
Real-world incidence and risk factors of pneumonitis in chemoradiation plus immune checkpoint inhibitors compared with chemoradiation alone in lung cancer: a retrospective cohort study
Highlight box
Key findings
• Immune checkpoint inhibitor (ICI) and V30 increase the incidence of grade ≥2 pneumonitis. A history of chronic lung disease is an independent risk factor in patients receiving combination therapy.
What is known and what is new?
• Radiation combined with ICI benefits patients.
• Dosimetric parameters were not correlated with pneumonitis in combination therapy.
What is the implication, and what should change now?
• Combination therapy should be applied with caution in patients with a history of chronic lung disease.
Introduction
Lung cancer is one of the leading causes of death worldwide. The introduction of immune checkpoint inhibitors (ICIs) has represented a milestone in lung cancer treatment that significantly improves the overall response and survival of lung cancer patients. They were recommended to use in advanced stage lung cancer patients in combination with chemotherapy or targeted therapy (1). But in locally advanced and inoperable non-small cell lung cancer (NSCLC) patients, concurrent chemoradiotherapy (cCRT) was still standard therapy according to National Comprehensive Cancer Network (NCCN) guideline version 4, 2023 (2). The mechanism of ICIs is to reinvigorate the function of immune cells by blocking immune checkpoints such as programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), thus in turn attacking cancer cells. However, enhanced immune activation can also attack healthy tissues, resulting in adverse events, including pneumonitis (3,4). Thoracic radiotherapy (RT) plays a vital role in the treatment of lung cancer. Large-scale clinical trials have shown significant survival benefit in patients receiving concurrent or sequential chemoradiation followed by ICI (5,6). Considering the synergy of chemotherapy, RT and ICI, studies have recommended that ICIs are initiated at the beginning of chemoradiotherapy. Some clinical trials have explored the efficacy and safety of ICI combined with chemoradiotherapy (7-9). However, whether chemoradiation combined with ICI would increase the risk of pneumonitis requires further investigation and the comparison of combination therapy and chemoradiation therapy in the real world has not yet been reported. In addition, relevant data of Asian patients are rare. Anti-PD-1 monoclonal antibody (mAb) and anti-PD-L1 mAb are the most commonly used ICIs and are approved for lung cancer. We retrospectively assessed the incidence and risk factors of pneumonitis within 6 months in combination therapy of chemoradiation and anti-PD-1/PD-L1 mAb in the real world and compared them with those chemoradiation therapy alone. Our primary objectives were to (I) compare the incidence of pneumonitis in chemoradiation therapy with or without ICIs and (II) identify the risk factors of pneumonitis in the combination group. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-756/rc).
Methods
Patients
We performed a retrospective cohort study to compare the incidence of pneumonitis in lung cancer patients who received conventional thoracic radiation and chemotherapy with or without anti-PD-1/PD-L1 mAb between January 2020 and December 2021 at West China Hospital and determine the risk factors. A minimum total radiation dose of 50 Gy by conventional fraction was an essential inclusion criterion. Patients who received chemotherapy combined with anti-PD-1/PD-L1 mAb as first line treatment concurrent with or sequenced by radical thoracic radiation were included in the combination group. Patients who received chemotherapy as first line treatment concurrent with or followed by thoracic radiation were included in the control group. Patients who received stereotactic body radiation therapy (SBRT) were excluded because of the relatively low risk of lung toxicity due to low irradiation volume. Patients who had received anti-PD-1/PD-L1 mAb after thoracic radiation, a lack of data, and a lack of follow-up were also excluded. The clinical characteristics were recorded from electronic medical records, including sex, age, smoking history, pathological diagnosis, baseline pulmonary disease [including chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD)], treatment strategy, and location of primary tumor. The radiological dosimetric parameters were also recorded, including percent volume of the lung receiving ≥5 Gy (V5), percent volume of lung receiving ≥20 Gy (V20), percent volume of lung receiving ≥30 Gy (V30), mean lung dose (MLD), lung volume, and planning target volume (PTV). The sample size calculation was based on previous reports estimating the probability of severe pneumonitis to be 7% in the chemoradiation group and 15% in the ICIs plus chemoradiation group, 1−β=0.8 and α=0.05. A total sample size of at least 60 patients was required, with at least 30 patients in each group. We retrospectively collected 152 patients who met our inclusion criteria. All of these patients were included in this study to address potential selected bias. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Committee of West China Hospital (No. 2023-1295) and individual consent for this retrospective analysis was waived.
