
Potential synergistic effects of cranial radiotherapy and atezolizumab in non-small cell lung cancer: an analysis of individual patient data from seven prospective trials
Highlight box
Key findings
• Overall survival (OS) in patients with metastatic non-small-cell lung cancer treated with atezolizumab was significantly better in patients with previously irradiated brain metastasis (BM) than that in those without BM.
What is known and what is new?
• Previous studies have mainly focused on investigating the therapeutic interaction between extracranial radiotherapy (RT) and programmed death 1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors. These studies have suggested a potential synergy between these two treatments.
• This study represents, to the best of our knowledge, a pilot investigation examining the effect of previous cranial RT on the efficacy of anti-PD-1/PD-L1 therapy based on the most compelling and largest body of clinical data available. The presence of baseline BM was not associated with poorer survival in patients on atezolizumab therapy. Intriguingly, in the atezolizumab group, OS was better among patients with previously irradiated BM than among those without BM. However, this survival advantage was not observed in the chemotherapy group.
What is the implication, and what should change now?
• Our findings challenge the classic view of immune privilege in the central nervous system and suggest a potential synergetic effect of cranial RT with PD-1/PD-L1 inhibitors. Cranial RT has the potential to help prime a more effective immune response to anti-PD-(L)1 therapy. Further clinical trials investigating this combination strategy for patients with BM are highly warranted.
Introduction
Brain metastasis (BM) is a frequent consequence of non-small cell lung cancer (NSCLC) (1), and traditional systemic treatments have limited efficacy against BM due to the blood-brain barrier, leading to generally unsatisfactory prognosis (2,3).
Programmed death 1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors have improved treatment for metastatic NSCLC. Studies have shown better overall survival (OS) with PD-1/PD-L1 inhibitors than with chemotherapy for patients with NSCLC BM (4,5). However, the impact of BM on OS and progression-free survival (PFS) for patients with NSCLC treated with anti-PD-1/PD-L1 therapy is still unclear. Some studies showed that BM did not impact OS or PFS (6-8), whereas others identify BM as a negative factor (9-11). Nevertheless, all of these studies are limited by small sample size and potential bias due to the nature of retrospective design.
The promising intracranial activity of PD-1/PD-L1 inhibitors also raises key questions about the clinical value of cranial radiotherapy (RT) in the era of immunotherapy. An increasing body of preclinical and clinical research has demonstrated the potential synergy of immunotherapy in combination with RT (12-14). However, much of the focus of previous clinical research has been on the therapeutic interaction between extracranial RT and PD-1/PD-L1 inhibitors, and there remains limited data regarding the clinical impact of previous cranial RT in patients treated with PD-1/PD-L1 inhibitors. A previous retrospective multi-institutional study showed that anti-PD-(L)1 therapy combined with upfront cranial RT prolonged OS for patients with brain-metastatic NSCLC than anti-PD-(L)1 therapy alone (15). Several case reports have demonstrated the extracranial abscopal effect induced by the combination of cranial RT and immune checkpoint inhibitors (ICIs) in melanoma (16) and NSCLC (17).
To fulfill this unmet need, we used Vivli, a global, neutral data-sharing platform that enables access to anonymized individual patient data from trials (18), to investigate the effect of previous cranial RT on treatment outcomes using individual patient data from seven prospective trials involving atezolizumab for the treatment of advanced NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-792/rc).
Methods
Study design and definitions
We searched the Vivli platform (https://vivli.org/; last accessed 7/30/2023) for prospective trials involving atezolizumab for the treatment of NSCLC. Seven clinical trials including BIRCH (NCT02031458) (19), FIR (NCT01846416) (20), IMpower130 (NCT02367781) (21), IMpower131 (NCT02367794) (22), IMpower150 (NCT02366143) (23), OAK (NCT02008227) (4), and POPLAR (NCT01903993) (24) were identified. All of the included trials obtained informed consent from participants and were done in full accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki (as revised in 2013). This study was deemed negligible risk research and exempt from review by the Institutional Review Board of Fudan University Shanghai Cancer Center.
