80) | 0.039 |
| Age, mean (SD) | 67.4 (11.0) | 70.8 (9.1) | 67.7 (9.6) | 0.231 |
| Sex, n (%) | 0.033 | |||
| Male | 9,268 (50.70) | 8,517 (51.00) | 2,091 (53.20) | |
| Female | 9,003 (49.30) | 8,180 (49.00) | 1,839 (46.80) | |
| Region of Ontario, n (%) | ||||
| Toronto | 1,026 (5.60) | 757 (4.50) | 172 (4.40) | 0.038 |
| Central | 4,327 (23.70) | 2,879 (17.20) | 783 (19.90) | 0.107 |
| East | 5,613 (30.70) | 5,295 (31.70) | 1,041 (26.50) | 0.077 |
| North | 1,609 (8.80) | 1,978 (11.80) | 473 (12.00) | 0.071 |
| West | 5,696 (31.20) | 5,788 (34.70) | 1,462 (37.20) | 0.084 |
| Immigration status, n (%) | ||||
| Recent immigrant (≤10 years) | 374 (2.00) | 51 (0.30) | 46 (1.20) | 0.111 |
| Long-term resident (>10 years) | 1,152 (6.30) | 402 (2.40) | 142 (3.60) | 0.129 |
| Non-immigrant | 16,745 (91.60) | 16,244 (97.30) | 3,742 (95.20) | 0.167 |
| Rurality & urban income quintile, n (%) | ||||
| Urban 1 (lowest) | 3,057 (16.70) | 3,598 (21.50) | 753 (19.20) | 0.082 |
| Urban 2 | 3,359 (18.40) | 3,259 (19.50) | 782 (19.90) | 0.026 |
| Urban 3 | 3,056 (16.70) | 2,710 (16.20) | 620 (15.80) | 0.017 |
| Urban 4 | 3,128 (17.10) | 2,215 (13.30) | 576 (14.70) | 0.072 |
| Urban 5 | 2,977 (16.30) | 1,954 (11.70) | 511 (13.00) | 0.088 |
| Rural | 2,694 (14.70) | 2,961 (17.70) | 688 (17.50) | 0.054 |
| Comorbidities, n (%) | ||||
| Median number of ADGs (IQR) | 8 (5 to 10) | 9 (7 to 12) | 8 (5 to 10) | 0.297 |
| High (>10 ADGs) | 5,710 (31.30) | 7,877 (47.20) | 1,185 (30.20) | 0.236 |
| Asthma | 1,475 (8.10) | 4,136 (24.80) | 369 (9.40) | 0.309 |
| Dementia | 377 (2.10) | 541 (3.20) | 59 (1.50) | 0.077 |
| Diabetes | 4,238 (23.20) | 4,811 (28.80) | 878 (22.30) | 0.099 |
| Congestive heart failure | 1,069 (5.90) | 2,486 (14.90) | 290 (7.40) | 0.201 |
COPD, chronic obstructive pulmonary disease; SMD, standardized mean difference; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; SD, standard deviation; ADGs, Aggregated Diagnosis Groups; IQR, interquartile range.
Prevalence of moderate or severe symptoms
Most patients (80%, n=30,994) reported at least one moderate to severe symptom within 90 days of lung cancer diagnosis. More patients with advanced-stage lung cancer reported moderate to severe symptoms (82%, n=24,618), however many patients with early-stage disease also reported moderate to severe symptoms (72%, n=6,376). Among patients with previously diagnosed COPD, 82% reported at least one moderate or severe symptom, with tiredness (54%), poor wellbeing (49%) and shortness of breath (46%) being most common (Figure 2A). Similarly, 81% of patients with newly diagnosed COPD reported at least one moderate to severe symptom, with moderate to severe tiredness (53%), poor wellbeing (49%) and shortness of breath (43%) being the most common (Figure 2A). A total of 77% of patients without COPD reported at least one moderate to severe symptom. Moderate to severe tiredness (50%) and poor wellbeing (48%) were also common among patients without COPD and fewer patients reported moderate to severe shortness of breath (34%) (Figure 2A).
