
Clinical characteristics and progression of pre-/minimally invasive lung adenocarcinoma harboring ALK or RET rearrangements: a retrospective cohort study
Highlight box
Key findings
• Anaplastic lymphoma kinase (ALK)/rearranged during transfection (RET)-positive pre-/minimally invasive lung adenocarcinoma has distinct radiologic features with rapid progression.
What is known and what is new?
• Patients harboring ALK or RET rearrangements are usually diagnosed at a relatively late stage with nodal and distant metastasis.
• ALK/RET-positive pre-/minimally invasive lung adenocarcinomas were mostly characterized as mixed ground-glass opacities with cystic airspace developing rapid nodule progression, and no recurrence occurred during long-term follow-up after resection.
What is the implication, and what should change now?
• This provides insights into proper curative surgery timing in the management of patients with gene fusions.
Introduction
Background
Anaplastic lymphoma kinase (ALK) and oncogenic rearranged during transfection (RET) fusions are two of the targetable driver events in non-small cell lung cancer (NSCLC), which are found in approximately 3–8% and 1–2% of non-squamous NSCLC (NS-NSCLC) populations (1-3), respectively. ALK fusions are reported to be very rare in lung squamous cancers with the rate of 0–1.5% (4-6), while RET rearrangements could also occur occasionally in about 3% of adenosquamous, large-cell carcinomas, and squamous-cell NSCLC (5), despite that none of this two gene alterations were reported to be prognostic factors for non-adenocarcinoma NSCLC (7). Clinically, the characteristics of non-adenocarcinoma NSCLC patients with ALK/RET rearrangements were similar with those with lung adenocarcinomas (7). ALK fusions are correlated with never-smokers and younger age patients (1,8), and scarcely are they detected in early-staged lung cancer (9). Next-generation tyrosine kinase inhibitors (TKIs) such as alectinib and lorlatinib result in significantly longer survival and provide impressive “brain-control” compared to the first-generation inhibitor crizotinib or chemotherapy (10,11). RET rearrangements are usually mutually exclusive with epidermal growth factor receptor (EGFR), Kirsten rat sarcoma viral oncogene (KRAS), ALK, or ROS proto-oncogene 1 (ROS1) alterations (3,5,12). Our previous study also revealed that RET fusions were more prevalent in never-smokers, younger age patients with more poorly differentiated tumors and distant metastatic disease (2), implicating a potential of rapid progression. Despite the superior efficacy and tolerability of TKIs, delayed diagnosis and treatment of such diseases may still lead to poorer prognosis and inability to receive radical surgical treatment for patients. Meanwhile, complex genetic heterogeneity could still lead to drug resistance and therefore rapid progression of tumor even after next-generation TKI treatments (13). On the contrast, adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) are reported to carry excellent prognosis and possess an indolent natural course, for which surgical time window is relatively wide.
Rationale and knowledge gap
Clinical characteristics and course of pre-/minimally invasive lung adenocarcinoma harboring ALK or RET fusions are poorly described. Identifying patients with gene fusions at early stage may offer surgical options that could cure those patients.
Objective
In this study, therefore, we summarized radiologic features, natural courses and survival outcomes of pre-/minimally invasive lung adenocarcinoma with ALK/RET rearrangements in an attempt to identify those nodules with potential of rapid progression at an early stage. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-517/rc).
Methods
Patients
This study was approved by the institutional review board [Fudan University Shanghai Cancer Center (FUSCC) IRB 2008223-9, date: 2020/07/14]. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of this study.
Medical records of patients with surgically resected AIS/MIA harboring EGFR mutations, ALK fusions or RET fusions at FUSCC from April 2008 to June 2021 were reviewed. Considering the relative low frequency and similar clinical characteristics, we combined patients with ALK fusions and RET fusions as a group, and compared it with EGFR-positive patients, the latter of which stands for the most common targetable driver mutation even in early-stage lung adenocarcinoma (14). Figure S1 showed the flowchart of patient recruitment. Patients with invasive adenocarcinoma or without EGFR or ALK/RET alternations were excluded. The sample size was determined by the actual cohort selection. Clinical characteristics such as age, gender, smoking history, and radiological and pathological features were prospectively evaluated for this study. The relapse-free survival (RFS) was defined as the interval from the date of surgery to the date of first recurrence or last follow-up. The overall survival (OS) was defined as the time between the date of surgery and the date of death or last follow-up. The lung cancer-specific survival (LCSS) was defined as the time from surgery to lung cancer-specific death or last follow-up.
