Is overall survival still the primary endpoint in maintenance non-small cell lung cancer studies? An analysis of phase III randomised trials
An increasing number of trials have evaluated the role of maintenance therapy in the treatment of non-small cell lung cancer (NSCLC) with various results. Taken individually, very few studies have led to an overall survival (OS) benefit; conversely, they invariably obtained a significant progression-free survival (PFS) gain. However, two updated meta-analyses (1,2) demonstrated that a maintenance strategy either with chemotherapy or targeted therapies (erlotinib or gefitinib) significantly improved the survival of NSCLC patients, not progressing after an induction, platinum-based chemotherapy. This is a common scenario, likely related to a high rate of crossover to the maintenance agent or different drugs as second-line therapy, in patients randomised to observation or placebo once they progress. In fact, in the two major randomised trials of switch maintenance therapy [with pemetrexed and erlotinib respectively (3,4)] 67 and 72 per cent of patients in the control arm crossed over to the active agent at progression. This could have diluted the survival benefit with the maintenance therapy and the one and two months in OS gain resulted was not significant. When all the switch maintenance trials were pooled in a meta-analysis, they resulted in a reduced risk of death of 10 and 13 per cent for chemotherapy and molecular-targeted agents according to the above mentioned Zhang meta-analysis (2).
Therefore, the question is: which correlation exists between OS and PFS and survival post-progression, defined as post-progression survival (PPS)? In other words, is the variation of OS with maintenance therapy more closely linked to PFS or PPS, and thus to second-line therapies implemented after progression to the maintenance phase? Which is the optimal endpoint for this type of trial is a matter of debate. In NSCLC (5,6), colorectal and breast cancer in particular (7,8), it has been demonstrated that when the survival post-progression is much longer, the median OS is more strongly correlated with PPS than with PFS, making OS a weak endpoint for phase III trials. This is especially true with targeted agents and in the trials designed in the last decade. In these cases, in few trials a PFS benefit will translate into an OS benefit.
As a result of the survival benefit from second- and even third-line chemotherapy, the routine practice of crossover in clinical trials severely limits the ability to detect an OS advantage of one treatment regimen over another, in particular in a maintenance setting. Therefore, the actual activity of a maintenance drug is probably better captured by progression-free survival (PFS). Improvements in PFS are not affected by survival after crossover or the start of subsequent therapies. To elucidate which is the optimal endpoint of a maintenance treatment in stage IV NSCLC, it is crucial to analyse the correlation existing between OS, PFS and PPS respectively. This correlation has still not been explored in NSCLC maintenance trials, in particular in trials involving biological agents. The effect of therapies instituted after disease progression on median OS in these clinical trials is thus of interest. However, little is known about PPS in stage IV NSCLC after a maintenance therapy.
In the present review, the median OS of modern (chemotherapy or molecular-targeted agents), phase III, and maintenance trials for patients with NSCLC not-progressing after 4-6 cycles of platinum-based therapy were divided into PFS and PPS. The correlation of each of them with OS was then assessed through a regression analysis. Evaluation of the PFS surrogacy of the OS was also performed.
Methods
Search strategy and selection of trials
A search for Pub Med, Embase, and the Cochrane Center Register of Controlled Trials citations until April 21, 2012 was carried out. A manual search of the annual meeting proceedings of the American Society of Clinical Oncology and European Society of Medical Oncology was also performed for additional trial retrieval. Keywords included in the search were ‘non small cell lung cancer or non small cell lung carcinoma or NSCLC’, ‘advanced or metastatic’, ‘maintenance or consolidation or early second line’. The search was limited to randomised controlled trials and articles published in English. The authors reviewed each publication, and phase III studies that compared a maintenance agent (including treatment with molecular-targeted agents) for advanced or metastatic NSCLC were selected. To find any additional trials, the reference lists of included trials were searched as well as large systematic reviews. Trials that provided data for both OS and either PFS or time to progression (TTP) were included, regardless of whether these parameters were explicitly defined.
Inclusion criteria were also: (I) comparisons of a maintenance drug (either continuing treatment with at least one of the agents given in the initial therapy or switching to a different agent) with observation or placebo or a maintenance biologic agent that is usually continued until progression during and after first-line therapy (e.g., bevacizumab), (II) clinical randomised phase III trials and (III) inclusion of patients not-progressing after 4-6 cycles of platinum-based chemotherapy only. To avoid bias, the authors independently abstracted the data from the trials. Trials were excluded if they included only patients with stage III cancer, if they used old drugs/radiotherapy or did not report survival data.