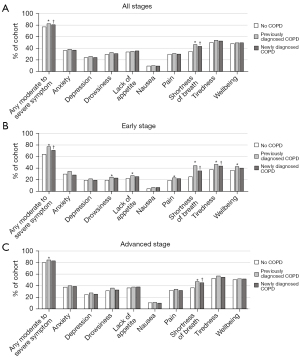
Differences in the prevalence of moderate to severe symptoms with respect to COPD status were greatest among early-stage patients (Figure 2B). Compared to early-stage patients without COPD, more patients with previously diagnosed COPD reported moderate to severe drowsiness, lack of appetite, pain, shortness of breath, tiredness, and poor wellbeing (SMD >0.1) (Figure 2B). Among early-stage patients with newly diagnosed COPD, only moderate to severe shortness of breath and tiredness were more common compared to patients without COPD (SMD >0.1) (Figure 2B). In advanced stage lung cancer, moderate to severe shortness of breath was the only symptom which was more prevalent among patients with COPD (previously diagnosed and newly diagnosed) compared to patients without COPD (SMD >0.1) (Figure 2C).
The results of the modified Poisson regression analyses are reported in Table 2. After adjusting for confounders, patients with previously diagnosed COPD had a higher risk of experiencing any moderate to severe symptom compared to patients without COPD (RR: 1.08, 95% CI: 1.07 to 1.09). The effect was greatest among patients with early-stage lung cancer (RR: 1.18, 95% CI: 1.15 to 1.22). The risk of experiencing any moderate to severe symptom was also slightly elevated for patients with newly diagnosed COPD compared to patients without COPD (RR: 1.05, 95% CI: 1.03 to 1.07).
Table 2
| Variables | Relative risk (95% CI) | |
|---|---|---|
| Unadjusted | Adjusted* | |
| Overall (all stages) | ||
| Previously diagnosed COPD | 1.07 (1.06 to 1.08) | 1.08 (1.07 to 1.09) |
| Newly diagnosed COPD | 1.05 (1.03 to 1.07) | 1.05 (1.03 to 1.07) |
| No COPD | Reference | Reference |
| Early stage | ||
| Previously diagnosed COPD | 1.21 (1.17 to 1.24) | 1.18 (1.15 to 1.22) |
| Newly diagnosed COPD | 1.11 (1.05 to 1.16) | 1.10 (1.04 to 1.15) |
| No COPD | Reference | Ref Reference |
| Advanced stage | ||
| Previously diagnosed COPD | 1.06 (1.05 to 1.07) | 1.04 (1.03 to 1.06) |
| Newly diagnosed COPD | 1.04 (1.02 to 1.06) | 1.04 (1.02 to 1.05) |
| No COPD | Reference | Reference |
*, model adjusted for age, sex, type of lung cancer, rurality & urban income quintile, immigration status, region of Ontario, and comorbidity (John Hopkins Aggregated Diagnostic Groups). The overall analysis was also adjusted for lung cancer stage. COPD, chronic obstructive pulmonary disease; CI, confidence interval.
Number of moderate to severe symptoms
Patients experienced a median of three moderate to severe symptoms (IQR, 1 to 5) which was consistent among the exposure groups. The results of the negative binomial regression analyses are provided in Table 3. Across all stages and after adjusting for confounding variables, the rate of moderate to severe symptoms was higher among lung cancer patients with COPD compared to patients without COPD. The greatest relative rate was among early-stage patients with previously diagnosed COPD (RR: 1.24, 95% CI: 1.19 to 1.30).