Radiologic evaluation
A 64 multi-detector helical scanner was used for lung computed tomography (CT) examination. The scanning section thickness and interval were 5.0 and 5.0 mm, respectively. And the reconstruction section width and interval were 1.0 and 1.0 mm, respectively. Two radiologists evaluated the patient radiological characteristics, including the tumor size, ground-glass component, cystic airspace, pleural attachment, lobulation, spiculation, and broncho-inflation. Each CT image with vision of the lesions before surgery was reviewed. The size of the nodule was defined as the maximum diameter on the axial plane measured on the lung window [window width: 1,600 Hounsfield unit (HU); window level: 600 HU; width and interval, 1.0 and 1.0 mm, respectively]. Progression of a nodule was defined as an increase of ≥2 mm or the appearance of solid component. This was based on a series of studies on the threshold to determine true nodule growth during follow-up (15,16).
Histological evaluation
Surgical resected specimens were fixed by formalin and stained with hematoxylin-eosin (H&E). The patholigical subtype diagnosis was subclassified into AIS, MIA, and invasive adenocarcinoma according to the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification.
Mutational analysis
Mutation analysis procedure was performed as described in previous studies (17,18). Briefly, EGFR hot spots (exons 18–22) was amplified and detected by Sanger sequencing. RET rearrangements were detected by quantitative real-time polymerase chain reaction (qRT-PCR)-based fusion detection methods with validation using fluorescent in situ hybridization (FISH). ALK rearrangements were analyzed by qRT-PCR-based fusion detection methods or Ventana immunohistochemistry (IHC) for ALK protein expression. IHC was performed on 3.5-µm-thick formalin-fixed paraffin-embedded (FFPE) specimens using the VENTANA ALK (Clone D5F3) CDx kit according to the manufacturer’s instructions.
Follow-up protocol
Routine follow-up of patients begins after surgery. Within 3 years after the operation, chest CT, ultrasonography, and brain magnetic resonance imaging or CT scans are performed every 4 months, and every 6 months for the next 2 years, and annually from then on. Bone scanning is performed annually.
Statistical analysis
Statistical analysis was performed using SPSS (version 24.0, Armonk, NY, USA) and R statistic language (version 3.6.2). The correlation between the two category variables was analyzed using the Pearson χ2 test or Fisher exact test. The student t-test is used to compare consecutive variables between the two groups. We investigated the survival results using Kaplan-Meier method, and examined the difference between the groups using log-rank test. All tests were two-tailed.
Results
After exclusion, a total of 238 patients with pre-/minimally invasive lung adenocarcinoma were recruited, 226 with EGFR mutations, 7 with ALK fusions, and 5 with RET fusions. Detailed clinicopathologic findings of patients with ALK/RET fusions are demonstrated in Table 1. The average age at surgery was 45.3 years, which was significantly younger than patients with EGFR mutations (P=0.049; Table S1). Among these patients, only one patient with ALK fusion had smoking history. Two patients received lobectomy and the remaining ten patients received sublobar resection. All of these 12 patients presented as MIA histologically.
