
Clinical and financial impact of immune checkpoint inhibitors following platinum chemotherapy in patients with advanced or metastatic non-small cell lung cancer: a nationwide population-based study
Highlight box
Key findings
• Immune checkpoint inhibitors (ICI) treatment led to longer survival and better prognosis in terms of overall survival and progression-free survival compared with cytotoxic chemotherapy (CC). However, the retention rate of ICIs was lower than targeted therapy (TT). Additionally, with ICI treatment, the increase in medical expenses for lung cancer treatment was higher than that with TT.
What is known and what is new?
• The survival benefit of subsequent ICI use after platinum-based CC is well established in patients with advanced non-small cell lung cancer.
• Our study found changes in the patterns of chemotherapy drug prescriptions after the coverage expanded, with a significant increase in annual medical expenses per patient.
What is the implication, and what should change now?
• Considering that a significantly higher medical cost was observed after ICI therapy, extensive profiling of TTs and ICIs is essential to improve the selection and sequencing of treatments, reduce the likelihood of ineffective therapies, and enhance patient outcomes.
Introduction
Background
Lung cancer is the most common carcinoma worldwide in terms of the morbidity and mortality associated with it (1,2). According to the Korea Cancer Registry, lung cancer has the third highest incidence among all carcinomas and is the most common cause of cancer-related death in the country (3,4). The crude incidence rate of lung cancer is increasing among both men and women every year, and it is expected to increase further after the introduction of the national lung cancer screening project in July 2018 (5).
Since the 2000s, research advances in biomarkers, such as EGFR, ALK, RET, BRAF, ROS1, NTRK, MET, and KRAS, to select patients for targeted and immunotherapy-based treatment have changed the treatment paradigm for non-small cell lung cancer (NSCLC) (6-8). Nevertheless, platinum-based chemotherapy regimens have been the mainstay treatments for most patients with NSCLC for whom an identifiable targeted therapy (TT) is not a treatment option. For decades, the median overall survival (OS) for advanced or metastatic disease has been less than 2 years (9,10).
The development of immune checkpoint inhibitors (ICIs) has dramatically changed the landscape of lung cancer treatment, demonstrating OS benefit (11,12). After ICIs were shown to improve OS and progression-free survival (PFS) when administered as second- and subsequent lines of treatment compared with chemotherapy, the US Food and Drug Administration approved nivolumab and pembrolizumab in 2015, the first two monoclonal antibodies targeting programmed cell death protein 1 (PD-1), for patients with advanced NSCLC (13-16). In the Republic of Korea, ICIs have been available as a second-line treatment for patients with advanced and metastatic lung cancer since July 2016.
Rationale and knowledge gap
Although various studies have demonstrated that the clinical efficacy of ICIs contributes to improving the prognosis of patients with NSCLC, more studies on the financial aspects based on large population-based data are needed (17-19).
Objective
This study aimed to analyze the differences in medical expenses and the effect of ICIs on prognosis improvement in patients with advanced or metastatic NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-686/rc).
Methods
Data source and study design
All Korean residents are enrolled in the Korean National Health Insurance Service (NHIS) and given a unique identification number at birth. Data accompany claims on fully adjudicated medical and pharmacy claims in the Republic of Korea, including general demographic data, the 10th revision of the International Statistical Classification of Diseases (ICD-10) and Related Health Problems codes, medical institution type, medications prescribed, medical cost, and mortality. This retrospective cohort study evaluated nationwide data from the Korean NHIS. All outpatients and hospitalized patients with lung cancer between 2010 and 2020 were identified.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Institutional Review Board of the NHIS Ilsan Hospital (NHIMC 2022-10-015). Individual consent for this retrospective analysis was waived.
Case identification
A flowchart of the identification of the study population is shown in Figure 1. Among the 346,505 patients with lung cancer who were initially screened, 102,964 assigned diagnostic codes for lung cancer (C34) before 2013 were excluded. Patients without a history of cancer-related diagnostic procedures or chemotherapeutic treatment and those with cancer diagnostic codes other than that for lung cancer were excluded. The billing codes used for the diagnosis were biopsy methods using fiberoptic bronchoscopy and computed tomography-guided needle aspiration. The treatment billing codes consisted of lung surgery and radiotherapy (body or brain), and the prescribed drugs were paclitaxel, pemetrexed, gemcitabine, docetaxel, erlotinib, afatinib, ceritinib, crizotinib, gefitinib, pembrolizumab, and nivolumab. The remaining 139,783 patients were selected as patients newly diagnosed with NSCLC between January 1, 2013, and December 31, 2020.