Pneumonitis assessment
Follow-up chest computed tomography (CT) scans were taken at least every 2 months until 6 months after radiation. Pneumonitis was determined and graded in consensus by an oncologist and a radiologist. Checkpoint inhibitor pneumonitis (CIP) and radiation pneumonitis (RP) were included because it was hard to distinguish which of them played a more important role in the incidence of pneumonitis after immunotherapy and radiation and the treatment strategy of CIP and RP were similar. Infectious pneumonitis was excluded. Infectious pneumonitis was carefully diagnosed based on symptoms of fever, aberrant white blood cell count, elevated procalcitonin (PCT), C-reactive protein (CRP), and positive for pathogenic microorganism. The grade of pneumonitis was based on the Common Terminology Criteria for Adverse Event Version (CTCAE) 5.0.
Statistical analysis
The software SPSS statistics version 27.0.1.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Descriptive statistics were applied to analyze the clinical features of enrolled patients. Chi-squared tests or Fisher’s exact tests were performed to analyze the difference between the combination group and control group for categorical variables and Mann-Whitney U test for continuous variables. The incidence rates of pneumonitis in the two groups were evaluated by Chi-squared tests. Univariate and multivariate analyses were performed by logistic regression to assess risk factors of pneumonitis. Variables that seemed to be significant by univariate analysis were taken into multivariate analysis, and “input” was selected for multivariate analysis. Statistical tests were 2-sided and P value less than 0.05 was considered statistically significant.
Results
Clinical characteristics
We retrospectively selected lung cancer patients in West China Hospital who received ≥50 Gy conventional radiation of the chest in 2020 and 2021. A total of 152 patients met our inclusion criteria (Figure 1). The clinical characteristics of the patients are listed in Table 1. The median age was 59 years (range, 31–81 years) and most patients were male (129/152, 84.9%). 75.7% patients were NSCLC (115/152), 14.5% (22/152) were small cell lung cancer (SCLC), and 9.9% (15/152) were other pathological types. Most patients received upper lobe irradiation (66.4%, 101/152).

Table 1
| Characteristics | Total (n=152) | Chemoradiation (n=94) | Chemoradiation + ICI (n=58) | P value |
|---|---|---|---|---|
| Age (years), median (IQR) | 59.0 (54.0–66.0) | 59 (53.0–66.3) | 62 (54.8–65.0) | 0.590 |
| Gender, n (%) | 0.103 | |||
| Female | 23 (15.1) | 18 (19.1) | 5 (8.6) | |
| Male | 129 (84.9) | 76 (80.9) | 53 (91.4) | |
| Histology, n (%) | 0.478 | |||
| Squamous | 65 (42.8) | 37 (39.4) | 28 (48.3) | |
| Adenocarcinoma | 50 (32.9) | 35 (37.2) | 15 (25.9) | |
| SCLC | 22 (14.5) | 14 (14.9) | 8 (13.8) | |
| Other | 15 (9.9) | 8 (8.5) | 7 (12.1) | |
| Stage, n (%) | <0.001 | |||
| III | 111 (73.0) | 81 (86.2) | 30 (51.7) | |
| IV | 41 (27.0) | 13 (13.8) | 28 (48.3) | |
| Smoking history, n (%) | 0.491 | |||
| Never | 84 (55.3) | 54 (57.4) | 30 (51.7) | |
| Former | 68 (44.7) | 40 (42.6) | 28 (48.3) | |
| ICI type, n (%) | ||||
| PD-1 inhibitor | 48 (82.8) | |||
| PD-L1 inhibitor | 10 (17.2) | |||
| History of chronic lung disease, n (%) | 0.451 | |||
| Yes | 19 (12.5) | 10 (10.6) | 9 (15.5) | |
| No | 133 (87.5) | 84 (89.4) | 49 (84.5) | |
| Irradiation site, n (%) | 0.586 | |||
| Upper lobe | 101 (66.4) | 64 (68.1) | 37 (63.8) | |
| Lower lobe | 51 (33.6) | 30 (31.9) | 21 (36.2) | |
| Dosimetric parameters, median (IQR) | ||||
| Dose (Gy) | 50 (50.0–60.0) | 60 (50.0–60.0) | 0.088 | |
| V5 (%) | 46.0 (40.8–54.0) | 46.0 (35.0–52.0) | 0.325 | |
| V10 (%) | 36.0 (28.0–40.3) | 35.0 (25.0–38.4) | 0.163 | |
| V20 (%) | 26.5 (20.8–30.0) | 25.0 (17.8–28.3) | 0.057 | |
| V30 (%) | 18.0 (14.0–20.0) | 17.0 (10.8–20.0) | 0.277 | |
| MLD (cc) | 1,374.5 (1,109.3–1,480.6) | 1,333.9 (1,049.8–1,476.1) | 0.397 | |
| PTV (cc) | 465.6 (309.8–667.3) | 433.7 (258.0–525.7) | 0.276 | |
| Lung all (cc) | 3,275.5 (2,931.5–3,826.8) | 3,590.0 (2,820.7–4,312.4) | 0.111 | |
ICI, immune checkpoint inhibitor; IQR, interquartile range; SCLC, small cell lung cancer; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; MLD, mean lung dose; PTV, planned target volume.