Table 3
| Variables | Relative rate (95% CI) | |
|---|---|---|
| Unadjusted | Adjusted* | |
| Overall (all stages) | ||
| Previously diagnosed COPD | 1.10 (1.08 to 1.12) | 1.13 (1.11 to 1.15) |
| Newly diagnosed COPD | 1.06 (1.03 to 1.09) | 1.07 (1.04 to 1.11) |
| No COPD | Reference | Reference |
| Early stage | ||
| Previously diagnosed COPD | 1.27 (1.21 to 1.33) | 1.24 (1.19 to 1.30) |
| Newly diagnosed COPD | 1.14 (1.05 to 1.24) | 1.13 (1.04 to 1.22) |
| No COPD | Reference | Reference |
| Advanced stage | ||
| Previously diagnosed COPD | 1.10 (1.08 to 1.12) | 1.08 (1.06 to 1.11) |
| Newly diagnosed COPD | 1.05 (1.02 to 1.09) | 1.05 (1.02 to 1.08) |
| No COPD | Reference | Reference |
*, model adjusted for age, sex, type of lung cancer, rurality & urban income quintile, immigration status, region of Ontario, and comorbidity (John Hopkins Aggregated Diagnostic Groups). The overall analysis was also adjusted for lung cancer stage. COPD, chronic obstructive pulmonary disease; CI, confidence interval.
Total symptom distress
Lung cancer patients with previously diagnosed COPD had a median total symptom distress score of 24 (IQR, 12 to 38). Patients with newly diagnosed COPD had a median total symptom distress score of 23 (IQR, 11 to 38) and the median score for patients without COPD was 21 (IQR, 10 to 36). Results of the linear regression analyses are provided in Table 4. The greatest difference in total symptom distress scores was observed among the early-stage cohort, where patients with previously diagnosed COPD scored, on average, 3.60 points higher (95% CI: 2.88 to 4.31) compared to patients without COPD (Table 4).
Table 4
| Variables | Unadjusted | Adjusted* | |||
|---|---|---|---|---|---|
| β (SE) | 95% CI | β (SE) | 95% CI | ||
| Overall (all stages) | |||||
| Previously diagnosed COPD | 2.08 (0.19) | 1.71 to 2.45 | 2.75 (0.19) | 2.37 to 3.12 | |
| Newly diagnosed COPD | 1.49 (0.31) | 0.88 to 2.10 | 1.74 (0.30) | 1.14 to 2.33 | |
| No COPD | Reference | Reference | |||
| Early stage | |||||
| Previously diagnosed COPD | 4.04 (0.36) | 3.33 to 4.74 | 3.60 (0.37) | 2.88 to 4.31 | |
| Newly diagnosed COPD | 2.00 (0.64) | 0.74 to 3.27 | 1.71 (0.64) | 0.46 to 2.96 | |
| No COPD | Reference | Reference | |||
| Advanced stage | |||||
| Previously diagnosed COPD | 2.43 (0.22) | 2.00 to 2.86 | 2.03 (0.23) | 1.59 to 2.47 | |
| Newly diagnosed COPD | 1.45 (0.35) | 0.77 to 2.14 | 1.40 (0.35) | 0.72 to 2.08 | |
| No COPD | Reference | Reference | |||
*, model adjusted for age, sex, type of lung cancer, rurality & urban income quintile, immigration status, region of Ontario, and comorbidity (John Hopkins Aggregated Diagnostic Groups). The overall analysis was also adjusted for lung cancer stage. COPD, chronic obstructive pulmonary disease; ESAS, Edmonton Symptom Assessment Scale; SE, standard error; CI, confidence interval.
Additional analyses
Among individuals with COPD, 23% (n=4,726) met the criteria for advanced COPD (24% of patients with previously diagnosed COPD and 18% of patients with previously undiagnosed COPD). The vast majority of patients with advanced COPD reported at least one moderate to severe symptom (91%, n=4,286). Overall, patients with advanced COPD had a higher risk of reporting any moderate to severe symptom compared to patients without COPD (RR: 1.16, 95% CI: 1.14 to 1.18) (Table 5). The impact of advanced COPD was greatest among patients with early-stage lung cancer (RR: 1.30, 95% CI: 1.24 to 1.35), yet still evident among patients with advanced-stage lung cancer (RR: 1.14, 95% CI: 1.12 to 1.16).