Table 1
| Patient no. | Age (years) | Sex | Smoking history | Radiologic tumor size (mm) | Radiologic subtype | Cystic airspace | Pleural attachment | Lobulation | Spiculation | Broncho-inflation | Operative procedure | Pathologic subtype | Lymph node status | Gene rearrangement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | M | N | 13 | mGGO | − | − | + | − | − | SEG | MIA | N0 | ALK fusion |
| 2 | 52 | M | N | 6 | mGGO | + | − | − | − | − | SEG | MIA | N0 | ALK fusion |
| 3 | 38 | F | N | 8 | Solid | − | − | − | + | − | WED | MIA | N0 | ALK fusion |
| 4 | 58 | M | N | 7 | mGGO | + | − | − | − | − | WED | MIA | N0 | ALK fusion |
| 5 | 25 | F | N | 12 | mGGO | + | − | − | − | − | SEG | MIA | N0 | ALK fusion |
| 6 | 51 | F | N | 18 | mGGO | − | − | − | − | − | LOB | MIA | N0 | ALK fusion |
| 7 | 34 | M | N | 9 | mGGO | + | − | − | − | − | WED | MIA | N0 | ALK fusion |
| 8 | 65 | M | Y | 10 | mGGO | + | − | − | − | − | SEG | MIA | N0 | RET fusion |
| 9 | 29 | M | N | 9 | mGGO | − | − | − | − | − | WED | MIA | N0 | RET fusion |
| 10 | 28 | F | N | 10 | mGGO | + | − | − | − | − | WED | MIA | N0 | RET fusion |
| 11 | 62 | M | N | 13 | mGGO | + | − | − | − | + | LOB | MIA | N0 | RET fusion |
| 12 | 56 | F | N | 9 | pGGO | + | − | − | − | − | WED | MIA | N0 | RET fusion |
ALK, anaplastic lymphoma kinase; RET, rearranged during transfection; M, male; N, no; mGGO, mixed ground-glass opacity; SEG, segmentation; MIA, minimal-invasive adenocarcinoma; F, female; WED, wedge resection; LOB, lobectomy; Y, yes; pGGO, pure ground-glass opacity.
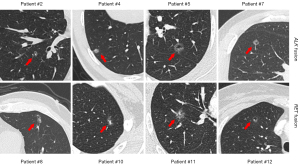
Radiologically, the average tumor size was 10.3±3.3 mm (Table 1). The majority of nodules were categorized as ground-glass opacities (GGOs) radiologically (11/12), and ten patients had mixed GGOs (mGGOs) (83.4%), which was significantly higher than that in EGFR-positive group (24.3%, P<0.001; Table S1). Interestingly, we found a substantial proportion of cystic airspace feature in ALK/RET-positive group (8/12, 66.7%; Figure 1) than in EGFR-positive group (32/226, 14.2%, P<0.001; Table S1). Other radiologic features such as pleural attachment, lobulation sign, spiculation sign and bronchoinflation sign were not specifically observed (Table 1).
In ALK/RET-positive group, four patients performed surveillance over 1 year before surgery, detailed follow-up length is demonstrated in Figure 2. Of note, two of them experienced significant nodule progression within approximately 12 months after the first detection, indicating rapid development of ALK/RET-positive adenocarcinomas even at pre-/minimally invasive stage. The 5-year OS was 98.6% and 100% for EGFR-positive group and for ALK/RET-positive group, respectively (P=0.758; Figure S2). The 5-year LCSS and RFS were all 100% for EGFR-positive group and for ALK/RET-positive group (Figure S2).

Discussion
The development of molecular detection of driver mutations has revolutionized the diagnose and treatment of advance staged lung cancer. Subsequently, molecular testing has been extensively applied clinically. Other than medication guidance, mutational profiles have also been applied in prognosis prediction and patient characterization. ALK and RET fusions have been characterized as younger aged and advanced-staged. There is noticeable difference in the incidence rate of ALK/RET rearrangements in advanced-staged and pre-invasive lung cancer (2,19), which indicates the rapid progression course of ALK/RET fusion-positive lung cancer even at early stage. Therefore, we assumed that performing radical resection for pre-/minimally invasive lung adenocarcinomas with ALK/RET fusions may offer a curative opportunity. In this study, we found that radiologic characteristics and natural course of AIS/MIA harboring ALK/RET fusions were distinct from patients with EGFR mutations. mGGOs with cystic airspace were significantly more prevalent in ALK/RET-positive AIS/MIAs than in EGFR-positive lesions, with relatively rapid progression during follow-up. After radical resection, however, the long-term survival was still excellent for pre-/minimally invasive lung adenocarcinomas with ALK/RET fusions, which validated our hypothesis.