Among the 152 patients, all received conventional fractionated chest radiation (1.8–2 Gy/fraction); 94 patients underwent chemotherapy, and 58 patients received chemotherapy combined with ICI before radiation. All cases continued with chemotherapy or combination therapy during irradiation or resumed immediately after irradiation. The baseline of clinicopathological features of the two groups was generally balanced, except for the pathological stages. In the combination group, 48.3% (28/58) were stage IV, whereas in the control group, 13.8% (13/94) were stage IV (P<0.001). A history of chronic lung disease was recorded in 15.5% (9/58) of the combination group and 10.6% (10/94) of the control group (P=0.451). In the combination group, 82.8% (48/58) of patients received PD-1 inhibitors, and 17.2% (10/58) received PD-L1 inhibitors. There was no significant difference between the two groups in terms of basic dosimetric parameters (Table 1). In the control group and the combination group, the median PTV was 465.6 and 433.7 cc (P=0.276), the volume of lung was 3,275.5 and 3,590.0 cc (P=0.111), and the median radiation dose was 50 and 60 Gy (P=0.088), respectively.
Incidence of pneumonitis
The median follow-up time was 8.4 months and at least 6 months for each patient. Retrospective analysis showed that 66.4% (101/152) patients had pneumonitis, 28.9% (44/152) had grade ≥2 and 9.9% (15/152) had grade ≥3 pneumonitis in the general population within 6 months (Table 2).
Table 2
| Grade | Total (n=152) | Chemoradiation (n=94) | Chemoradiation + ICI (n=58) | P value |
|---|---|---|---|---|
| 0 | 51 (33.6) | 39 (41.5) | 12 (20.7) | |
| ≥1 | 101 (66.4) | 55 (58.5) | 46 (79.3) | 0.013 |
| ≥2 | 44 (28.9) | 21 (22.3) | 23 (39.7) | 0.028 |
| ≥3 | 15 (9.9) | 6 (6.4) | 9 (15.5) | 0.092 |
Data are presented as n (%). ICI, immune checkpoint inhibitor.
The incidence of any grade pneumonitis was significantly higher in the combination group than in the control group (79.3% vs. 58.5%, P=0.013), and the incidence of grade ≥2 pneumonitis was also higher in the combination group (39.7% vs. 22.3%, P=0.028). The incidence of grade ≥3 pneumonitis had no statistical difference, but the combination group showed a higher trend (6.4% vs. 15.5%, P=0.092). There was 1 treatment-related death in each group (1.1% vs. 1.7%) (Table 2).
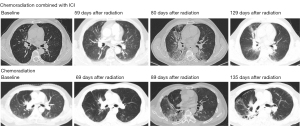
From the start of RT, the median time of onset of grade ≥3 pneumonia was 108 days (range, 55–157 days) in the combination group and 89 days (range, 82–204 days) in the chemoradiotherapy group. The median time for corticosteroid treatment was 32 days. Of the 9 patients with grade ≥3 pneumonia in the combination group, 1 used PD-L1 inhibitor and 8 used PD-1 [10% (1/10) vs. 16.7% (8/48), P=0.601]. The CT presentations of pneumonitis in the two groups were similar, with a patchy infiltration during the acute phase followed by lung consolidation and interstitial fibrosis. The range of the lesion was mainly within the radiation field (Figure 2). There was 1 death in each group, due to radiation pneumonia with pathogenic infection, presenting as an effusion within the radiation field and then evolving into a widespread effusion. The clinical presentation was irreversible type I respiratory failure.
Risk factors of pneumonitis
In all patients, univariate analysis showed that several factors were associated with incidence of grade ≥2 pneumonitis, including stage (P=0.041), application of ICI (P=0.024), V5 (P=0.034), V20 (P=0.030), and V30 (P=0.014). Continuous variables were sorted into ordered categorical variables by quartile. Multivariate analysis showed that ICI application [odds ratio (OR): 2.641, 95% confidence interval (CI): 1.244–5.608, P=0.011] and V30 (OR: 1.728, 95% CI: 1.214–2.460, P=0.002) were independent risk factors (Table 3). Univariate analysis showed that history of chronic lung disease (P=0.002) and PTV volume (P=0.016) were correlated with grade ≥3 pneumonitis. Variables with P value less than 0.1 were included in multivariate analysis. Multivariate analysis showed that the history of chronic lung disease was the independent risk factor (OR: 6.359, 95% CI: 1.953–20.705, P=0.002). Notably, the application of ICI showed an increased trend, but did not reach statistical significance (P=0.099) (Table 4).