Table 5
| Variables | Relative risk (95% CI) | |
|---|---|---|
| Unadjusted | Adjusted* | |
| Advanced COPD | ||
| Overall (all stages) | ||
| Advanced COPD (n=4,726) | 1.18 (1.16 to 1.20) | 1.16 (1.14 to 1.18) |
| Non-advanced COPD (n=15,901) | 1.03 (1.02 to 1.04) | 1.05 (1.03 to 1.06) |
| No COPD (n=18,271) | Reference | Reference |
| Early stage | ||
| Advanced COPD (n=939) | 1.33 (1.28 to 1.39) | 1.30 (1.24 to 1.35) |
| Non-advanced COPD (n=4,608) | 1.16 (1.13 to 1.20) | 1.14 (1.11 to 1.18) |
| No COPD (n=3,356) | Reference | Reference |
| Advanced stage | ||
| Advanced COPD (n=3,787) | 1.15 (1.13 to 1.17) | 1.14 (1.12 to 1.16) |
| Non-advanced COPD (n=11,293) | 1.02 (1.01 to 1.03) | 1.01 (1.00 to 1.02) |
| No COPD (n=14,915) | Reference | Reference |
| Previously diagnosed COPD (age ≥66) | ||
| Overall (all stages) | ||
| COPD medication (n=7,161) | 1.08 (1.06 to 1.10) | 1.09 (1.07 to 1.10) |
| No COPD medication (n=4,741) | Reference | Reference |
| Early stage | ||
| COPD medication (n=2,457) | 1.13 (1.08 to 1.17) | 1.12 (1.08 to 1.16) |
| No COPD medication (n=1,299) | Reference | Reference |
| Advanced stage | ||
| COPD medication (n=4,704) | 1.08 (1.06 to 1.10) | 1.07 (1.05 to 1.09) |
| No COPD medication (n=3,442) | Reference | Reference |
| COPD case definition with higher specificity | ||
| Overall (all stages) | ||
| COPD (n=11,104) | 1.08 (1.07 to 1.09) | 1.09 (1.08 to 1.10) |
| No COPD (n=27,794) | Reference | Reference |
| Early stage | ||
| COPD (n=3,506) | 1.20 (1.17 to 1.23) | 1.18 (1.15 to 1.21) |
| No COPD (n-5,397) | Reference | Reference |
| Advanced stage | ||
| COPD (n=7,598) | 1.06 (1.05 to 1.08) | 1.05 (1.04 to 1.06) |
| No COPD (n=22,397) | Reference | Reference |
*, models adjusted for age, sex, type of lung cancer, rurality & urban income quintile, immigration status, region of Ontario, and comorbidity (John Hopkins Aggregated Diagnostic Groups). The overall analysis was also adjusted for lung cancer stage. COPD, chronic obstructive pulmonary disease; CI, confidence interval.
Among individuals with previously diagnosed COPD, 11,902 were aged 66 and over and had medication data available, of which 60.2% were prescribed COPD medication in the year prior to lung cancer diagnosis. Among this subgroup, individuals prescribed COPD medication had a higher risk of reporting any moderate to severe symptom compared to patients who were not prescribed COPD medication (RR: 1.09, 95% CI: 1.07 to 1.10) (Table 5).
When using a case definition with higher specificity to account for potential misclassification of COPD, our findings were similar to the primary analysis (Table 5).
Discussion
In this population-based study, we utilized PROMs to understand the impact of underlying COPD on symptom burden among lung cancer patients. We found that lung cancer patients with COPD experienced more severe symptom burden. This association was most evident among patients with early-stage lung cancer, and not as pronounced in patients with advanced stage lung cancer. Previously diagnosed COPD had the greatest impact on symptom burden, however even individuals with newly diagnosed COPD had worse symptom burden compared to individuals without COPD. These findings speak to the importance of considering comorbid respiratory disease when caring for lung cancer patients and the need for interventions that can effectively alleviate symptom burden for patients with underlying COPD.