Details of the therapeutic regimens and eligibility of the seven clinical trials have been published previously (4,19-24). Briefly, there were two phase II single-arm trials evaluating atezolizumab (1,200 mg intravenous administration every three weeks) as first-line or subsequent therapy in locally advanced or metastatic NSCLC patients with PD-L1 expressing tumors (BIRCH and FIR) (19,20); one phase II and one phase III randomized controlled trial (RCT) evaluating atezolizumab versus docetaxel as second-line or third-line therapy in patients with advanced or metastatic NSCLC (POPLAR and OAK) (4,24); two phase III RCTs evaluating atezolizumab combined with chemotherapy versus chemotherapy alone as first-line therapy in patients with stage IV non-squamous NSCLC (IMpower130) (21) or squamous NSCLC (IMpower131) (22); one three-arm phase III RCT evaluating atezolizumab combined with chemotherapy versus bevacizumab plus chemotherapy versus atezolizumab combined with bevacizumab plus chemotherapy as first-line therapy in patients with stage IV non-squamous NSCLC (IMpower150) (23). In these trials, patients with BM were eligible provided that the BM is asymptomatic or treated, and non-progressive with no ongoing requirement for corticosteroids. Brain monitoring (computed-tomography or magnetic resonance imaging) was mandatory before inclusion in the trials.
All patients who received at least one cycle of per-protocol treatment were included in the analysis. The patients were divided into two groups: those who received atezolizumab monotherapy or atezolizumab-chemoimmunotherapy combinations (atezolizumab group) and those who received comparator chemotherapies (chemotherapy group). For this study, we used an expanded definition of “baseline BM” that included patients who presented with metastatic brain lesions at baseline, as well as those with a history of BM (25). Patients with baseline BM were further divided into two subgroups based on whether they received cranial RT before starting per-protocol treatment: patients with previously irradiated BM (iBM) and patients with non-irradiated BM (niBM).
Adverse events (AEs) were assessed per the National Cancer Institute Common Terminology Criteria for AEs version 4.0. Serious AE was defined according to the International Conference on Harmonisation guideline for Good Clinical Practice, as any event that causes death, is life‐threatening, requires or prolongs hospitalization, results in persistent or significant disability, or leads to congenital anomaly/birth defect.
Statistical analysis
The primary aim of this study was the comparison of prognosis, in terms of OS and PFS, between patients with iBM, niBM and without BM in the two treatment groups (atezolizumab-containing group and chemotherapy group). OS was defined as the time from the date of initiation of per-protocol treatment to the date of death from any cause. PFS was defined as the time from the date of initiation of per-protocol treatment to the first occurrence of disease progression or death. An additional analysis was conducted to assess the neurological safety profiles of atezolizumab in patients with iBM, niBM, and without BM in the two treatment groups.
Pearson’s χ2 test or Fisher’s exact test was used to assess the differences in baseline characteristics between subgroups. OS and PFS were estimated using the Kaplan-Meier method and compared with the log-rank test. Univariable and multivariable Cox proportional hazards models with adjustment for key clinical-pathological factors [age, sex, race, history of tobacco use, tumor histology, Eastern Cooperative Oncology Group performance status (ECOG PS), treatment regimen, and PD-L1 expression] were used to report hazard ratios (HRs) and 95% confidence intervals (CIs). Interactions between BM status and treatment assignment (atezolizumab vs. chemotherapy) were tested with the Cox model to assess differences in the impact of BM status on survival outcomes, including PFS and OS, between the atezolizumab group and the chemotherapy group.
To adjust for the possible bias induced by the retrospective design of this study, 1:2 nearest-neighbor propensity score matching (PSM) with a caliper of 0.2 was used, accounting for age, sex, race, ECOG PS, tumor histology, tobacco use history, treatment regimen, previous extracranial RT, and PD-L1 expression. We evaluated the balance of covariates between arms using standardized mean difference (SMD); an SMD of 0.1 or less was deemed to be minimally different.
All statistical analyses were performed with R software, version 4.0. A two-sided P values less than 0.05 was considered statistically significant. Due to the exploratory nature of this study, no adjustment for multiple comparisons was applied.
Results
Patient characteristics
Table S1 provides an overview of the included seven clinical trials. Figure 1 shows the patient disposition for the per-protocol population.