Table 3
| Characteristics | Total (N=152) | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Grade <2 (n=108) | Grade ≥2 (n=44) | P value | OR | 95% CI | P value | |||
| Age (years), median (IQR) | 58.5 (53.0–65.0) | 62.5 (55.0–67.0) | 0.334 | |||||
| Gender, n | ||||||||
| Female | 23 | 18 | 5 | 0.411 | ||||
| Male | 129 | 90 | 39 | |||||
| Histology, n | ||||||||
| Squamous | 65 | 45 | 20 | 0.341 | ||||
| Adenocarcinoma | 50 | 33 | 17 | |||||
| SCLC | 22 | 19 | 3 | |||||
| Other | 15 | 11 | 4 | |||||
| Stage, n | ||||||||
| III | 111 | 84 | 27 | 0.041 | 0.211 | |||
| IV | 41 | 24 | 17 | |||||
| Smoking history, n | ||||||||
| Never | 84 | 58 | 26 | 0.545 | ||||
| Former | 68 | 50 | 18 | |||||
| ICI, n | ||||||||
| Yes | 58 | 35 | 23 | 0.024 | 2.641 | 1.244–5.608 | 0.011 | |
| No | 94 | 73 | 21 | |||||
| History of chronic lung disease, n | ||||||||
| Yes | 19 | 12 | 7 | 0.420 | ||||
| No | 133 | 96 | 37 | |||||
| Irradiation site, n | ||||||||
| Upper lobe | 101 | 71 | 30 | 0.773 | ||||
| Lower lobe | 51 | 37 | 14 | |||||
| Dosimetric parameters, median (IQR) | ||||||||
| Dose (Gy) | 60.0 (50.0–60.0) | 60.0 (50.0–60.0) | 0.725 | |||||
| V5 (%) | 45.0 (35.3–53.0) | 50.0 (42.3–55.0) | 0.034 | 0.355 | ||||
| V10 (%) | 35.0 (26.0–40.0) | 36.0 (33.3–40.0) | 0.053 | |||||
| V20 (%) | 25.0 (19.0–29.0) | 27.5 (23.0–30.0) | 0.030 | 0.976 | ||||
| V30 (%) | 17.0 (13.0–19.0) | 20.0 (16.0–21.0) | 0.014 | 1.728 | 1.214–2.460 | 0.002 | ||
| MLD (cc) | 1,333.5 (1,059.1–1,471.3) | 1,393.0 (1,237.3–1,526.8) | 0.107 | |||||
| PTV (cc) | 439.1 (289.5–654.4) | 484.2 (308.9–600.5) | 0.423 | |||||
| Lung all (cc) | 3,275.8 (2,855.3–3,900.0) | 3,627.5 (2,811.0–4,045.3) | 0.385 | |||||
OR, odds ratio; CI, confidence interval; IQR, interquartile range; SCLC, small cell lung cancer; ICI, immune checkpoint inhibitor; MLD, mean lung dose; PTV, planned target volume.
Table 4
| Characteristics | Total (N=152) | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Grade <3 (n=137) | Grade ≥3 (n=15) | P value | OR | 95% CI | P value | |||
| Age (years), median (IQR) | 59.0 (53.0–65.0) | 63.0 (56.0–71.0) | 0.085 | 0.434 | ||||
| Gender, n | ||||||||
| Female | 23 | 23 | 0 | 0.998 | ||||
| Male | 129 | 114 | 15 | |||||
| Histology, n | ||||||||
| Squamous | 65 | 57 | 8 | 0.307 | ||||
| Adenocarcinoma | 50 | 45 | 5 | |||||
| SCLC | 22 | 21 | 1 | |||||
| Other | 15 | 14 | 1 | |||||
| Stage, n | ||||||||
| III | 111 | 102 | 9 | 0.238 | ||||
| IV | 41 | 35 | 6 | |||||
| Smoking history, n | ||||||||
| Never | 84 | 76 | 8 | 0.874 | ||||
| Former | 68 | 61 | 7 | |||||
| ICI, n | ||||||||
| Yes | 58 | 49 | 9 | 0.075 | 0.099 | |||
| No | 94 | 88 | 6 | |||||
| History of chronic lung disease, n | ||||||||
| Yes | 19 | 13 | 6 | 0.002 | 6.359 | 1.953–20.705 | 0.002 | |
| No | 133 | 124 | 9 | |||||
| Irradiation site, n | ||||||||
| Upper lobe | 101 | 92 | 9 | 0.579 | ||||
| Lower lobe | 51 | 45 | 6 | |||||
| Dosimetric parameters, median (IQR) | ||||||||
| Dose (Gy) | 60.0 (50.0–60.0) | 50.0 (50.0–60.0) | 0.377 | |||||
| V5 (%) | 46.0 (39.0–53.0) | 50.0 (43.0–55.0) | 0.183 | |||||
| V10 (%) | 36.0 (27.0–40.0) | 36.0 (32.0–41.0) | 0.253 | |||||
| V20 (%) | 26.0 (20.0–30.0) | 28.0 (18.0–29.0) | 0.736 | |||||
| V30 (%) | 17.0 (13.5–20.0) | 20.0 (12.0–21.0) | 0.975 | |||||
| MLD (cc) | 1,347.0 (1,106.4–1,479.2) | 1,386.6 (1,038.4–1,475.5) | 0.981 | |||||
| PTV (cc) | 430.0 (290.0–596.3) | 516.0 (366.2–733.4) | 0.016 | 0.130 | ||||
| Lung all (cc) | 3,288.0 (2,828.0–3,905.3) | 3,697.8 (3,365.0–4,037.0) | 0.083 | 0.610 | ||||
OR, odds ratio; CI, confidence interval; IQR, interquartile range; SCLC, small cell lung cancer; ICI, immune checkpoint inhibitor; MLD, mean lung dose; PTV, planned target volume.