Our findings were largely driven by differences in symptoms with the greatest overlap between lung cancer and COPD (e.g., tiredness and shortness of breath). Even patients with newly diagnosed COPD had worse shortness of breath and, in the early-stages, worse tiredness compared to patients without COPD. This finding supports the consideration of and investigation for COPD among lung cancer patients who are symptomatic. It is important to note that patients with ‘newly diagnosed COPD’ were likely diagnosed with COPD due to the clinical assessment for lung cancer and presumably their COPD may have remained undiagnosed otherwise. It is commonly assumed that ‘undiagnosed’ COPD is mild and not clinically meaningful. However, our findings show that individuals with ‘undiagnosed’ COPD (or in our case, ‘newly diagnosed’ COPD) are symptomatic, which is consistent with prior studies (11-13). Given that nearly half of patients with newly diagnosed COPD reported moderate to severe shortness of breath and tiredness, it is pertinent to ensure that these patients are receiving COPD-related care in addition to their lung cancer treatment. For lung cancer patients with comorbid COPD, integrating respirology care has been shown to improve guideline-based management of COPD (31), however, the impact of this approach on symptom burden remains unclear. When compared to usual care, integrated respirologist management for lung cancer patients with COPD, with an emphasis on inhaled therapy, showed non-significant trends towards improvement in dyspnea and fatigue with no difference in global quality of life scores (32). The results of our sensitivity analysis suggest COPD medication heightened the risk of reporting any moderate to severe symptom however this may be a reflection of inappropriate treatment, or simply that individuals with more severe COPD were more likely to be prescribed COPD medication. Further studies are needed to determine whether integrated approaches to caring for patients with lung cancer and COPD can improve patient-oriented outcomes. Additional comorbidities should also be considered when designing interventions to alleviate symptom burden among this population.
A higher comorbidity index is associated with reporting moderate to severe symptoms among lung cancer patients (9,10). In our study, individuals with previously diagnosed COPD had a higher comorbidity index and were more likely to have asthma and congestive heart failure compared to individuals with newly diagnosed COPD or individuals without COPD, which may have contributed to their collective symptom burden. Additionally, lung cancer treatment or medications for other chronic diseases can elicit symptoms such as drowsiness or lack of appetite and this may have influenced our results. To our knowledge, only two studies (3,4) have reported on non-respiratory symptoms among lung patients with and without COPD and did not find any significant differences in the prevalence of pain or fatigue, however these studies did not consider the symptom severity or stratify by stage which may explain our conflicting results. Regardless of the complex relationship between lung cancer symptoms and comorbidities, our findings suggest that attention should also be given to non-respiratory symptoms, such as drowsiness, lack of appetite, pain, and tiredness when caring for early-stage lung cancer patients with comorbid COPD. Their overall well-being should also be considered.
Since COPD has the greatest impact on symptom burden in the early-stages of lung cancer, our findings support the need for early and ongoing symptom monitoring and management of symptoms among this population. Prior research has shown that symptoms continue to persist over time among lung cancer patients (9,10), emphasizing the need for routine symptom monitoring among this population. Since the implementation of routine symptom monitoring with the ESAS questionnaire by Cancer Care Ontario in 2007, the proportion of cancer patients being screened for symptoms has been steadily increasing (33). Yet differences between ESAS completers and non-completers are evident (9,10,34). In our study, regional differences were apparent among ESAS completers and non-completers, but there were also additional disparities, including fewer patients with early-stage disease. This emphasizes the need for earlier integration of symptom monitoring in lung cancer care. Routine symptom monitoring with the ESAS in Ontario has been associated with reduced healthcare utilization and improved survival among cancer patients (35,36). Additional benefits of PROM feedback interventions include improved physician-patient communication and quality of life, albeit there appears to be no impact on symptom burden (37). However, PROMs can still be implemented successfully into clinical practice and serve as indicators of when to escalate care. In Ontario, efforts should be undertaken to have universal completion of symptom screening by all cancer patients, early in their disease trajectory. Cancer centres or hospitals outside of this province should also consider early implementation of symptom screening to quickly identify and manage patient’s needs.
Among the strengths of this study, the most notable is the large sample size and inclusive, population-based, real-world approach. We assessed symptom burden using a validated questionnaire, including reporting the prevalence of specific symptoms as well as using regression analyses to account for potential confounders impacting symptom burden. Additionally, we stratified by previously diagnosed COPD and newly diagnosed COPD to provide insights into the needs of these unique populations.