Patients who underwent lung surgery were considered as having early-stage NSCLC; those who received concurrent chemoradiation therapy as having locally advanced NSCLC; and those who received only systemic cytotoxic chemotherapy (CC), TT, or ICI appropriate for NSCLC as having metastatic or recurrent disease. Therefore, to identify patients with stage IIIB or IV NSCLC, the following operational definitions were used: (I) first-ever administration of TTs such as afatinib, ceritinib, crizotinib, erlotinib, or gefitinib; (II) use of CC such as paclitaxel, pemetrexed, gemcitabine, docetaxel, irinotecan, or etoposide administered at least 180 days after radiation therapy or resection surgery of the lung parenchyma; (III) first-ever administration of CC without a history of radiation therapy or resection surgery of the lung parenchyma; (IV) conditions (I), (II), and (III) were started after January 1, 2013; and (V) the observation duration was at least 6 months long. Overall, 49,842 patients were found to have been diagnosed with stage IIIB or IV NSCLC and received palliative chemotherapy.
To evaluate the effect of ICIs as second-line chemotherapeutics, patients who met the following criteria were further screened as patients treated with second-line chemotherapeutics: (I) initial chemotherapy started after January 2016, (II) ICI was not administered as first-line chemotherapy, (III) TT was not administered as first-line chemotherapy, (IV) history of second-line chemotherapy, (V) ICIs were not administered as third (or higher)-line chemotherapy and (VI) atezolizumab was not administered. A total of 7,297 patients who were diagnosed with stage IIIB or IV NSCLC and received second-line chemotherapy were included in the final study population. Among them, 2,485 and 4,812 patients were divided into groups depending on their history of ICI administration as second-line chemotherapeutics.
Charlson comorbidity index (CCI)
The CCI is a widely used prognostic model that predicts the 1-year mortality risk, depending on individual comorbidities. Each comorbidity was scored, and the CCI was calculated by summing the comorbidity scores (Table S1). Because of its usefulness in measuring the effects of comorbidities on mortality by using an administrative database, including ICD-10 codes, the CCI was adopted as a variable (20,21).
Covariates
Age, sex, income level, type of hospital, hypertension, diabetes, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), congestive heart failure (CHF), cerebrovascular disease, stage, and brain metastasis were used as adjustment variables. Additionally, sensitivity analysis was conducted on 4,644 individuals with available smoking history data to examine the impact of smoking. The results of this analysis have been provided in the supplementary tables (Tables S2,S3).
Clinical outcomes
The primary outcome was the difference in overall mortality between patients with advanced NSCLC who did and did not use ICIs subsequently after platinum-based CC. Secondary outcomes included PFS and medical expenditure. In the analysis related to PFS, disease progression was defined as when a patient’s regimen was changed again during the secondary regimen.
Statistical analysis
The variables in each group were compared using a chi-squared test. Analysis of variance (ANOVA) was used to analyze the differences in OS, PFS, and cost differences between groups. An interrupted time series (ITS) analysis was used to evaluate the longitudinal impact of introducing a cost exemption policy. ITS is regarded as one of the most robust quasi-experimental designs to assess the effect of an intervention (22,23). In an ITS analysis, data are arranged at evenly spaced time intervals and separated into segments by the intervention. The analysis assessed the short-term impact of the intervention, as measured by a change in the level, and the over-time effect, as measured by a change in the trend (i.e., slope) after the intervention (24). Cox proportional hazard models were fitted to estimate the mortality and disease progression. The results are reported as adjusted hazard ratio (HR) with a 95% confidence interval (CI). All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) at a significance level of 5%.
Results
Baseline characteristics of the study population
Overall, 7,297 patients newly diagnosed with advanced NSCLC after 2016 were included in this study. Patients who received ICIs as second-line chemotherapeutics (n=2,485) were compared with those who did not receive ICIs (Figure 1). Older male patients over 60s accounted for a large proportion of both groups. The proportion of patients with stage IV disease was significantly higher in the ICI group than in the non-ICI’s (98.2% vs. 94.7%, P<0.0001). However, the two groups did not differ significantly in income level, care level, or CCI (Table 1). In the ICI group, 961 and 1,524 patients were treated with nivolumab and pembrolizumab, respectively. In the non-ICI group, 3,665 and 1,147 patients received CC and TT, respectively, as second-line treatments (Table S4).