Risk factors of pneumonitis in the combination group
In the combination group, univariate and multivariate analyses showed that a history of chronic lung disease was correlated with grade ≥3 pneumonitis (P=0.017). There was no significant correlation between dosimetric parameters and the incidence of grade ≥2 or 3 pneumonitis (Tables 5,6). Therefore, in patients who undergo chemoradiation in combination with ICI, more strictly limiting dosimetric parameters such as V5, V20, V30, MLD, or PTV cannot reduce the incidence of pneumonitis. Instead, the combination treatment should be applied with caution in patients with a history of chronic lung disease.
Table 5
| Characteristics | Univariate analysis | |||||
|---|---|---|---|---|---|---|
| Grade <2 (n=35) | Grade ≥2 (n=23) | P value | Grade <3 (n=49) | Grade ≥3 (n=9) | P value | |
| Age (years), median (IQR) | 68.0 (53.0–65.0) | 63.0 (56.0–67.0) | 0.111 | 60.0 (54.5–65.0) | 63.0 (55.5–74.0) | 0.190 |
| Gender, n | 0.343 | 0.999 | ||||
| Female | 2 | 3 | 5 | 0 | ||
| Male | 33 | 20 | 44 | 9 | ||
| Histology, n | 0.678 | 0.477 | ||||
| Squamous | 17 | 11 | 23 | 5 | ||
| Adenocarcinoma | 7 | 8 | 12 | 3 | ||
| SCLC | 7 | 1 | 8 | 0 | ||
| Other | 4 | 3 | 6 | 1 | ||
| Stage, n | 0.123 | 0.239 | ||||
| III | 21 | 9 | 27 | 3 | ||
| IV | 14 | 14 | 22 | 6 | ||
| Smoking history, n | 0.261 | 0.635 | ||||
| Never | 16 | 14 | 26 | 4 | ||
| Former | 19 | 9 | 23 | 5 | ||
| ICI type, n | 0.178 | 0.601 | ||||
| PD-1 inhibitor | 27 | 21 | 40 | 8 | ||
| PD-L1 inhibitor | 8 | 2 | 9 | 1 | ||
| History of chronic lung disease, n | 0.296 | 0.017 | ||||
| Yes | 4 | 5 | 5 | 4 | ||
| No | 31 | 18 | 44 | 5 | ||
| Irradiation site, n | 0.855 | 0.577 | ||||
| Upper lobe | 22 | 15 | 32 | 5 | ||
| Lower lobe | 13 | 8 | 17 | 4 | ||
| Dosimetric parameters, median (IQR) | ||||||
| Dose (Gy) | 60.0 (54.0–60.0) | 60.0 (50.0–60.0) | 0.051 | 60.0 (50.0–60.0) | 50.0 (50.0–60.0) | 0.123 |
| V5 (%) | 46.0 (32.6–50.0) | 50.0 (42.0–55.0) | 0.094 | 46.0 (34.0–51.5) | 50.0 (42.5–55.0) | 0.308 |
| V10 (%) | 33.0 (22.0–38.0) | 36.0 (26.0–40.0) | 0.067 | 35.0 (24.5–38.0) | 34.0 (25.0–42.5) | 0.515 |
| V20 (%) | 24.0 (16.0–27.0) | 27.0 (20.0–30.0) | 0.101 | 25.0 (18.5–29.0) | 20.0 (15.5–28.0) | 0.615 |
| V30 (%) | 16.0 (10.0–19.0) | 18.5 (13.0–20.7) | 0.158 | 17.5 (11.5–20.0) | 13.0 (9–20.0) | 0.490 |
| MLD (cc) | 1,333.0 (1,049.0–1,452.0) |
1,353.0 (1,098.4–1,502.0) |
0.415 | 1,337.9 (1,144.6–1,479.5) |
1,098.4 (900–1,457.3) |
0.291 |
| PTV (cc) | 408.7 (261.6–502.0) | 489.9 (229.7–586.8) | 0.499 | 430.0 (256.9–526.8) | 502.0 (263.3–837.5) | 0.132 |
| Lung all (cc) | 3,360.0 (2,828.0–4,055.4) |
3,654.9 (2,798.9–4,668.3) |
0.408 | 3,574.2 (2,813.4–4,276.5) |
3,654.9 (3,008.9–4,593.5) |
0.421 |
ICI, immune checkpoint inhibitor; IQR, interquartile range; SCLC, small cell lung cancer; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; MLD, mean lung dose; PTV, planned target volume.