Although our sample size was very large, the generalizability of our findings is limited by the exclusion of lung cancer patients with missing stage information and those who did not complete the ESAS within 90 days of lung cancer diagnosis, which was a significant proportion of the population. We chose to focus on symptoms within 90 days of diagnosis to compare symptoms at a similar time point for all patients and because early identification of symptoms can be used to guide clinical decisions. We also restricted the population to those with stage information which allowed us to identify important differences in symptom burden with respect to lung cancer stage. Patients with missing stage information were on average, older, more likely to live in Central Ontario, had a higher comorbidity index and were more likely to have dementia, congestive heart failure. Individuals with missing stage were also more likely to have previously diagnosed COPD but less likely to have newly diagnosed COPD. If individuals with more severe COPD, and subsequently more severe symptom burden, were excluded, this means our study may underestimate the impact of COPD on symptom burden. Another limitation of our study was the inability to ascertain smoking status or history and thus we could not address its impact on symptom burden. Additionally, we were unable to stratify by the severity of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (38) due to a lack of spirometry results in our databases. However, we stratified by advanced and non-advanced COPD with a definition used previously by our team to identify individuals in need of palliative care (30). Findings were consistent in this stratified analysis and suggested that advanced COPD had an even greater impact on symptom severity. Our results also reflect a real-world population since spirometry is widely underutilized (39) and there are significant barriers to spirometry testing (39,40). Using health administrative data to identify individuals also introduces the possibility of misclassification of COPD. We also addressed this limitation by using a case definition with higher specificity and found consistent results with our primary analysis.
The findings of our cohort study suggest differences in symptom burden among patients with and without COPD around the time of lung cancer diagnosis in a real-world setting. However, given that time of ESAS completion was not uniform among the population, we cannot comment on whether these differences are affected by lung cancer treatment or persist in the survivorship stage. It is also difficult to ascertain the cause of the symptoms (e.g., lung cancer, COPD, other comorbidities or life situations). We also cannot comment on the impact of symptom burden on the quality of life for lung cancer patients with and without COPD or the impact on healthcare utilization or mortality, which are important directions that should be explored further. Future studies should also consider incorporating the patient experience, since the symptom which is rated as most severe on the ESAS does not always correlate with what patients perceive to be the most bothersome symptom (41). Our findings also support the need for early symptom monitoring among lung cancer patients and evaluating interventions which integrate respiratory care for patients with comorbid COPD to alleviate symptom burden.
Conclusions
Symptom burden is worse among lung cancer patients with underlying COPD. There is a need to develop and test interventions that aim to alleviate symptom burden among this population.
Acknowledgments
The authors would like to thank Drew Wilton, Senior Research Analyst at ICES, for his expertise and assistance. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from ©Canada Post Corporation and Statistics Canada. Parts of this material are based on data and information compiled and provided by: the Canadian Institute for Health Information (CIHI), the Ontario Ministry of Health, Ontario Health (OH), and IRCC current to March 2020, Statistics Canada. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. We thank IQVIA Solutions Canada Inc. for use of their Drug Information File.