Table 1
| Variables | Total | ICI group | Non-ICI group | P value |
|---|---|---|---|---|
| Total | 7,297 | 2,485 | 4,812 | <0.0001 |
| Age, years (mean ± SD) | 65.6±9.5 | 66.8±9.5 | 65.1±9.6 | <0.0001 |
| <40 | 83 (1.1) | 20 (0.8) | 63 (1.3) | |
| 40–49 | 338 (4.6) | 98 (3.9) | 240 (4.9) | |
| 50–59 | 1,346 (18.4) | 400 (16.1) | 946 (19.6) | |
| 60–69 | 2,789 (38.2) | 908 (36.5) | 1,881 (39.0) | |
| ≥70 | 2,741 (37.6) | 1,059 (42.6) | 1,682 (34.9) | |
| Sex | ||||
| Male | 5,931 (81.3) | 2,104 (84.6) | 3,827 (79.5) | <0.0001 |
| Female | 1,366 (18.7) | 381 (15.3) | 985 (20.4) | |
| Income level | ||||
| Medical-aid | 394 (5.4) | 141 (5.6) | 253 (5.2) | 0.738 |
| 1Q | 1,340 (18.4) | 449 (18.0) | 891 (18.5) | |
| 2Q | 1,447 (19.8) | 479 (19.2) | 968 (20.1) | |
| 3Q | 1,819 (24.9) | 618 (24.8) | 1,201 (24.9) | |
| 4Q (richest) | 2,297 (31.5) | 798 (32.1) | 1,499 (31.1) | |
| Level of care | ||||
| Secondary hospital | 2,388 (32.7) | 841 (33.8) | 1,547 (32.1) | 0.333 |
| Tertiary hospital | 4,909 (67.3) | 1,644 (66.1) | 3,265 (67.8) | |
| Comorbidity | ||||
| Hypertension | 3,910 (53.5) | 1,344 (54.0) | 2,566 (53.3) | 0.006 |
| Diabetes | 3,139 (43.0) | 1,101 (44.3) | 2,038 (42.3) | 0.001 |
| COPD | 1,553 (21.2) | 534 (21.4) | 1,019 (21.1) | 0.374 |
| CKD | 153 (2.0) | 67 (2.7) | 86 (1.7) | <0.0001 |
| CHF | 870 (11.9) | 318 (12.8) | 552 (11.4) | 0.012 |
| CVD | 1,337 (18.3) | 464 (18.6) | 873 (18.1) | 0.007 |
| CCI | ||||
| 6 | 449 (6.1) | 154 (6.2) | 295 (6.1) | 0.440 |
| 7 | 783 (10.7) | 269 (10.8) | 514 (10.6) | |
| ≥8 | 6,065 (83.1) | 2,062 (82.9) | 4,003 (83.1) | |
| Cancer stage | ||||
| Stage IIIB | 295 (4.0) | 44 (1.7) | 251 (5.2) | <0.0001 |
| Stage IV | 7,002 (95.9) | 2,441 (98.2) | 4,561 (94.7) | |
| Brain metastasis | 106 (1.4) | 38 (1.5) | 68 (1.4) | 0.267 |
Data are presented as n (%) unless otherwise stated. ICI, immune checkpoint inhibitor; 1Q, first quintile; 2Q, second quintile; 3Q, third quintile; 4Q, fourth quintile (richest); COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; CHF, congestive heart failure; CVD, cerebrovascular disease; CCI, Charlson comorbidity index.