Radiologic features of lung adenocarcinoma with gene fusions have been reported in previous studies, solid masses and lobulated margins were summarized as key characteristics (20,21). Yet, patient cohorts in those studies were composed of advanced-staged lung cancer. Our study firstly identified mGGO with cystic airspace as specific radiologic feature in a subgroup of early-staged adenocarcinoma with ALK/RET fusions. The mechanism of cystic airspace formation in lung cancer is not fully understood. Some researchers believe that cystic airspace is correlated with rapid tumor growth that oversteps the blood supply of the tumor, thus creating necrosis in the middle of the tumor (22). Clinically, cystic airspace has been reported to be an independent factor for poor prognosis in patients with resected early-staged lung cancer (23). Furthermore, cystic airspace is also reported to be lower in AIS/MIA when compared with invasive adenocarcinoma (24), indicating its predictive ability of tumor invasiveness. In our study, up to 66.7% of cystic airspace was found in patients with AIS/MIA harboring ALK/RET fusions compared with 14.9% in EGFR-positive group, which was consistent with the aggressive behavior of ALK/RET rearrangements in lung adenocarcinoma.
GGO-featured lung adenocarcinoma is generally considered as an indolent subtype. Studies reported a rather small proportion of GGOs would progress in long-term follow-up. Kakinuma et al. reported a probability of 11% for GGOs to progress during 5-year follow-up (25). In our cohort, however, four patients with ALK/RET fusions performed surveillance over 1 year before surgery, two of them developed rapid radiologic progression of the nodules. Identifying radiologic features of those patients that may progress rapidly could give hints on the proper timing for intervention.
Our study has several limitations. First, only 12 patients with AIS/MIA with ALK/RET rearrangements were included in this study, and these findings must be treated with caution and validated in future multi-center studies with larger sample size. Second, this was a retrospective, single-institution study. Thus, selection bias and time-trend bias are inevitable.
Conclusions
ALK/RET-positive pre-/minimally invasive lung adenocarcinomas were mostly characterized as mGGOs with cystic airspace developing rapid nodule progression, and no recurrence occurred during long-term follow-up after resection. This provides insights into proper curative surgery timing in the management of patients with gene fusions.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-517/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-517/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-517/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-517/coif). C.D. reports the funding from the Shanghai Anticancer Association EYAS PROJECT (No. SACA-CY21B07). Y.Z. reports the funding from the Shanghai Rising-Star Program (No. 21QC1400600). H.C. reports the funding from the National Natural Science Foundation of China (No. 81930073). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board [Fudan University Shanghai Cancer Center (FUSCC) IRB 2008223-9, date: 2020/07/14]. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived, because this was a retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012;18:382-4. [Crossref] [PubMed]
- Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012;30:4352-9. [Crossref] [PubMed]
- Feng J, Li Y, Wei B, et al. Clinicopathologic characteristics and diagnostic methods of RET rearrangement in Chinese non-small cell lung cancer patients. Transl Lung Cancer Res 2022;11:617-31. [Crossref] [PubMed]
- Zhao W, Choi YL, Song JY, et al. ALK, ROS1 and RET rearrangements in lung squamous cell carcinoma are very rare. Lung Cancer 2016;94:22-7. [Crossref] [PubMed]
- Griesinger F, Eberhardt W, Nusch A, et al. Biomarker testing in non-small cell lung cancer in routine care: Analysis of the first 3,717 patients in the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021;152:174-84. [Crossref] [PubMed]