The per-protocol population consisted of 4,714 patients, including 3,176 in the atezolizumab group and 1,538 in the chemotherapy group. Of the patients in the atezolizumab group and the chemotherapy group, 308 (9.7%) and 182 (11.8%) presented with baseline BM, respectively. Baseline demographics and clinical characteristics for patients with and without BM are shown in Table 1. In patients with baseline BM, 280 (90.9%) in the atezolizumab group and 165 (90.7%) in the chemotherapy group had previously received cranial RT before initiation of per-protocol treatment. The median interval between previous cranial RT and the initiation of per-protocol treatment was 1.4 months [interquartile range (IQR), 0.9–3.0 months] in the atezolizumab group and 1.8 months (IQR, 1.0–9.1 months) in the chemotherapy group.
Table 1
| Baseline characteristics | Chemotherapy | Atezolizumab | |||||
|---|---|---|---|---|---|---|---|
| Patients without baseline BM (n=1,356) | Patients with baseline BM (n=182) | P value† | Patients without baseline BM (n=2,868) | Patients with baseline BM (n=308) | P value† | ||
| Age (years) | 0.036 | 0.003 | |||||
| ≥65 | 641 (47%) | 71 (39%) | 1,388 (48%) | 122 (40%) | |||
| <65 | 715 (53%) | 111 (61%) | 1,480 (52%) | 186 (60%) | |||
| Sex | <0.001 | 0.004 | |||||
| Male | 898 (66%) | 95 (52%) | 1,857 (65%) | 174 (56%) | |||
| Female | 458 (34%) | 87 (48%) | 1,011 (35%) | 134 (44%) | |||
| Race | 0.464 | 0.268 | |||||
| White | 1,100 (81%) | 139 (76%) | 2,358 (82%) | 263 (85%) | |||
| Asian | 164 (12%) | 28 (15%) | 330 (12%) | 35 (11%) | |||
| Black | 39 (3%) | 6 (3%) | 60 (2%) | 2 (1%) | |||
| Other | 17 (1%) | 4 (2%) | 45 (2%) | 4 (1%) | |||
| Missing | 36 (3%) | 5 (3%) | 75 (3%) | 4 (1%) | |||
| ECOG PS | 0.650 | 0.895 | |||||
| 0 | 544 (40%) | 67 (37%) | 1,121 (39%) | 118 (38%) | |||
| ≥1 | 808 (60%) | 115 (63%) | 1,741 (61%) | 190 (62%) | |||
| Missing | 4 (0.3%) | 0 | 6 (0.2%) | 0 | |||
| Histology | <0.001 | <0.001 | |||||
| Squamous | 459 (34%) | 28 (15%) | 950 (33%) | 44 (14%) | |||
| Non-squamous | 891 (66%) | 154 (85%) | 1,912 (67%) | 264 (86%) | |||
| Missing | 6 (0.4%) | 0 | 6 (0.2%) | 0 | |||
| History of tobacco use | 0.707 | 0.711 | |||||
| Never | 180 (13%) | 26 (14%) | 461 (16%) | 47 (15%) | |||
| Current or previous | 1,176 (87%) | 156 (86%) | 2,407 (84%) | 261 (85%) | |||
| Number of prior lines of systemic therapies | 0.136 | <0.001 | |||||
| 0 | 816 (60%) | 99 (54%) | 1,724 (60%) | 217 (70%) | |||
| ≥1 | 540 (40%) | 83 (46%) | 1,144 (40%) | 91 (30%) | |||
| PD-L1 expression | 0.201 | 0.001 | |||||
| TC2/3 or IC2/3 | 427 (31%) | 69 (38%) | 1,344 (47%) | 111 (36%) | |||
| TC0/1 or IC0/1 | 927 (68%) | 113 (62%) | 1,517 (53%) | 197 (64%) | |||
| Missing | 2 (0.1%) | 0 | 7 (0.2%) | 0 | |||
†, patients with and without brain metastases are compared. BM, brain metastasis; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed death ligand-1; TC, tumor cell (TC2/3: ≥5% and <50%/≥50% PD-L1 staining; TC0/1: <1%/≥1% and <5% PD-L1 staining); IC, tumor-infiltrating immune cell (IC2/3: ≥5% and <10%/≥10% PD-L1 staining; IC0/1: <1%/≥1% and <5% PD-L1 staining).
Among patients with BM, OS and PFS were significantly better in the atezolizumab group compared to the chemotherapy group (median OS 18.7 vs. 12.8 months, HR: 0.62; 95% CI: 0.49–0.80; P=0.00012; Figure S1; median PFS 6.8 vs. 4.1 months, HR: 0.57; 95% CI: 0.47–0.70; P<0.0001; Figure S2), which is consistent with previous reports of anti-PD-1/PD-L1 treatment in patients with baseline BM (4,22).