Survival outcomes
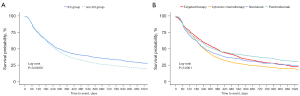
Table 2 showed survival outcomes including OS and PFS. The median OS duration was the longest in the TT group (15.5±16.7 months) and shortest in the CC group (11.2±13.2 months). In the ICI group, the median OS durations for nivolumab (11.7±12.0 months) and pembrolizumab therapy (12.9±12.3 months) were shorter than those for TT and longer than those for CC. Likewise, the PFS duration was the longest in the TT group (9.4±12.3 months) and the shortest in the CC group (6.7±9.2 months). In the ICI group, the median PFS durations for nivolumab (7.7±10.1 months) and pembrolizumab (9.0±10.9 months) therapy were shorter than those for TT and longer than those for CC. Kaplan‑Meier analysis revealed that the ICI group had a significantly longer survival time than the non-ICI group. When analyzed by detailed drug, the survival time of pembrolizumab and nivolumab was longer than that of CC, and TT showed a similar survival rate to pembrolizumab (Figure 2). Kaplan-Meier analysis of PFS also showed that the ICI group was superior to the non-ICI group, and pembrolizumab had the highest proportion of patients with long-term survival without progression (Figure 3).
Table 2
| Outcomes | Cytotoxic chemotherapy (N=3,665) | Targeted therapy (N=1,147) | Nivolumab (N=961) | Pembrolizumab (N=1,524) | P value* |
|---|---|---|---|---|---|
| Overall survival (months) | 11.2±13.2 | 15.5±16.7 | 11.7±12.0 | 12.9±12.3 | <0.0001 |
| Progression-free survival (months) | 6.7±9.2 | 9.4±12.3 | 7.7±10.1 | 9.0±10.9 | <0.0001 |
| Individual annual medical costs (USD) | |||||
| Total treatment | 39.51K | 41.12K | 54.11K | 69.83K | <0.0001 |
| Cancer-related treatment | 38.43K | 39.84K | 52.81K | 68.53K | <0.0001 |
*, The P values were calculated using ANOVA (analysis of variance).

Risk factors associated with survival outcomes
Old age (≥60 years) and male sex were identified as risk factors for an increased risk of death. However, the ICI group had a lower risk of death than the non-ICI group (HR: 0.79; 95% CI: 0.75–0.84) (Table 3). Moreover, ICI group had a lower risk of disease progression than the non-ICI use group (HR 0.92; 95% CI: 0.85–0.99) (Table 4). When analyze with specific drugs, TT and pembrolizumab were associated with lower risks of disease progression (OR: 0.81, P<0.0001; OR: 0.81, P<0.0001) and mortality than CC (OR: 0.78, P<0.0001; OR: 0.70, P<0.0001). However, nivolumab only reduced the risk of death (OR: 0.84, P<0.0001) (Tables S5,S6).
Table 3
| Variables | Overall survival | P value | ||
|---|---|---|---|---|
| Adjusted HR |
95% CI | |||
| Low | High | |||
| ICI use | 0.79 | 0.75 | 0.84 | <0.0001 |
| Age (years) | ||||
| <40 | Ref. | |||
| 40–49 | 0.97 | 0.74 | 1.27 | 0.828 |
| 50–59 | 1.11 | 0.86 | 1.42 | 0.403 |
| 60–69 | 1.28 | 1.00 | 1.64 | 0.044 |
| ≥70 | 1.48 | 1.16 | 1.90 | 0.001 |
| Sex | ||||
| Male | 1.27 | 1.18 | 1.36 | <0.0001 |
| Female | Ref. | |||
| Income level | ||||
| Medical-aid | 1.11 | 0.99 | 1.25 | 0.070 |
| 1Q | 1.06 | 0.99 | 1.15 | 0.088 |
| 2Q | 1.04 | 0.97 | 1.12 | 0.231 |
| 3Q | 1.04 | 0.97 | 1.12 | 0.185 |
| 4Q (richest) | Ref. | - | - | |
| Type of hospital | ||||
| Secondary hospital | 1.00 | 0.95 | 1.06 | 0.738 |
| Tertiary hospital | Ref. | |||
| Comorbidity | ||||
| Hypertension | 1.01 | 0.95 | 1.06 | 0.727 |
| Diabetes | 1.03 | 0.97 | 1.09 | 0.259 |
| COPD | 1.02 | 0.96 | 1.09 | 0.404 |
| CKD | 1.14 | 0.96 | 1.36 | 0.120 |
| CHF | 1.01 | 0.93 | 1.09 | 0.806 |
| CVD | 1.01 | 0.94 | 1.08 | 0.662 |
| CCI | ||||
| 6 | 1.00 | |||
| 7 | 0.98 | 0.86 | 1.12 | 0.850 |
| ≥8 | 0.99 | 0.88 | 1.10 | 0.884 |
| Cancer stage | ||||
| Stage IIIB | Ref. | |||
| Stage IV | 1.28 | 1.12 | 1.46 | 0.001 |
| Brain metastasis | 0.95 | 0.77 | 1.18 | 0.693 |
HR, hazard ratio; CI, confidence interval; 1Q, first quintile; 2Q, second quintile; 3Q, third quintile; 4Q, fourth quintile (richest); ICI, immune checkpoint inhibitor; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; CHF, congestive heart failure; CVD, cerebrovascular disease; CCI, Charlson comorbidity index.
