
PD-1 expression on tumor cells: a new target for cancer therapy
In this editorial commentary, we discussed the potential of programmed death-1 (PD-1) expression on tumor cells as a novel target for the treatment of non-small cell lung cancer (NSCLC) patients based mainly on the findings reported by Rotolo and colleagues (1). We focused on PD-1 expression on tumor cells as a biomarker for combination chemotherapy with anti-PD-1 antibodies and cytotoxic agents as well as future perspectives for PD-1 targeted cancer therapy (Figure 1).
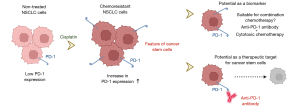
Anti-PD-1 blockade is one of the most frequently used immune checkpoint inhibitors in multiple settings, including monotherapy, combination therapy with cytotoxic agents (2), neoadjuvant therapy (3), and post-chemoradiation therapy (4), for patients with NSCLC. While the most common mechanism for the anti-tumor effects of anti-PD-1 blockade is an increase in T cell activity in the tumor microenvironment (5-7), a lymphocyte-independent function was recently reported in melanoma (8-10). Kleffel and colleagues reported that human melanoma tumor tissues frequently contained PD-1-expressing tumor cell subsets. Moreover, an anti-PD-1 antibody inhibited lymphocyte-independent tumor growth in murine models via the mTOR pathway (8). Sanlorenzo and colleagues demonstrated that BRAF and MEK inhibitors increased the number of PD-1-positive melanoma cells, indicating the potential of lymphocyte-independent synergism with anti-PD-1 antibodies (9). In the study that is the focus of this editorial commentary, Rotolo and colleagues reported the novel lymphocyte-independent anti-tumor efficacy of an anti-PD-1 antibody in human and murine NSCLC cell lines (1). The baseline expression of PD-1 in NSCLC cell lines was low and increased after the administration of cisplatin. PD-1 expression was enhanced in stem-like NSCLC pneumospheres, derived from NSCLC cell lines cultured within a defined serum-free medium, exhibiting characteristics reminiscent of stem cells. The anti-PD-1 antibody showed lymphocyte-independent anti-tumor efficacy in vitro and in vivo, and its combination with cisplatin exerted synergistic anti-tumor effects. A question refers to the variance in downstream signaling of PD-1 observed between T cells and tumor cells. Anti-PD1 antibody blocks the growth of PD-1-positive tumor cells, but not PD-1-expressing T cells. Comparison of downstream signaling of PD-1 between T cells and tumor cells will clarify the rational of anti-PD-1 therapy.
The study by Rotolo and colleagues focused on the expression of PD-1, not programmed death-ligand 1 (PD-L1), in NSCLC cells. PD-L1 is currently used as a biomarker to predict the efficacy of anti-PD-1 antibody therapy for NSCLC patients (11,12), whereas PD-1 expression has not been reported to be a predictive biomarker of anti-PD-1 treatment outcome in clinical settings. A critical issue for NSCLC patients without driver mutations is that there are currently no useful biomarkers for combination therapy with anti-PD-1 antibodies and cytotoxic agents. PD-L1 expression is not used as a biomarker for the combination therapy with anti-PD-1 antibodies. The findings obtained by Rotolo and colleagues suggested that PD-1-positive NSCLC cells exhibited resistance to cytotoxic chemotherapy. Anti-PD-1 may be used more effectively when NSCLC relapses after the administration of cytotoxic agents for NSCLC highly expressing PD-1. The study by Rotolo and colleagues showed that the concurrent combination of an anti-PD-1 antibody with cisplatin exerted synergistic lymphocyte-independent effects on NSCLC in vivo. The synergistic effects of the combination of anti-PD-1 antibodies with cytotoxic agents have been attributed to immunogenic cell death induced by cytotoxic agents (13). On the other hand, the findings of lymphocyte-independent experiments conducted by Rotolo and colleagues provide a novel viewpoint of anti-PD-1 providing additional efficacy to cytotoxic agents by targeting PD-1-positive NSCLC cells. Therefore, PD-1 expression on NSCLC cells has potential as a predictive biomarker for the efficacy of anti-PD-1 therapy with cytotoxic agents (Figure 1). Although the study by Rotolo and colleagues mainly focused on PD-1 mRNA detection in cell lines, further investigation of PD-1 expression by immunohistochemistry (IHC) staining of human tumor tissues is needed for the development of PD-1 as a novel biomarker. Furthermore, protein profiling platforms such as digital spatial profiling (DSP) also provide insights into PD-1/PD-L1 axis in the tumor microenvironment.
The study by Rotolo and colleagues suggested that PD-1-positive NSCLC cells had stem-like features that indicated chemoresistance (Figure 1). The stemness of tumor cells is an important issue that needs to be overcome because it has become clear that the targeted destruction of cancer stem cells (CSCs) is required for a radical cure for malignant tumors (14,15). CSCs are a small subset of tumor cells in various cancer types that have intrinsic self-renewal and tumorigenic characteristics, which lead to chemoresistance (14). CSCs possess these properties via signaling pathways, including the Notch, WNT, Hedgehog, and Hippo pathways (14). While agents that target CSCs in all cancer types, including lung cancer, are not currently available in clinical settings, previous studies reported the development of drugs that target CSCs in NSCLC (14,16-19). For example, inhibitory antibodies against delta-like ligands 3 and 4, which are involved in the Notch signaling pathway, have been developed as novel drugs targeting CSCs and are being used in phase I and II clinical trials for NSCLC and small cell lung cancer (SCLC) patients (16,17). The anti-tumor efficacy of napabucasin has also been demonstrated via its inhibition of CSCs by targeting STAT3, a critical mediator for the maintenance of cancer stemness in NSCLC (18). While phase III clinical trials on the efficacy of napabucasin for NSCLC, colorectal cancer, and gastric cancer are now underway according to ClinicalTrials.gov (https://clinicaltrials.gov), the addition of napabucasin to conventional cytotoxic chemotherapy did not improve treatment efficacy in patients with untreated metastatic pancreatic adenocarcinoma (20). Besides the development of drugs that target CSCs, anti-PD-1 antibodies have been suggested to disrupt interactions between CSCs and immune cells. Various interactions between CSCs and immune cells prevent the detection of CSCs by immune cells (14). Therefore, in CSC targeted treatments, anti-PD-1 antibodies may target not only cancer stemness, but also interactions with immune cells, thereby preventing immune evasion by CSCs.