OS
We compared the OS of patients with and without BM in the atezolizumab group and the chemotherapy group. As shown in Figure 2A, in the atezolizumab group, OS was better in patients with baseline BM compared to those without BM (median OS 18.7 vs. 15.8 months; HR: 0.83; 95% CI: 0.70–0.98; P=0.028), while in the chemotherapy group, OS was similar between patients with BM and those without BM (median OS 12.8 vs. 13.5 months; HR: 1.11; 95% CI: 0.92–1.35; P=0.273; Figure 2B). Interaction tests suggested a statistically significant difference in the impact of BM on OS between the atezolizumab group and the chemotherapy group (Pinteraction=0.024).
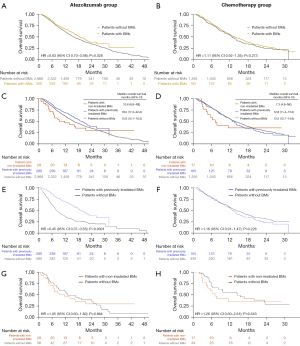
Patients with BM were divided into two subgroups according to their history of cranial RT, namely, patients with previously iBM and patients with niBM (Table S2). Figure 2C,2D shows the Kaplan-Meier survival curves for patients with iBM, patients with niBM, and patients without BM. In the atezolizumab group, patients with iBM had a trend toward improved OS than those with niBM (median OS 19.2 vs. 10.6 months; adjusted HR: 0.66; 95% CI: 0.41–1.07; P=0.090). Intriguingly, patients with iBM had longer OS compared to patients without BM (median OS 19.2 vs. 15.8 months; adjusted HR: 0.83; 95% CI: 0.70–0.99; P=0.043). There was no difference in OS between patients with niBM and those without BM (median OS 10.6 vs. 15.8 months; adjusted HR: 1.15; 95% CI: 0.73–1.80; P=0.551). In the chemotherapy group, there was no significant difference in OS between patients with iBM and those without BM (median OS 12.8 vs. 13.5 months; adjusted HR: 1.11; 95% CI: 0.90–1.36; P=0.320) or between patients with iBM and those with niBM (median OS 12.8 vs. 7.3 months; adjusted HR: 0.91; 95% CI: 0.48–1.76; P=0.788).
We applied PSM to reduce the bias due to confounding variables (age, sex, race, ECOG PS, tumor histology, tobacco use history, treatment regimen, previous extracranial RT, and PD-L1 expression) between patients without BM and patients with iBM (Tables S3,S4). A total of 19 (0.60%) patients in the atezolizumab group and 12 (0.79%) in the chemotherapy group were excluded due to missing relevant baseline characteristic data. In the atezolizumab group, OS was still significantly better in patients with iBM compared to patients without BM (unadjusted HR: 0.45, 95% CI: 0.37–0.55, P<0.0001; adjusted HR: 0.40, 95% CI: 0.32–0.49, P<0.0001; Figure 2E); in the chemotherapy group, patients with iBM had similar OS compared with those without BM (unadjusted HR: 1.16, 95% CI: 0.91–1.47, P=0.226; adjusted HR: 1.19, 95% CI: 0.94–1.51, P=0.156; Figure 2F). There was still a significant interaction between treatment groups and BM status (Pinteraction<0.001). Patients with niBM had numerically shorter OS compared with those without BM in the atezolizumab group (unadjusted HR: 1.05, 95% CI: 0.60–1.82, P=0.864; adjusted HR: 1.07, 95% CI: 0.61–1.87, P=0.814) and the chemotherapy group (unadjusted HR: 1.26, 95% CI: 0.60–2.61, P=0.543; adjusted HR: 0.86, 95% CI: 0.38–1.97, P=0.724) after PSM (Tables S5,S6, Figure 2G,2H).