Table 6
| Characteristics | Pneumonitis grade ≥2 | Pneumonitis grade ≥3 | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Median age | 1.062 | 0.985–1.145 | 0.118 | ||||
| Stage (III vs. IV) | 2.082 | 0.625–6.934 | 0.232 | ||||
| ICI (PD-1 vs. PD-L1) | |||||||
| History of chronic lung disease (no vs. yes) | 8.351 | 1.469–47.484 | 0.017 | ||||
| Median dose | 0.318 | 0.094–1.081 | 0.318 | 0.293 | 0.61–1.414 | 0.126 | |
| V5 | 1.100 | 0.562–2.155 | 0.780 | ||||
| V20 | 1.382 | 0.708–2.698 | 0.343 | ||||
| V30 | |||||||
| PTV | 0.924 | 0.451–1.892 | 0.829 | ||||
ICI, immune checkpoint inhibitor; OR, odds ratio; CI, confidence interval; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; PTV, planned target volume.
Discussion
In recent years, many studies have attempted to improve the survival of locally advanced and inoperable lung cancer patients (7,9). The PACIFIC study explored the use of durvalumab, a PD-L1 inhibitor, after cCRT as consolidation therapy in stage III inoperable NSCLC and reported an outstanding increase in median progression-free survival (mPFS) and overall survival (OS), compared with cCRT alone (5). Meanwhile, the PACIFIC study indicated that starting durvalumab within 2 weeks after radiation (hazard ratio: 0.39, 95% CI: 0.26–0.58) gained preferable mPFS than its initiation beyond 2 weeks (hazard ratio: 0.63, 95% CI: 0.49–0.80) (10). Furthermore, a retrospective analysis showed OS improvement when ICI was started at least 1 month before RT, indicating a benefit of “induction effect” of ICI prior to RT (11). So, concern about the time to initiate ICIs and the treatment-related lung injury had arisen. In the PACIFIC-R study, an international, retrospective study of a cohort of patients who received at least one dose of durvalumab after chemoradiotherapy enrolled 250 patients, showed 17.9% incidence of pneumonitis or ILD (250 of 1,399). In all, 4.0% (n=56), 8.4% (n=118), 2.9% (n=41), and 0.4% (n=5) of the patients in the full analysis set had pneumonitis or ILD events classified as mild, moderate, severe, and life threatening or fatal, respectively (12).
For lung cancer, the most worrying side effect of combining ICI with chemoradiation therapy is pneumonitis. ICI treatment alone can induce pneumonitis, with an incidence of 5–6% according to clinical trials (13,14). Meanwhile, in a real-world study, the rate reached 19% (15) and was suggested to correlate to race, smoking status, history of ILD and COPD (16), and combined therapy (17). Thoracic RT also causes pneumonitis. The incidence of cCRT-induced grade ≥3 pneumonitis in NSCLC was shown to be 7.85% in platinum-based double chemotherapy (18). So, physicians worried that the combination of chemoradiation and ICI would increase the risk of pneumonitis, but it remained unconfirmed. The NICOLAS study was the first prospective trial to evaluate the safety and efficacy of adding nivolumab (a kind of ICI) concurrently to chemoradiotherapy in stage III NSCLC patients (8). Among 77 patients, 9 (11.7%) experienced pneumonitis of grade 3 or higher. Keynote-799, a phase 2 non-randomized trial, demonstrated a 6.9–8% incidence of grade ≥3 pneumonitis in patients who received pembrolizumab plus cCRT (7). However, both of these prospective studies were single-arm studies without comparison with chemoradiation, and due to more complicated situations in real-world patients, it was unclear how much the results of these clinical trials could be extrapolated to clinical practice. Many studies have pointed out the disparate results between clinical trials and real-world applications (19,20). A few retrospective studies analyzed pneumonitis in patients who received ICIs and radiation (21-24). Most of them reported the recalled RP in patients receiving ICI after cCRT (21,23,24). Bi et al. reported pneumonitis in patients receiving ICI before radiation (22). The RP of grade ≥2 was present in 40% (16/40) patients, and that of grade ≥3 was 22.5% (9/40), but it also did not compare to the chemoradiation group.