Data abstraction
Two endpoints (PFS and TTP) based on tumor assessment are collectively referred to as PFS in the present analysis, similar to the approach adopted in recent reports (9,10). Median OS and median PFS were extracted from all trials that provided data for each treatment group. Median PPS was defined as median OS minus median PFS for each trial. The following information was also obtained from each report: year of publication of trial, number of patients randomised, number of patients in each treatment arm, number of treatment arms in each trial, type of agents, second lines rate, rate of PS 0-1, response rate (RR) and stable disease (SD) rate during first line, and rate of non-squamous tumors in each arm.
Data analysis
The authors summarised the survival data (median OS, median PFS, median PPS, and median PFS/median OS, differences in median PFS and median OS of control and experimental arms) as the median for all, experimental and control arms. To assess the relation between median OS and either median PFS or median PPS in the experimental arms, the researchers used the coefficient of determination (R2) and Spearman’s rank correlation coefficient (r). The coefficient of determination R2 is used in the context of statistical models whose main purpose is the prediction of future outcomes on the basis of other related information. It is the proportion of variability in a data set that is accounted for by the statistical model. Spearman’s rank correlation coefficient or Spearman’s rho, is a non-parametric measure of statistical dependence between two variables. To account for differences in sample size amongst trial arms, all analyses were weighted by the number of patients in each experimental arm. In addition, all trials were divided into two groups on the basis of the use of biological agents or chemotherapy. The differences of PFS between the experimental and control arms (ΔPFS) were also calculated in each trial and correlated with the differences in median OS derived from the same arms (ΔOS) to evaluate the surrogacy PFS/OS. Differences in OS (ΔOS) and in the surrogate endpoint (ΔPFS) were calculated as the median estimate in the experimental arm(s) minus the median estimate in the control arm. The Spearman rank correlation coefficient (r) was used as a measure of correlation between the differences in PFS and the difference in OS.
All reported P-values correspond to two-sided tests and those of P-values <0.05 were considered statistically significant. Analyses were carried out with NCSS 2007 version 07.1.20 (released February 19, 2010;
Results
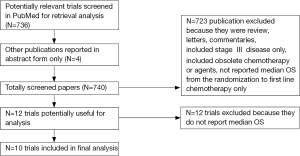
The search yielded a total of 736 potentially relevant publications, with a further four studies derived from ASCO and ESMO meetings for a total of 740 screened papers. Initially, 723 studies were excluded for various reasons. The selection process for the randomised controlled trials is shown in Figure 1. A review of the remaining 17 publications yielded 12 trials (with 25 arms) that were considered to be highly relevant for the present study. Amongst them two were finally excluded from inclusion in the analysis because they have not yet reported median OS.
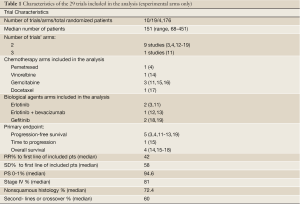
The main characteristics of the ten included phases III trials (3,4,11-19) included in the analysis are listed in Table 1. A total of 4,176 patients with advanced NSCLC were enrolled, with a median number of patients per study arm of 151 (range, 68-451). All trials had a prevalent proportion of non-squamous NSCLCs. Nine trials randomised patients to maintenance chemotherapy (5 experimental arms) or maintenance with molecular-targeted agents (4 experimental arms). One trial randomised patients to observation, chemotherapy or erlotinib (2 experimental arms). A total of 11 arms were included in the analysis.
Full table
Median OS, PFS and PPS in all trials in subgroups based on the use of molecular-targeted agents and chemotherapy
The median OS was 10.65 months for control arms (n=10). The median PFS and PPS were 2.73 and 8.1 months. For all maintenance arms (n=11), the median OS, PFS and PPS were 12.1, 4.3 and 8.3 months. For the chemotherapy maintenance arms (n=6), the median OS, PFS and PPS were 12.2, 4.65 and 6.95 months. For arms including biological-targeted agents (n=5) the median OS, PFS and PPS were 12, 4.1 and 8.9 months, respectively.
Relation between OS and either PFS and PPS
The relation between median OS and either median PFS or median PPS for the 11 treatment arms of the whole trials is shown in Figures 2,3 respectively. The median PPS was strongly associated and correlated with median OS (R2=0.83, P<0.0001 and r=0.75, P=0.007) on the basis of Spearman’s correlation coefficient, whereas median PFS was not associated nor correlated with median OS (R2=0.00007, P=0.97 and r=0.37, P=0.26). This means that the proportions of the variations in median OS that can be accounted for by variation in PPS and PFS are 83 per cent and 0.007 per cent, respectively.