Funding: This study received funding from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-560/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-560/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-560/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-560/coif). S.J.B. and A.S.G. report that this study received Godfrey S. Pettit Block Term Grant from the Division of Respirology at the University of Toronto. S.J.B. received stipend support from the Canadian Institutes of Health Research. A.V.L. has received speaker fees from AstraZeneca and is a member of the advisory board, and has received clinician-scientist funding from the MOH via the Ontario Association of Radiation Oncologists. A.V.L. serves as an unpaid editorial board member of Translational Lung Cancer Research from November 2022 to October 2024. L.P. has received clinician-scientist funding from the MOH via the Ontario Association of Radiation Oncologists. A.S.G. has received funding support from the Government of Ontario and the PSI Foundation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This project was approved by the University of Toronto Health Sciences Research Ethics Board (protocol #40390) and individual consent for this retrospective analysis was waived. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology 2016;21:269-79. [Crossref] [PubMed]
- Mohan A, Singh P, Singh S, et al. Quality of life in lung cancer patients: impact of baseline clinical profile and respiratory status. Eur J Cancer Care (Engl) 2007;16:268-76. [Crossref] [PubMed]
- Yi YS, Ban WH, Sohng KY. Effect of COPD on symptoms, quality of life and prognosis in patients with advanced non-small cell lung cancer. BMC Cancer 2018;18:1053. [Crossref] [PubMed]
- Yuan L, Guo T, Hu C, et al. Clinical characteristics and gene mutation profiles of chronic obstructive pulmonary disease in non-small cell lung cancer. Front Oncol 2022;12:946881. [Crossref] [PubMed]
- Lee AL, Harrison SL, Goldstein RS, et al. Pain and its clinical associations in individuals with COPD: a systematic review. Chest 2015;147:1246-58. [Crossref] [PubMed]
- Ebadi Z, Goërtz YMJ, Van Herck M, et al. The prevalence and related factors of fatigue in patients with COPD: a systematic review. Eur Respir Rev 2021;30:200298. [Crossref] [PubMed]
- Zareifopoulos N, Bellou A, Spiropoulou A, et al. Prevalence, Contribution to Disease Burden and Management of Comorbid Depression and Anxiety in Chronic Obstructive Pulmonary Disease: A Narrative Review. COPD 2019;16:406-17. [Crossref] [PubMed]
- Kuon J, Vogt J, Mehnert A, et al. Symptoms and Needs of Patients with Advanced Lung Cancer: Early Prevalence Assessment. Oncol Res Treat 2019;42:650-9. [Crossref] [PubMed]
- Hirpara DH, Gupta V, Davis LE, et al. Severe symptoms persist for Up to one year after diagnosis of stage I-III lung cancer: An analysis of province-wide patient reported outcomes. Lung Cancer 2020;142:80-9. [Crossref] [PubMed]
- Tjong MC, Doherty M, Tan H, et al. Province-Wide Analysis of Patient-Reported Outcomes for Stage IV Non-Small Cell Lung Cancer. Oncologist 2021;26:e1800-e1811. [Crossref] [PubMed]
- Çolak Y, Afzal S, Nordestgaard BG, et al. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med 2017;5:426-34. [Crossref] [PubMed]
- Alhabeeb FF, Whitmore GA, Vandemheen KL, et al. Disease burden in individuals with symptomatic undiagnosed asthma or COPD. Respir Med 2022;200:106917. [Crossref] [PubMed]
- Ruparel M, Quaife SL, Dickson JL, et al. Prevalence, Symptom Burden, and Underdiagnosis of Chronic Obstructive Pulmonary Disease in a Lung Cancer Screening Cohort. Ann Am Thorac Soc 2020;17:869-78. [Crossref] [PubMed]
- Bradley C, Alexandris P, Baldwin DR, et al. Measuring spirometry in a lung cancer screening cohort highlights possible underdiagnosis and misdiagnosis of COPD. ERJ Open Res 2023;9:00203-2023. [Crossref] [PubMed]
- Zhang J, Zhou JB, Lin XF, et al. Prevalence of undiagnosed and undertreated chronic obstructive pulmonary disease in lung cancer population. Respirology 2013;18:297-302. [Crossref] [PubMed]
- Butler SJ, Louie AV, Sutradhar R, et al. Association between COPD and Stage of Lung Cancer Diagnosis: A Population-Based Study. Curr Oncol 2023;30:6397-410. [Crossref] [PubMed]
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9.