To our knowledge, this is the largest retrospective concurrent controlled study comparing the incidence of pneumonitis in chemoradiation alone and chemoradiation combined with ICI. The comparison of patients at the same center during the same period was more reliable. This study presented a comprehensive pneumonitis profile of chemoradiation with or without ICI of lung cancer from a Chinese cohort. We found that the rate of grade ≥2 pneumonitis in the combination group was 39.7%, in similarity with other reports (8,22,25); it was significantly higher than that in the chemoradiation group (39.7% vs. 22.3%, P=0.028). Although the incidence of grade ≥3 pneumonitis in the combination group showed an increasing trend, it did not reach statistical significance (15.5% vs. 6.4%, P=0.092). The rate of grade ≥3 pneumonitis in our report was slightly higher than in Wang et al. (26), because our study contained both concurrent and sequential chemoradiation with ICI, whereas all patients received sequential chemoradiation with ICI in Wang’s report. Although the incidence of symptomatic pneumonitis was increased in the combination group, the majority recovered after receiving corticosteroids and did not increase treatment-related death (1.7% vs. 1.1%). Multivariate analysis showed that the only impact factor of grade ≥3 pneumonitis in the combination group was the history of chronic lung disease. So, the combination treatment should be applied with caution in patients with a history of chronic lung disease.
Decades ago, Madani et al. reported that radiation dosimetry could predict the incidence of pneumonitis (27). In our study, the application of ICI and V30 were the independent risk factors of grade ≥2 pneumonitis. Whether it remained appropriate in the context of ICI is controversial. The results showed no correlation of V5, V20, V30, and MLD with grade ≥2 pneumonitis, which was in accordance with a prospective phase I study (25). In other words, regular radiation dosimetry parameters did not affect the incidence of pneumonitis under the current chest radiation criterion of West China Hospital (V5 <50%, V20 <30%, V30 <20%, MLD <1,500 cc). In clinical practice, physicians found that RP also occurred in patients receiving low dose radiation after ICI treatment. This could not be explained by high dose radiation, but must have been due to some other uncertain reasons.
So far, the standard treatment of unresectable stage III and stage IV NSCLC is chemoradiation and chemotherapy with ICI, respectively. Therefore, almost half of the combination group of our study was stage IV. The baseline of stage in the two groups was not balanced, so the survival could not be evaluated in this retrospective study and we mainly focused on the safety. Our results suggested that chemoradiation combined with ICI was generally safe and did not increase treatment-related death, but whether it is preferable in unresectable NSCLC, especially unresectable stage III, needs further study. Other publications aiming to address the safety of radiation in the context of ICI have been limited by heterogeneities of populations, radiation modes (radical, palliative), and fractional dose (conventional, hypofractionated). In most former studies (23,24), patients who received SBRT in lung lesions were also taken into analyzes, which might reduce high grade pneumonitis due to smaller and more conformal irradiated volume, whereas radical radiation usually used conventional fraction rather than SBRT in advanced stage NSCLC. In this study, we only included conventional chest radiation cases and the patients’ features were mainly balanced in two groups. However, we only observed that acute RP occurred within 6 months after radiation; the chronic pulmonary injury was not estimated. In addition, we only enrolled patients with full data, which inevitably introduced selected bias. We also demonstrated an increasing trend of grade ≥3 pneumonitis in the combination group that needed corticosteroid treatment (28). Whether its occurrence warrants suspension of ICI application and impacts OS needs further study.