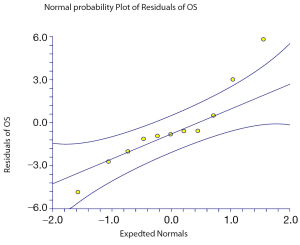 Figure 2. Correlation of OS with PFS in all maintenance arms (Spearman correlation=0.37; P=0.26). The middle line is the regression line; the 95% CIs are indicated by the outside lines
Figure 2. Correlation of OS with PFS in all maintenance arms (Spearman correlation=0.37; P=0.26). The middle line is the regression line; the 95% CIs are indicated by the outside lines
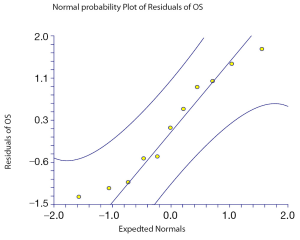 Figure 3. Correlation of OS with PPS in all maintenance arms (Spearman correlation=0.75; P=0.007). The middle line is the regression line; the 95% CIs are indicated by the outside lines
Figure 3. Correlation of OS with PPS in all maintenance arms (Spearman correlation=0.75; P=0.007). The middle line is the regression line; the 95% CIs are indicated by the outside lines
The association and correlation between median OS with median PPS and PFS in molecular-targeted agents trials are respectively R2=0.94 (P=0.005) and r=1 (P<0.0001) and R2=0.74 (P=0.06); r=0.74 (P=0.14). Conversely, the association and correlation between median OS with median PPS and PFS in chemotherapy trials are respectively R2=0.85 (P=0.008) with r=0.77 (P=0.07) and R2=0.32 (P=0.23) with r=-0.06 (P=0.9).
The rate of non-squamous NSCLCs correlate best with PFS (r=0.66; P=0.035). Post-progression survival correlates strongly with the patients’ rate of PS 0-1 (r=0.82; P=0.001).
Correlation of OS with PFS in all maintenance arms (Spearman correlation=0.37; P=0.26). The middle line is the regression line; the 95% CIs are indicated by the outside lines
Correlation of OS with PPS in all maintenance arms (Spearman correlation=0.75; P=0.007). The middle line is the regression line; the 95% CIs are indicated by the outside lines
Correlation between ΔPFS and ΔOS within trials
Overall, there was a moderate correlation between ΔPFS and ΔOS after inclusion of these ten trials. The Spearman rank correlation coefficient was 0.64 (95% CI, 0.06 to 0.88; P=0.0326) between ΔPFS and ΔOS (n=11 arm comparisons). The results also suggest that the one-month difference in PFS is associated with approximately a three-week difference in OS (slope 0.76; Figure 4).
 Figure 4. Correlation between differences in progression-free survival (∆PFS) and differences in overall survival (∆OS). The middle line is the regression line; the 95% CIs are indicated by the outside lines
Figure 4. Correlation between differences in progression-free survival (∆PFS) and differences in overall survival (∆OS). The middle line is the regression line; the 95% CIs are indicated by the outside lines
Correlation between differences in progression-free survival (∆PFS) and differences in overall survival (∆OS). The middle line is the regression line; the 95% CIs are indicated by the outside lines
Discussion
The survival rate of NSCLC has progressively improved in recent years. This improvement has to be correlated to new agents introduced in first line, maintenance and second or further lines of therapy. In particular, in recent phase III (first-line) trials, OS appears more strongly correlated to PPS than to PFS (10). Our results show that maintenance agents (either chemotherapy or biological agents) overall delay median OS by approximately 1.5 months and that, even in the maintenance phase, OS is more closely correlated to PPS than to PFS. The survival benefit is greater for trials including molecular-targeted agents where the median OS is approximately 2.6 months longer than the median OS of the corresponding control arms. Chemotherapy trials instead delayed the median OS by 0.2 months only.
No single trial, except Cappuzzo (erlotinib-based) and Ciuleanu (pemetrexed-based) studies, revealed a significant survival gain. Overall, the median further lines rate in particular in erlotinib or gefitinib trials (where this data is available), is 60 per cent; this means that two-thirds of patients received some second-line therapies.
The overall correlation of median OS with PFS is weak (r=0.37). Conversely, this correlation is very strong (r=0.75) for PPS. The correlation of OS with PPS is similar for chemotherapy and targeted therapies trials but stronger and significant only for these last trials (r=0.77 and r=1 respectively). In particular, PPS is closely correlated to a good performance status of patients, which makes it possible to offer them subsequent lines of therapies.