The study by Rotolo and colleagues showed the limited expression of PD-1 in NSCLC cell lines, particularly before the administration of cisplatin; however, a previous study reported that human melanoma cells frequently included cancer cell subsets that highly expressed PD-1 without the prior administration of cytotoxic agents (8). Furthermore, anti-PD-1 antibodies inhibited lymphocyte-independent tumor growth in PD-1-expressing melanoma regardless of the administration of cytotoxic agents. The analysis of PD-1 expression in tumor cells across a large cohort of human samples representing various tumor types is required. The next question refers to the impact of co-expression of PD-1 and PD-L1 in tumor cells on the tumor microenvironment. The interaction between PD-1 and PD-L1 within tumor cells potentially influences the efficacy of anti-PD-1 therapy. Concerning hyperproliferative disease following anti-PD-1 therapy, the impact of PD-1 expression in tumor cells on the pathogenesis of these conditions needs assessment. Further investigations for the application of PD-1 expression on tumor cells as a therapeutic target in various cancer types are anticipated.
In summary, Rotolo and colleagues reported increases in PD-1 expression in chemoresistant NSCLC cells and the novel lymphocyte-independent anti-tumor efficacy of anti-PD-1 antibodies against NSCLC via the inhibition of cancer stemness. Further studies will broaden the possibilities of anti-PD-1 therapy for cancer patients.
Acknowledgments
We would like to sincerely thank the editorial board of Translational Lung Cancer Research for inviting us to write this editorial.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-683/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-683/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rotolo R, Leuci V, Donini C, et al. Novel Lymphocyte-Independent Antitumor Activity by PD-1 Blocking Antibody against PD-1+ Chemoresistant Lung Cancer Cells. Clin Cancer Res 2023;29:621-34. [Crossref] [PubMed]
- Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol 2021;18:625-44. [Crossref] [PubMed]
- Akinboro O, Drezner N, Amatya A, et al. US Food and Drug Administration Approval Summary: Nivolumab Plus Platinum-Doublet Chemotherapy for the Neoadjuvant Treatment of Patients With Resectable Non-Small-Cell Lung Cancer. J Clin Oncol 2023;41:3249-59. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Lin Q, Wang X, Hu Y. The opportunities and challenges in immunotherapy: Insights from the regulation of PD-L1 in cancer cells. Cancer Lett 2023;569:216318. [Crossref] [PubMed]
- Kleffel S, Posch C, Barthel SR, et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell 2015;162:1242-56. [Crossref] [PubMed]
- Sanlorenzo M, Vujic I, Floris A, et al. BRAF and MEK Inhibitors Increase PD-1-Positive Melanoma Cells Leading to a Potential Lymphocyte-Independent Synergism with Anti-PD-1 Antibody. Clin Cancer Res 2018;24:3377-85. [Crossref] [PubMed]
- Chen M, Bie L, Ying J. Cancer cell-intrinsic PD-1: Its role in malignant progression and immunotherapy. Biomed Pharmacother 2023;167:115514. [Crossref] [PubMed]
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064-74. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J Clin Oncol 2021;39:2339-49. [Crossref] [PubMed]
- Opzoomer JW, Sosnowska D, Anstee JE, et al. Cytotoxic Chemotherapy as an Immune Stimulus: A Molecular Perspective on Turning Up the Immunological Heat on Cancer. Front Immunol 2019;10:1654. [Crossref] [PubMed]
- Clara JA, Monge C, Yang Y, et al. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol 2020;17:204-32. [Crossref] [PubMed]
- Phi LTH, Sari IN, Yang YG, et al. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int 2018;2018:5416923. [Crossref] [PubMed]
- McKeage MJ, Kotasek D, Markman B, et al. Phase IB Trial of the Anti-Cancer Stem Cell DLL4-Binding Agent Demcizumab with Pemetrexed and Carboplatin as First-Line Treatment of Metastatic Non-Squamous NSCLC. Target Oncol 2018;13:89-98. [Crossref] [PubMed]
- Morgensztern D, Besse B, Greillier L, et al. Efficacy and Safety of Rovalpituzumab Tesirine in Third-Line and Beyond Patients with DLL3-Expressing, Relapsed/Refractory Small-Cell Lung Cancer: Results From the Phase II TRINITY Study. Clin Cancer Res 2019;25:6958-66. [Crossref] [PubMed]
- MacDonagh L, Gray SG, Breen E, et al. BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett 2018;428:117-26. [Crossref] [PubMed]
- Liu M, Wu H, Xu C. Targeting cancer stem cell pathways for lung cancer therapy. Curr Opin Oncol 2023;35:78-85. [Crossref] [PubMed]
- Bekaii-Saab T, Okusaka T, Goldstein D, et al. Napabucasin plus nab-paclitaxel with gemcitabine versus nab-paclitaxel with gemcitabine in previously untreated metastatic pancreatic adenocarcinoma: an adaptive multicentre, randomised, open-label, phase 3, superiority trial. EClinicalMedicine 2023;58:101897. [Crossref] [PubMed]