PFS
We compared the PFS of patients with and without BM in the atezolizumab group and the chemotherapy group. As shown in Figure 3A,3B, in the atezolizumab group, PFS was numerically longer in patients with baseline BM compared to those without BM (median PFS 6.8 vs. 5.6 months; HR: 0.91; 95% CI: 0.80–1.04; P=0.149), while in the chemotherapy group, PFS was significantly shorter in patients with BM compared to those without BM (median PFS 4.1 vs. 5.6 months; HR: 1.37; 95% CI: 1.16–1.61; P<0.001). Interaction tests suggested a statistically significant difference in the impact of BM on PFS between the atezolizumab group and the chemotherapy group (Pinteraction<0.001).
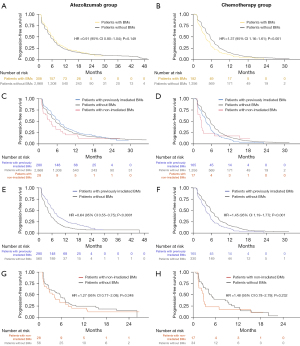
We further compared the PFS of patients with iBM, patients with niBM, and those without baseline BM in the two treatment groups (Figure 3C,3D). In the atezolizumab group, patients with iBM had a trend toward improved PFS than those with niBM (median PFS 6.9 vs. 3.9 months; adjusted HR: 0.66; 95% CI: 0.43–1.01; P=0.058). Patients with iBM had longer PFS compared to patients without BM (median PFS 6.9 vs. 5.6 months; adjusted HR: 0.86; 95% CI: 0.75–0.99; P=0.030). There was no difference in PFS between patients with niBM and those without BM (median PFS 3.9 vs. 5.6 months; adjusted HR: 1.29; 95% CI: 0.86–1.93; P=0.212). In the chemotherapy group, patients with iBM had shorter PFS compared with those without BM (median PFS 4.2 vs. 5.6 months; adjusted HR: 1.39; 95% CI: 1.16–1.64; P<0.001). There was no difference in PFS between patients with iBM and those with niBM (median PFS 4.2 vs. 2.7 months; adjusted HR: 0.82; 95% CI: 0.46–1.46; P=0.498).
PSM was applied to control for the heterogeneity between patients with iBM and those without BM (Tables S3,S4). In the atezolizumab group, PFS was still significantly better in patients with iBM compared to patients without BM (unadjusted HR: 0.64, 95% CI: 0.55–0.75, P<0.0001; adjusted HR: 0.60, 95% CI: 0.51–0.70, P<0.0001; Figure 3E) in the propensity-score matched cohort; in the chemotherapy group, patients with iBM had shorter PFS compared with those without BM (unadjusted HR: 1.45, 95% CI: 1.19–1.77, P<0.001; adjusted HR: 1.50, 95% CI: 1.23–1.83, P<0.001; Figure 3F). There was still a significant interaction between treatment groups and BM status (Pinteraction<0.001). Patients with niBM still had similar PFS compared with those without BM in the atezolizumab group (unadjusted HR: 1.27, 95% CI: 0.77–2.08, P=0.348; adjusted HR: 1.24, 95% CI: 0.74–2.07, P=0.409; Figure 3G) and the chemotherapy group (unadjusted HR: 1.48, 95% CI: 0.78–2.79, P=0.232; adjusted HR: 1.51, 95% CI: 0.73–3.13, P=0.269; Figure 3H) after PSM.
Neurological AEs
Atezolizumab-related neurological AEs of any grade were reported in 352 patients (12%) without baseline BMs, 5 patients (18%) with niBMs, and 44 patients (16%) with previously iBMs (Table 2). Atezolizumab-related grade 3/4 neurological AEs occurred in 24 patients (1%) without BMs, no patient with niBMs, and 7 patients (3%) with previously iBMs. The incidence of atezolizumab-related serious neurological AEs was similar between patients without BMs and patients with previously irradiated BMs (0.6% vs. 0.7%). The most common atezolizumab-related neurological AE was headaches (Table S7).
Table 2
| Safety characteristic | Patients without baseline BMs (n=2,868) | Patients with non-irradiated BMs (n=28) | Patients with previously irradiated BMs (n=280) |
|---|---|---|---|
| Any neurological AE | 1,426 (50%) | 12 (43%) | 181 (65%) |
| Any serious neurological AE | 89 (3%) | 1 (4%) | 21 (8%) |
| Any atezolizumab-related neurological AE | 352 (12%) | 5 (18%) | 44 (16%) |
| Grade 3/4 | 24 (1%) | 0 | 7 (3%) |
| Grade 5 | 0 | 0 | 0 |
| Any atezolizumab-related serious neurological AE | 16 (0.6%) | 0 | 2 (0.7%) |
AE, adverse event; BMs, brain metastases.