- Gershon AS, Wang C, Guan J, et al. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD 2009;6:388-94. [Crossref] [PubMed]
- Cho EE, Mecredy GC, Wong HH, et al. Which Physicians Are Taking Care of People With COPD? Chest 2019;155:771-7. [Crossref] [PubMed]
- Gershon AS, Pequeno P, Alberga Machado A, et al. Factors Associated With Nonreceipt of Recommended COPD Medications: A Population Study. Chest 2021;160:1670-80. [Crossref] [PubMed]
- Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000;88:2164-71. [Crossref] [PubMed]
- Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991--2006). Palliat Med 2008;22:111-22. [Crossref] [PubMed]
- Hui D, Bruera E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. J Pain Symptom Manage 2017;53:630-43. [Crossref] [PubMed]
- Selby D, Cascella A, Gardiner K, et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage 2010;39:241-9. [Crossref] [PubMed]
- Cancer Care Ontario. Staging data [Internet]. [cited 2023 Oct 4]. Available from: https://www.cancercareontario.ca/en/data-research/submitting-data/staging-data
- Gershon AS, Wang C, Guan J, et al. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J 2009;16:183-8. [Crossref] [PubMed]
- Jaakkimainen RL, Bronskill SE, Tierney MC, et al. Identification of Physician-Diagnosed Alzheimer's Disease and Related Dementias in Population-Based Administrative Data: A Validation Study Using Family Physicians' Electronic Medical Records. J Alzheimers Dis 2016;54:337-49. [Crossref] [PubMed]
- Schultz SE, Rothwell DM, Chen Z, et al. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can 2013;33:160-6.
- Lipscombe LL, Hwee J, Webster L, et al. Identifying diabetes cases from administrative data: a population-based validation study. BMC Health Serv Res 2018;18:316. [Crossref] [PubMed]
- Gershon AS, Maclagan LC, Luo J, et al. End-of-Life Strategies among Patients with Advanced Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2018;198:1389-96. [Crossref] [PubMed]
- Digby GC, Robinson A. Quality Improvement Initiatives to Optimize the Management of Chronic Obstructive Pulmonary Disease in Patients With Lung Cancer. J Oncol Pract 2017;13:e957-65. [Crossref] [PubMed]
- Gottlieb M, Mellemgaard A, Marsaa K, et al. Optimizing COPD treatment in patients with lung- or head and neck cancer does not improve quality of life - a randomized, pilot, clinical trial. Eur Clin Respir J 2020;7:1731277.
- Barbera L, Lee F, Sutradhar R. Use of patient-reported outcomes in regional cancer centres over time: a retrospective study. CMAJ Open 2019;7:E101-8. [Crossref] [PubMed]
- Mahar AL, Davis LE, Bubis LD, et al. Factors associated with receipt of symptom screening in the year after cancer diagnosis in a universal health care system: a retrospective cohort study. Curr Oncol 2019;26:e8-e16. [Crossref] [PubMed]
- Barbera L, Sutradhar R, Seow H, et al. The impact of routine Edmonton Symptom Assessment System (ESAS) use on overall survival in cancer patients: Results of a population-based retrospective matched cohort analysis. Cancer Med 2020;9:7107-15. [Crossref] [PubMed]
- Barbera L, Sutradhar R, Howell D, et al. Does routine symptom screening with ESAS decrease ED visits in breast cancer patients undergoing adjuvant chemotherapy? Support Care Cancer 2015;23:3025-32. [Crossref] [PubMed]
- Lu SC, Porter I, Valderas JM, et al. Effectiveness of routine provision of feedback from patient-reported outcome measurements for cancer care improvement: a systematic review and meta-analysis. J Patient Rep Outcomes 2023;7:54. [Crossref] [PubMed]
- Agustí A, Celli BR, Criner GJ, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur Respir J 2023;61:2300239. [Crossref] [PubMed]
- Gershon AS, Hwee J, Croxford R, et al. Patient and physician factors associated with pulmonary function testing for COPD: a population study. Chest 2014;145:272-81. [Crossref] [PubMed]
- Yamada J, Lam Shin Cheung J, Gagne M, et al. Barriers and Enablers to Objective Testing for Asthma and COPD in Primary Care: A Systematic Review Using the Theoretical Domains Framework. Chest 2022;161:888-905. [Crossref] [PubMed]
- Li B, Mah K, Swami N, et al. Symptom Assessment in Patients with Advanced Cancer: Are the Most Severe Symptoms the Most Bothersome? J Palliat Med 2019;22:1252-9. [Crossref] [PubMed]