Thus, the point that rises from this analysis is: which is the optimal endpoint of maintenance trials? Given that the maintenance strategy treatment is directly related to first-line therapy (that is, maintaining the results of upfront treatment), the obvious answer is OS. However, the data show that the weight of median OS from the start of the maintenance phase is mainly linked to PPS, that is, survival from the progression to maintenance therapy to deaths. Survival post-progression is likely related to second- or third-line therapies that are prescribed by clinicians at disease progression. The docetaxel study of Fidias demonstrated that patients who received chemotherapy in both arms have identical survival of 12.5 months. Although this is a biased analysis, it may suggest that timing is less important than the ability to receive some form of therapy at the time of progression versus earlier. In other words, the benefit of docetaxel is similar in patients who receive it after progression. The ability of a progression-delaying agent to produce an OS benefit is not actually a concrete possibility with modern trials of chemotherapy or targeted agent trials.
Therefore, is OS the ideal endpoint for maintenance trials or is PFS more suitable? Overall, PFS requires a lower number of patients, it directly measures the efficacy of the study drug and it is less influenced by the confounding impact of post-study treatment. On the other hand, PFS is potentially influenced by inter-observer bias related to the interval of disease evaluation (other than by independent versus investigator-based evaluation). Consequently, small absolute improvements in PFS may not translate into an OS benefit, as shown in the studies included in this analysis. Conversely, OS is often considered the primary endpoint in NSCLC but requires large patient numbers to demonstrate small differences and is invariably affected by post-study treatment. However, this analysis collectively confirmed that the improvements in PFS were significantly associated with improvements in OS in randomised controlled trials of maintenance therapy for advanced NSCLC. Overall, these results confirm that PFS may represent a valid surrogate endpoint in this setting. Infact a moderate correlation ΔPFS/ΔOS exists.
It seems that PFS could be an alternative endpoint for these types of trials. This is probably more true for targeted agents than chemotherapy. In this analysis, the correlation between PPS and OS is strong for both chemotherapy and molecular-targeted agent trials. For the last studies, the correlation between PFS and OS is stronger than for chemotherapy trials (r=0.74 vs. r=-0.06). In this case, the progression-delaying effect of anti-epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) is probably a carryover effect translating into a survival benefit. It seems that the extended use of biological agents as maintenance permits a larger use of second and further lines of treatment post-progression. This is confirmed by the greater rate of treatment upon progression in arms with erlotinib or gefitinib (63% versus 46% of patients compared to chemotherapy maintenance arms). This translates into a two-month gain in survival after progression with biological agents. Conversely, PFS only accounts only for minimal variation of OS in chemotherapy arms. Progression-free survival more than objective response rate also correlates better with the final outcome of phase III trials in the colorectal cancer setting, in particular when phase III studies include biological agents (20,21).
The present study has several limitations. First, this analysis includes literature-based data only. The use of individual patient data might be expected to allow a better characterisation of the relation between OS and other endpoints based on tumor assessment, including PFS or TTP. Second, the results of this study potentially have several confounders due to the selection of many heterogeneous trials for analysis. Third, the assessment of disease progression is potentially subject to measurement error and bias in individual patients, and the quality of measurement for endpoints based on tumor assessment can vary between centres and trials. Finally, short follow-up is another limitation of this analysis. In fact, the median follow-up ranged between 10 and 20 months.
Overall, the confounding effect of second- and third-line therapies has now been commonly observed in solid tumors. This has led to a debate about the optimal endpoint of first-line trials (22). Composite measures comprising patient-reported outcomes (e.g., quality of life and symptoms control) and intermediate endpoints such as PFS could be the solution and could be investigated, in particular when maintenance agents are implemented in NSCLC. Progression-free survival is a measure of a drug’s effect on tumor growth whilst it is administered; it is also a surrogate for OS in this analysis, even if it is only moderately correlated with median survival due to confounding lines of therapy after the failure of maintenance treatments.
Stage IV NSCLC has been converted into a relatively chronic disease with an increase in the duration of median OS and with new agents introduced in clinical trials. Thus, for ethical reasons, it is not possible to renounce to these active second lines. For these reasons, modern trials, have to statistically balance for post-study treatment or permit equal access to post-study treatment in both the experimental and control arm for a more precise assessment of efficacy. In the meantime, it seems reasonable to no more consider OS as the only endpoint to validate the benefit of agents in the maintenance phase, at least with targeted agents. This is particularly true when these agents are applied in an unselected population of lung cancer patients enrolled in the published trials included in this analysis.