Conclusions
In the real world, the incidence of pneumonitis of chemoradiation combined with ICI was higher than chemoradiation alone, but severe pneumonitis was only associated with the history of chronic lung disease. In the combination group, dosimetric parameters, including V5, V20, V30, and MLD, were not correlated to the development of pneumonitis under the current chest radiation criterion of West China Hospital (V5 <50%, V20 <30%, V30 <20%, MLD <1,500 cc). Thus, combination therapy was generally safe, but not for patients with the history of chronic lung disease.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-756/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-756/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-756/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-756/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Committee of West China Hospital (No. 2023-1295) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shi Y, Ji M, Jiang Y, et al. A cohort study of the efficacy and safety of immune checkpoint inhibitors plus anlotinib versus immune checkpoint inhibitors alone as the treatment of advanced non-small cell lung cancer in the real world. Transl Lung Cancer Res 2022;11:1051-68. [Crossref] [PubMed]
- NCCN. NCCN guidelines Version 4, 2023. Non-Small Cell Lung Cancer. 2023. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Shibata Y, Murakami S, Kato T. Overview of checkpoint inhibitor pneumonitis: incidence and associated risk factors. Expert Opin Drug Saf 2021;20:537-47. [Crossref] [PubMed]
- Jayakrishnan B, Al-Moundhri M, Burney I, et al. Pulmonary toxicities of immune check point inhibitors in the management of cancer: mini review. Adv Respir Med 2022;90:219-29. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. [Crossref] [PubMed]
- Zhou Q, Chen M, Jiang O, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2022;23:209-19. [Crossref] [PubMed]
- Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol 2021; Epub ahead of print. [Crossref]
- Peters S, Felip E, Dafni U, et al. Progression-Free and Overall Survival for Concurrent Nivolumab With Standard Concurrent Chemoradiotherapy in Locally Advanced Stage IIIA-B NSCLC: Results From the European Thoracic Oncology Platform NICOLAS Phase II Trial (European Thoracic Oncology Platform 6-14). J Thorac Oncol 2021;16:278-88. [Crossref] [PubMed]
- Manapov F, Kenndoff S, Käsmann L. NICOLAS, DETERRED and KEYNOTE 799: focus on escalation of conventionally fractionated chemoradiotherapy by immune checkpoint inhibition in unresectable stage III non-small cell lung cancer. Transl Lung Cancer Res 2022;11:702-5. [Crossref] [PubMed]
- Uemura T, Hida T. Durvalumab showed long and durable effects after chemoradiotherapy in stage III non-small cell lung cancer: results of the PACIFIC study. J Thorac Dis 2018;10:S1108-12. [Crossref] [PubMed]
- Samstein R, Rimner A, Barker CA, et al. Combined Immune Checkpoint Blockade and Radiation Therapy: Timing and Dose Fractionation Associated with Greatest Survival Duration Among Over 750 Treated Patients. Int J Radiat Oncol Biol Phys 2017;99:S129-30.
- Girard N, Bar J, Garrido P, et al. Treatment Characteristics and Real-World Progression-Free Survival in Patients With Unresectable Stage III NSCLC Who Received Durvalumab After Chemoradiotherapy: Findings From the PACIFIC-R Study. J Thorac Oncol 2023;18:181-93. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref] [PubMed]
- Tiu BC, Zubiri L, Iheke J, et al. Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: a multi-institutional cohort study. J Immunother Cancer 2022;10:e004670. [Crossref] [PubMed]
- Chao Y, Zhou J, Hsu S, et al. Risk factors for immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer. Transl Lung Cancer Res 2022;11:295-306. [Crossref] [PubMed]
- Naidoo J, Nishino M, Patel SP, et al. Immune-Related Pneumonitis After Chemoradiotherapy and Subsequent Immune Checkpoint Blockade in Unresectable Stage III Non-Small-Cell Lung Cancer. Clin Lung Cancer 2020;21:e435-44. [Crossref] [PubMed]
- Kuang Y, Pierce CM, Chang HC, et al. Chemoradiation-induced pneumonitis in patients with unresectable stage III non-small cell lung cancer: A systematic literature review and meta-analysis. Lung Cancer 2022;174:174-85. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Jung HA, Noh JM, Sun JM, et al. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer 2020;146:23-9. [Crossref] [PubMed]
- Jang JY, Kim SS, Song SY, et al. Radiation pneumonitis in patients with non-small-cell lung cancer receiving chemoradiotherapy and an immune checkpoint inhibitor: a retrospective study. Radiat Oncol 2021;16:231. [Crossref] [PubMed]
- Bi J, Qian J, Yang D, et al. Dosimetric Risk Factors for Acute Radiation Pneumonitis in Patients With Prior Receipt of Immune Checkpoint Inhibitors. Front Immunol 2021;12:828858. [Crossref] [PubMed]
- Lu X, Wang J, Zhang T, et al. Comprehensive Pneumonitis Profile of Thoracic Radiotherapy Followed by Immune Checkpoint Inhibitor and Risk Factors for Radiation Recall Pneumonitis in Lung Cancer. Front Immunol 2022;13:918787. [Crossref] [PubMed]
- Cousin F, Desir C, Ben Mustapha S, et al. Incidence, risk factors, and CT characteristics of radiation recall pneumonitis induced by immune checkpoint inhibitor in lung cancer. Radiother Oncol 2021;157:47-55. [Crossref] [PubMed]
- Jabbour SK, Berman AT, Decker RH, et al. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol 2020;6:848-55. [Crossref] [PubMed]
- Wang Y, Zhang T, Wang J, et al. Induction Immune Checkpoint Inhibitors and Chemotherapy Before Definitive Chemoradiation Therapy for Patients With Bulky Unresectable Stage III Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2023;116:590-600. [Crossref] [PubMed]
- Madani I, De Ruyck K, Goeminne H, et al. Predicting risk of radiation-induced lung injury. J Thorac Oncol 2007;2:864-74. [Crossref] [PubMed]
- Tao H, Li F, Wu D, et al. Rate and risk factors of recurrent immune checkpoint inhibitor-related pneumonitis in patients with lung cancer. Transl Lung Cancer Res 2022;11:381-92. [Crossref] [PubMed]