- Watanabe J, Togo S, Sumiyoshi I, et al. Clinical features of squamous cell lung cancer with anaplastic lymphoma kinase (ALK)-rearrangement: a retrospective analysis and review. Oncotarget 2018;9:24000-13. [Crossref] [PubMed]
- Song Z, Yu X, Zhang Y. Clinicopathological characteristics and survival of ALK, ROS1 and RET rearrangements in non-adenocarcinoma non-small cell lung cancer patients. Cancer Biol Ther 2017;18:883-7. [Crossref] [PubMed]
- Kim HR, Shim HS, Chung JH, et al. Distinct clinical features and outcomes in never-smokers with nonsmall cell lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement. Cancer 2012;118:729-39. [Crossref] [PubMed]
- Chen MF, Chaft JE. Early-stage anaplastic lymphoma kinase (ALK)-positive lung cancer: a narrative review. Transl Lung Cancer Res 2023;12:337-45. [Crossref] [PubMed]
- Kuang S, Leighl NB. Lorlatinib in ALK-Rearranged Lung Cancer. Cancer Cell 2021;39:25-7. [Crossref] [PubMed]
- Zia V, Lengyel CG, Tajima CC, et al. Advancements of ALK inhibition of non-small cell lung cancer: a literature review. Transl Lung Cancer Res 2023;12:1563-74. [Crossref] [PubMed]
- Ferrara R, Auger N, Auclin E, et al. Clinical and Translational Implications of RET Rearrangements in Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:27-45. [Crossref] [PubMed]
- Long X, Wu H, Yang C, et al. Complex genetic alterations contribute to rapid disease progression in an ALK rearrangement lung adenocarcinoma patient: a case report. Transl Cancer Res 2021;10:3081-6. [Crossref] [PubMed]
- Chen H, Carrot-Zhang J, Zhao Y, et al. Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat Commun 2019;10:5472. [Crossref] [PubMed]
- Solomon J, Ebner L, Christe A, et al. Minimum perceivable size difference: how well can radiologists visually detect a change in lung nodule size from CT images? Eur Radiol 2021;31:1947-55. [Crossref] [PubMed]
- Kim H, Park CM, Song YS, et al. Measurement Variability of Persistent Pulmonary Subsolid Nodules on Same-Day Repeat CT: What Is the Threshold to Determine True Nodule Growth during Follow-Up? PLoS One 2016;11:e0148853. [Crossref] [PubMed]
- Deng C, Zheng Q, Zhang Y, et al. Validation of the Novel International Association for the Study of Lung Cancer Grading System for Invasive Pulmonary Adenocarcinoma and Association With Common Driver Mutations. J Thorac Oncol 2021;16:1684-93. [Crossref] [PubMed]
- Pan Y, Zhang Y, Ye T, et al. Detection of Novel NRG1, EGFR, and MET Fusions in Lung Adenocarcinomas in the Chinese Population. J Thorac Oncol 2019;14:2003-8. [Crossref] [PubMed]
- Zhu J, Wang W, Xiong Y, et al. Evolution of lung adenocarcinoma from preneoplasia to invasive adenocarcinoma. Cancer Med 2023;12:5545-57. [Crossref] [PubMed]
- Li H, Zhang R, Wang S, et al. CT-Based Radiomic Signature as a Prognostic Factor in Stage IV ALK-Positive Non-small-cell Lung Cancer Treated With TKI Crizotinib: A Proof-of-Concept Study. Front Oncol 2020;10:57. [Crossref] [PubMed]
- Wang H, Schabath MB, Liu Y, et al. Clinical and CT characteristics of surgically resected lung adenocarcinomas harboring ALK rearrangements or EGFR mutations. Eur J Radiol 2016;85:1934-40. [Crossref] [PubMed]
- Wang M, Zhao J, Pan Y, et al. Do tumor cavitation and sex in resected stage I non-small-cell lung cancer correlate with prognosis? World J Surg 2009;33:497-504. [Crossref] [PubMed]
- Tomizawa K, Shimizu S, Ohara S, et al. Clinical significance of tumor cavitation in surgically resected early-stage primary lung cancer. Lung Cancer 2017;112:57-61. [Crossref] [PubMed]
- Li X, Zhang W, Yu Y, et al. CT features and quantitative analysis of subsolid nodule lung adenocarcinoma for pathological classification prediction. BMC Cancer 2020;20:60. [Crossref] [PubMed]
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural History of Pulmonary Subsolid Nodules: A Prospective Multicenter Study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]