Discussion
In this pooled analysis of individual patient data from seven prospective trials involving atezolizumab for the treatment of NSCLC, we observed superior OS with atezolizumab in patients with BM versus those without BM. Furthermore, patients with iBM had better OS than those without BM, while patients with niBM had numerically shorter OS compared with those without BM. This survival advantage of patients with iBM over those without BM was not observed in the chemotherapy group. These results suggest that the superior survival outcomes observed in patients with BM, as compared to those without, in the present study may be due to the effect of previous cranial RT. This finding points to a potential synergy between cranial RT and atezolizumab. Additionally, this study represents, to the best of our knowledge, the first effort to report the effects of previous cranial RT on the neurological toxicity of anti-PD-(L)1 therapy using prospectively collected data. The neurological safety profile of atezolizumab in patients who previously received cranial RT for NSCLC was generally acceptable.
Up till now, there are several studies on the prognostic significance of BM in patients with metastatic NSCLC treated with anti-PD-(L)1 therapy (6,26,27). In a retrospective, multicenter analysis comparing the outcome of patients with NSCLC BM receiving ICIs (n=255) with the outcome of those without BM (n=770), the median PFS and OS were shorter for patients with BM than for those without BM (6). In multivariate analysis, presence of BM did not significantly impact PFS or OS (6). Notably, 39.2% of patients with BM had nonirradiated and/or progressive BM in this study (6). A pooled analysis of KEYNOTE-001, 010, 024, and 042 showed that the median PFS with pembrolizumab monotherapy was shorter for patients with stable BM than for those without BM (26). Consistently, another pooled analysis of KEYNOTE-021, -189, and -407 showed that the median PFS and OS with pembrolizumab plus platinum-based chemotherapy was shorter for patients with stable BM than for those without BM (27). Notably, only 10.5% of patients with BM had previously received cranial RT in this study. A unique feature of the present study, compared to previous investigations, is that the majority (90.9%) of the included patients with BM had previously irradiated stable BM. Therefore, we speculate that the superior survival outcomes of patients with BM, versus those without, in the present study may be attributed to the effect of previous cranial RT.
Although the immune environment of BMs from NSCLC has been demonstrated to be immunosuppressed with lower densities of T cells and lower adaptive immune responses compared with primary tumors (28), several trials have shown promising activity of PD-1/PD-L1 inhibitors in patients with BMs from NSCLC (4,5). Given the promising intracranial activity of PD-1/PD-L1 inhibitors, we speculate that the combination of anti-PD-(L)1 therapy and cranial RT might provide synergistic antitumor effects. Upregulated PD-L1 expression (29) and higher densities of tumor-infiltrating lymphocytes (30) in surgically resected specimens of BMs after cranial RT also suggest a potential synergy of PD-1/PD-L1 inhibitors and RT in the central nervous system, and intriguingly, several case reports have detailed the extracranial abscopal effect induced by the combination of cranial RT and ICIs in melanoma (16) and NSCLC (17). A recent study also suggested that cranial RT may prime a more effective systemic immune response to immunotherapy compared with RT to other organs (31).
One possible mechanism for the synergism between atezolizumab treatment and cranial RT is their combined effect on the immune system and tumor microenvironment. Cranial RT can enhance the release of tumor antigens, making them more accessible to the immune system. Atezolizumab, by blocking the PD-L1 pathway, can further enhance the immune response, preventing cancer cells from evading immune detection and destruction. This combined approach has the potential to increase tumor cell killing and promote a stronger anti-tumor immune response, potentially improving treatment outcomes for patients. It is important to note that further research is still needed to fully understand the mechanism and optimize the combination of atezolizumab treatment and cranial RT for maximum efficacy and safety.