Conclusions
This is the first study that has explored the correlation of median OS with PFS and PPS in trials exploring maintenance therapy with both chemotherapy and molecular-targeted agents. The results indicate that, especially for trials including anti-EGFR TKIs, PPS is highly associated with OS (r=1). This correlation is strong even for chemotherapy arms (r=0.77). There is a weaker correlation between OS and PFS (r=0.37) and in particular, no correlation in chemotherapy trials (r=-0.06). Summarising all studies, however, PFS is a surrogate endpoint of survival for these trials exploring maintenance agents, and it represents a practical and reliable endpoint of activity.
In relation to the strong effect of PPS on OS, PFS could be considered an appropriate endpoint for maintenance trials. However, a precise assessment of the disease course with a preplanned second-line therapy or an adjustment for treatments prescribed beyond progression is crucial.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Behera M, Owonikoko TK, Chen Z, et al. Single agent maintenance therapy for advanced stage non-small cell lung cancer: a meta-analysis. Lung Cancer 2012;77:331-8.
- Zhang X, Zang J, Xu J, et al. Maintenance therapy with continuous or switch strategy in advanced non-small cell lung cancer: a systematic review and meta-analysis. Chest 2011;140:117-26.
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9.
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40.
- Zietemann VD, Schuster T, Duell TH. Post-study therapy as a source of confounding in survival analysis of firstline studies in patients with advanced non-small-cell lung cancer. J Thorac Dis 2011;3:88-98.
- Hotta K, Kiura K, Fujiwara Y, et al. Role of survival postprogression in phase III trials of systemic chemotherapy in advanced non-small-cell lung cancer: a systematic review. PLoS One 2011;6:e26646.
- Buyse M, Burzykowski T, Carroll K, et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol 2007;25:5218-24.
- Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 2009;101:1642-9.
- Saad ED, Katz A, Buyse M. Overall survival and postprogression survival in advanced breast cancer: a review of recent randomized clinical trials. J Clin Oncol 2010;28:1958-62.
- Hayashi H, Okamoto I, Morita S, et al. Postprogression survival for first-line chemotherapy of patients with advanced non-small-cell lung cancer. Ann Oncol 2012;23:1537-41.
- Pérol M , Zalcman G , Monnet I , et al . Final results from the IFCT-GFPC 0502 phase III study: maintenance therapy in advanced NSCLC with either gemcitabine (G) or erlotinib (E) versus observation (O) after cisplatin-gemcitabine induction chemotherapy (CT), with predefined second-line treatment. J Clin Oncol 2010;21:abstr 4766.
- Miller VA, O’Connor P, Soh C, et al. A randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2009;27:abstr LBA8002.
- Kabbinavar FF, Miller VA, Johnson BE, et al. Overall survival (OS) in ATLAS, a phase IIIb trial comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy (chemo) with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2010;28:abstr 7526.
- Westeel V, Quoix E, Moro-Sibilot D, et al. Randomized study of maintenance vinorelbine in responders with advanced non-small-cell lung cancer. J Natl Cancer Inst 2005;97:499-506.
- Brodowicz T, Krzakowski M, Zwitter M, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer 2006;52:155-63.
- Belani CP, Waterhouse DM, Ghazal H, et al. Phase III study of maintenance gemcitabine (G) and best supportive care (BSC) versus BSC, following standard combination therapy with gemcitabine-carboplatin (G-Cb) for patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2012;28:abstr 7506.
- Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after frontline therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:591-8.
- Gaafar RM, Surmont VF, Scagliotti GV, et al. A double-blind, randomised, placebo-controlled phase III intergroup study of gefitinib in patients with advanced NSCLC, non-progressing after first line platinum-based chemotherapy (EORTC 08021/ ILCP 01/03). Eur J Cancer 2011;47:2331-40.
- Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466-75.
- Grothey A, Hedrick EE, Mass RD, et al. Responseindependent survival benefit in metastatic colorectal cancer: a comparative analysis of N9741 and AVF2107. J Clin Oncol 2008;26:183-9.
- Siena S, Peeters M, Van Cutsem E, et al. Association of progression-free survival with patient-reported outcomes and survival: results from a randomised phase 3 trial of panitumumab. Br J Cancer 2007;97:1469-74.
- Amir E, Seruga B, Kwong R, et al. Poor correlation between progression-free and overall survival in modern clinical trials: are composite endpoints the answer? Eur J Cancer 2012;48:385-8.