Table 4
| Variables | Disease progression | P value | ||
|---|---|---|---|---|
| Adjusted HR |
95% CI | |||
| Low | High | |||
| ICI use | 0.92 | 0.85 | 0.99 | 0.035 |
| Age (years) | ||||
| <40 | Ref. | – | – | |
| 40–49 | 1.03 | 0.75 | 1.40 | 0.841 |
| 50–59 | 1.00 | 0.75 | 1.34 | 0.971 |
| 60–69 | 0.91 | 0.68 | 1.22 | 0.547 |
| ≥70 | 0.74 | 0.55 | 1.00 | 0.050 |
| Sex | ||||
| Male | 1.01 | 0.93 | 1.10 | 0.689 |
| Female | Ref. | – | – | |
| Income level | ||||
| Medical-aid | 1.04 | 0.88 | 1.23 | 0.599 |
| 1Q | 0.99 | 0.90 | 1.10 | 0.960 |
| 2Q | 1.06 | 0.96 | 1.17 | 0.230 |
| 3Q | 1.01 | 0.92 | 1.10 | 0.821 |
| 4Q (richest) | Ref. | – | – | |
| Type of hospital | ||||
| Secondary hospital | 0.96 | 0.89 | 1.03 | 0.311 |
| Tertiary hospital | Ref. | – | – | |
| Comorbidity | ||||
| Hypertension | 1.03 | 0.96 | 1.12 | 0.340 |
| Diabetes | 0.96 | 0.88 | 1.03 | 0.313 |
| COPD | 0.90 | 0.82 | 0.99 | 0.032 |
| CKD | 0.94 | 0.72 | 1.24 | 0.709 |
| CHF | 0.96 | 0.85 | 1.07 | 0.493 |
| CVD | 0.95 | 0.87 | 1.05 | 0.389 |
| CCI | ||||
| 6 | Ref. | – | – | |
| 7 | 0.98 | 0.84 | 1.16 | 0.883 |
| ≥8 | 0.94 | 0.82 | 1.09 | 0.462 |
| Cancer stage | ||||
| Stage IIIB | Ref. | – | – | |
| Stage IV | 0.84 | 0.72 | 0.97 | 0.024 |
| Brain metastasis | 0.89 | 0.67 | 1.18 | 0.426 |
HR, hazard ratio; CI, confidence interval; ICI, immune checkpoint inhibitor; 1Q, first quintile; 2Q, second quintile; 3Q, third quintile; 4Q, fourth quintile (richest); COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; CHF, congestive heart failure; CVD, cerebrovascular disease; CCI, Charlson comorbidity index.
Trends in chemotherapeutic use and medical expenses
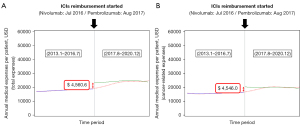
After the NHIS-approved ICI reimbursement in 2017, the number of patients receiving ICIs following platinum chemotherapy gradually increased, while the number of patients receiving CC alone decreased (Figure 4). Regarding medical expenses, patients who received ICIs had higher overall medical expenses than those who did not receive them. Compared with non-ICI treatment groups, the total medical expenses were higher in patients receiving ICI treatment ($69.83K in the pembrolizumab group and $54.11K in the nivolumab group) (Table 2). Both total and cancer-related costs increased after the introduction of reimbursement for ICIs (total cost: β, $4.56K; SE: $0.27K; P<0.0001 and cancer-related cost: β, $4.54K; SE: $0.27K; P<0.0001) (Table 5, Figure 5).