To date, PD-1/PD-L1 inhibitor-related neurological disorders remain largely unexplored. To our knowledge, this pooled analysis represents the first effort to investigate the neurological safety profile of PD-1/PD-L1 inhibitors in a large patient population. Atezolizumab-related neurological AEs of any grade occurred in 12% of patients without baseline BMs in the atezolizumab group, which is consistent with the previously reported incidence of neurological immune-related AEs (0.3–14.0%) (32). The neurologic safety profile of atezolizumab in patients who had previously received cranial RT for NSCLC was generally acceptable. Due to the small sample size of patients with niBM, we did not compare the neurological safety profile between patients with iBM and those with niBM. There were more cases of atezolizumab-related grade 3/4 neurological AEs reported in patients with iBM than in patients without BM (3% vs. 1%). The incidence of atezolizumab-related serious neurological AEs was similar between patients without BM and patients with iBM (0.6% vs. 0.7%). Note that our finding should not be generalized to concurrent/consolidative cranial RT with anti-PD-(L)1 therapy. Caution is warranted with concurrent PD-1/PD-L1 inhibitors and stereotactic radiosurgery (SRS), as some previous reports have suggested that this combination strategy may increase the risk of radiation necrosis (33,34). Since information on RT techniques was not available for many patients, the effect of previous SRS on neurologic toxicity in patients treated with PD-1/PD-L1 inhibitors was not investigated.
While this study included a large patient population and high-quality data, there were some limitations. First, the small sample size of patients with niBM may have limited our power to detect significant survival differences between patients with niBM and those with iBM. However, patients with smaller BM may have been less likely to receive cranial RT and thus may have had better prognosis than the patients with cranial RT. In this regard, the better, albeit not significantly better, survival of patients with iBM compared to those with niBM in the atezolizumab group is especially notable. Secondly, there is a selection bias in the group of patients who received RT compared to those who did not. It is important to consider that patients with fewer and smaller BMs were more likely to receive treatment such as SRS. On the other hand, patients with more and larger BMs may opt for immunotherapy instead of whole brain radiation therapy (WBRT) to minimize potential toxicity. Similarly, patients with limited extracranial disease may have been more inclined to choose cranial RT as a more aggressive treatment approach. Third, most of the included trials did not provide details on the cranial RT regimen and patient’s BM status, such as number of baseline BM and the diameter of the largest BM. Therefore, the potential impact of these factors on OS could not be assessed. Fourth, the 7 trials included in this study did not specify the type of cranial RT (SRS or WBRT). As a result, we are unable to compare the survival outcomes between patients who previously received WBRT or SRS for BM and those without BM. Fifth, this analysis is based on a selective group of patients with treated, stable BM, which limits the generalizability of our findings. Further investigations on the combination of cranial RT and PD-1/PD-L1 inhibitors for active BM are warranted. Sixth, the 7 trials included did not provide specific information about the sites of the progressive disease (i.e. intracranial vs. extracranial progression). Therefore, intracranial PFS or extracranial PFS could not be analyzed.
Conclusions
In conclusion, this study demonstrated that OS in patients on atezolizumab therapy was significantly better in patients with previously iBM than that in patients without BM. The neurological safety profile of atezolizumab in patients who previously received cranial RT for NSCLC was generally acceptable. These findings suggest that even though anti-PD-(L)1 therapy has activity against BMs, patients may benefit from having cranial RT prior to anti-PD-(L)1 therapy, which merits further investigation.
Acknowledgments
This publication is based on research using data from Roche that has been made available through Vivli, Inc. Vivli has not contributed to nor approved, and is not in any way responsible for, the contents of this publication.