Table 5
| Variables | Total cost | Cancer-related cost | |||||
|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | ||
| Intervention | |||||||
| Before | Ref | – | Ref | – | |||
| After | 4,560.6 | 278.6 | <0.0001 | 4,546.0 | 275.2 | <0.0001 | |
| Trend before policy | 52.5 | 8.1 | <0.0001 | 49.0 | 8.0 | <0.0001 | |
| Trend after policy | −4.4 | 12.0 | 0.717 | −11.3 | 11.9 | 0.342 | |
| Age (years) | |||||||
| <40 | Ref | – | Ref | – | |||
| 40–49 | −2,038.9 | 699.6 | 0.003 | −2,179.1 | 691.0 | 0.001 | |
| 50–59 | −2,724.7 | 652.4 | <0.0001 | −2,827.5 | 644.3 | <0.0001 | |
| 60–69 | −3,838.7 | 647.1 | <0.0001 | −4,004.1 | 639.2 | <0.0001 | |
| ≥70 | −7,884.6 | 649.7 | <0.0001 | −8,053.8 | 641.7 | <0.0001 | |
| Sex | 3,327.6 | 153.6 | <0.0001 | 3,416.5 | 151.7 | <0.0001 | |
| Male | |||||||
| Female | Ref. | – | Ref. | – | |||
| Income level | 174.0 | 324.6 | 0.591 | −39.4 | 320.6 | 0.902 | |
| Medical-aid | |||||||
| 1Q | 648.6 | 206.7 | 0.001 | 663.6 | 204.1 | 0.001 | |
| 2Q | 339.4 | 207.6 | 0.102 | 324.3 | 205.1 | 0.113 | |
| 3Q | 611.9 | 192.7 | 0.001 | 599.3 | 190.4 | 0.001 | |
| 4Q (richest) | Ref. | – | Ref. | – | |||
| Type of hospital | |||||||
| Secondary hospital | 684.8 | 151.6 | <0.0001 | 671.3 | 149.7 | <0.0001 | |
| Tertiary hospital | Ref. | – | Ref. | – | |||
| Comorbidities | |||||||
| Hypertension | 407.3 | 162.8 | 0.012 | 300.9 | 160.8 | 0.061 | |
| Diabetes | 584.8 | 159.0 | 0.0002 | 469.7 | 157.0 | 0.002 | |
| COPD | 394.8 | 186.3 | 0.034 | 347.7 | 184.0 | 0.058 | |
| CKD | 1,578.4 | 469.4 | 0.0008 | −65.3 | 463.6 | 0.888 | |
| CHF | −2.2 | 233.5 | 0.992 | −215.1 | 230.7 | 0.351 | |
| CVD | −153.9 | 185.2 | 0.406 | −262.2 | 182.9 | 0.151 | |
| CCI | |||||||
| 6 | Ref | – | Ref | – | |||
| 7 | 410.8 | 351.0 | 0.241 | 363.6 | 346.7 | 0.294 | |
| ≥8 | −63.8 | 302.4 | 0.832 | −168.3 | 298.7 | 0.573 | |
| Cancer stage | |||||||
| Stage IIIB | Ref | – | Ref | – | |||
| Stage IV | −10,153.8 | 506.6 | <0.0001 | −10,107.7 | 500.4 | <0.0001 | |
| Brain metastasis | 84.8 | 568.7 | 0.881 | 205.0 | 561.7 | 0.715 | |
SE, standard error; USD, United States dollar; 1Q, first quintile; 2Q, second quintile; 3Q, third quintile; 4Q, fourth quintile (richest); COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; CHF, congestive heart failure; CVD, cerebrovascular disease; CCI, Charlson comorbidity index.
Discussion
Key findings
In this study, we found that the cost and drug retention rate among patients with NSCLC receiving second-line treatment can vary depending on the type of treatment received. ICI treatment led to longer survival and a better prognosis in terms of OS and PFS compared with CC. However, the retention rate of ICI appeared to be lower than that of TT. Additionally, when using ICIs, the increase in medical expenses for lung cancer treatment was higher than that for TT. Our study found changes in the patterns of chemotherapy drug prescriptions after coverage expanded with a significant increase in annual medical expenses per patient.