Funding: This research was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-792/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-792/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-792/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All of the included trials obtained informed consent from participants and were done in full accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki (as revised in 2013). This study was deemed negligible risk research and exempt from review by the Institutional Review Board of Fudan University Shanghai Cancer Center.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fenske DC, Price GL, Hess LM, et al. Systematic Review of Brain Metastases in Patients With Non-Small-Cell Lung Cancer in the United States, European Union, and Japan. Clin Lung Cancer 2017;18:607-14. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Oh Y, Taylor S, Bekele BN, et al. Number of metastatic sites is a strong predictor of survival in patients with nonsmall cell lung cancer with or without brain metastases. Cancer 2009;115:2930-8. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Hendriks LEL, Henon C, Auclin E, et al. Outcome of Patients with Non-Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors. J Thorac Oncol 2019;14:1244-54. [Crossref] [PubMed]
- Dudnik E, Moskovitz M, Daher S, et al. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: The real-life data. Lung Cancer 2018;126:217-23. [Crossref] [PubMed]
- Scott SC, Pennell NA. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J Thorac Oncol 2018;13:1771-5. [Crossref] [PubMed]
- Arbour KC, Mezquita L, Long N, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2872-8. [Crossref] [PubMed]
- Juergens RA, Mariano C, Jolivet J, et al. Real-world benefit of nivolumab in a Canadian non-small-cell lung cancer cohort. Curr Oncol 2018;25:384-92. [Crossref] [PubMed]
- Mountzios G, de Toma A, Economopoulou P, et al. Steroid Use Independently Predicts for Poor Outcomes in Patients With Advanced NSCLC and High PD-L1 Expression Receiving First-Line Pembrolizumab Monotherapy. Clin Lung Cancer 2021;22:e180-92. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med 2021;9:467-75. [Crossref] [PubMed]
- Gong X, Li X, Jiang T, et al. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1085-97. [Crossref] [PubMed]
- Guo T, Chu L, Chu X, et al. Brain metastases, patterns of intracranial progression, and the clinical value of upfront cranial radiotherapy in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors. Transl Lung Cancer Res 2022;11:173-87. [Crossref] [PubMed]
- Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014;3:e28780. [Crossref] [PubMed]
- Lin X, Lu T, Xie Z, et al. Extracranial abscopal effect induced by combining immunotherapy with brain radiotherapy in a patient with lung adenocarcinoma: A case report and literature review. Thorac Cancer 2019;10:1272-5. [Crossref] [PubMed]
- Bierer BE, Li R, Barnes M, et al. A Global, Neutral Platform for Sharing Trial Data. N Engl J Med 2016;374:2411-3. [Crossref] [PubMed]
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]
- Spigel DR, Chaft JE, Gettinger S, et al. FIR: Efficacy, Safety, and Biomarker Analysis of a Phase II Open-Label Study of Atezolizumab in PD-L1-Selected Patients With NSCLC. J Thorac Oncol 2018;13:1733-42. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J Thorac Oncol 2020;15:1351-60. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Gadgeel SM, Lukas RV, Goldschmidt J, et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase III OAK study. Lung Cancer 2019;128:105-12. [Crossref] [PubMed]
- Mansfield AS, Herbst RS, de Castro G Jr, et al. Outcomes With Pembrolizumab Monotherapy in Patients With Programmed Death-Ligand 1-Positive NSCLC With Brain Metastases: Pooled Analysis of KEYNOTE-001, 010, 024, and 042. JTO Clin Res Rep 2021;2:100205. [Crossref] [PubMed]
- Powell SF, Rodríguez-Abreu D, Langer CJ, et al. Outcomes With Pembrolizumab Plus Platinum-Based Chemotherapy for Patients With NSCLC and Stable Brain Metastases: Pooled Analysis of KEYNOTE-021, -189, and -407. J Thorac Oncol 2021;16:1883-92. [Crossref] [PubMed]
- Kudo Y, Haymaker C, Zhang J, et al. Suppressed immune microenvironment and repertoire in brain metastases from patients with resected non-small-cell lung cancer. Ann Oncol 2019;30:1521-30. [Crossref] [PubMed]
- Takamori S, Toyokawa G, Okamoto I, et al. Clinical Significance of PD-L1 Expression in Brain Metastases from Non-small Cell Lung Cancer. Anticancer Res 2018;38:553-7. [Crossref] [PubMed]
- Ikarashi D, Okimoto T, Shukuya T, et al. Comparison of Tumor Microenvironments Between Primary Tumors and Brain Metastases in Patients With NSCLC. JTO Clin Res Rep 2021;2:100230. [Crossref] [PubMed]
- Wu M, Liu J, Wu S, et al. Systemic Immune Activation and Responses of Irradiation to Different Metastatic Sites Combined With Immunotherapy in Advanced Non-Small Cell Lung Cancer. Front Immunol 2021;12:803247. [Crossref] [PubMed]
- Sechi E, Zekeridou A. Neurologic Complications of Immune Checkpoint Inhibitors in Thoracic Malignancies. J Thorac Oncol 2021;16:381-94. [Crossref] [PubMed]
- Alomari AK, Cohen J, Vortmeyer AO, et al. Possible Interaction of Anti-PD-1 Therapy with the Effects of Radiosurgery on Brain Metastases. Cancer Immunol Res 2016;4:481-7. [Crossref] [PubMed]
- Colaco RJ, Martin P, Kluger HM, et al. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg 2016;125:17-23. [Crossref] [PubMed]