Strengths and limitations
To our knowledge, this is the first analysis of medical resource use and costs based on reimbursed claims for second-line immunotherapy for treating NSCLC in Korean patients. However, this study had some limitations. First, this was a retrospective cohort analysis of reimbursed claims data. Although predefined and strict operational definitions using diagnostic codes can help identify patients with lung cancer, they are not sufficiently detailed to accurately determine specific tumor types, disease stages, treatment responses, or adverse events. Further, there is a small possibility that stage IIIA patients who received palliative chemotherapy without definitive radiotherapy were also included in the study group. The efficacy and cost analyses did not include patients who received ICIs without reimbursement or primary treatment. In addition, considering the recent reimbursement approval, this study only analyzed the clinical efficacy and cost of immunotherapy as a secondary drug treatment. Additionally, regarding medical expenses, the cause of the cost difference was not accurately explained. However, since March 2022, the reimbursement criteria have been expanded to include ICIs as first-line therapy. The impact of this expansion on health insurance funds, including changes in prescription rates and costs, requires further analysis.
Comparison with similar research
The five-year survival update from Keynote 010 showed superior outcomes of ICI therapy for 16.9 months compared to Docetaxel for 8.2 months in programmed death-ligand 1 (PD-L1) in 50% of patients (25). Our research similarly demonstrated an OS and PFS advantage of ICI treatment over chemotherapy. However, it is important to note that our findings differ from Keynote 010’s, indicating a shorter duration. This inconsistency is supported by other real-world studies (26,27), consistently aligning with our study results. Clinical trials focusing on internal validity often enroll patients with optimal organ function and good performance status, excluding or underrepresenting those encountered in daily practice. This creates a gap between the efficacy in randomized controlled trials and in the real world (28). Several studies have reported results similar to those of our study regarding the efficacy of ICIs according to PD-L1 testing (29-31). Khozin et al. analyzed claims data to examine treatment patterns and found that most patients received nivolumab and pembrolizumab in a community practice setting (31). That study also reported low rates of PD-L1 immunohistochemistry testing and a shorter PFS than that reported in pivotal trials of these therapies. However, long PFS and OS were associated with increased PD-L1 staining in previous multicenter real-world studies (32). These findings suggested that although ICIs are effective in clinical trials, there may be challenges in replicating these outcomes in real-world clinical practice. The efficacy of ICIs in patients with NSCLC is limited by a lack of strongly predictive response markers, resulting in potential underutilization of effective alternative treatments, increased risk of suboptimal care, and excessive medical care costs.
Previous studies have revealed that up to 60% of patients with NSCLC do not benefit from ICIs (14,16,33-35). The KEYNOTE-024 (33) and CheckMate-026 (36) trials investigated the efficacy of pembrolizumab and nivolumab in previously untreated patients with NSCLC. While pembrolizumab resulted in significantly longer PFS and OS than platinum-based chemotherapy in the KEYNOTE-024 trial, the CheckMate-026 trial did not show any differences in efficacy between nivolumab and chemotherapy. The KEYNOTE-024 trial used a PD-L1 threshold of ≥50%, while the CheckMate-026 trial used a cut-off of ≥1%. The effectiveness of pembrolizumab can be attributed to the selection of patients with high PD-L1 levels, inherently leading to the selection of patients who were more likely to benefit from immunotherapy than the drug itself. Therefore, patient selection is crucial for determining the success of immunotherapy, and high PD-L1 thresholds should be used to select patients who would highly benefit from PD-1/PD-L1-TT.
Explanations of findings
Our findings regarding medical expenditure differed from those of a previous study on costs and medical resource use associated with NSCLC before and after the approval of ICIs (37). The study showed that although the cost of treatment with ICIs was higher than that associated with other treatments, the total cost of care decreased following the US Food and Drug Administration’s approval of ICIs, owing to a reduction in emergency room visits and hospitalizations among patients with NSCLC. Therefore, the authors concluded that although ICIs are more expensive than other drugs, they may reduce overall medical costs by reducing the use of other healthcare resources. Our study confirmed that overall medical and cancer-related medical expenses increased after reimbursement for ICIs. However, annual cancer-related medical expenses decreased after reimbursement, although the difference was insignificant.
Implications and actions needed
Identifying potential responders to ICIs from among patients with NSCLC after identifying patients with oncogenic driver mutations in the EGFR and ALK genes is recommended because patients with these mutations have much lower response rates to ICIs and are more likely to experience increased toxicity. Accordingly, the American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines state that patients with NSCLC should receive oral therapies targeting the EGFR and ALK genes before receiving ICIs (38-40). In particular, to achieve better response rates and identify patients who will show satisfactory responses following TT, NSCLC practice guidelines recommend additional testing for a range of genetic mutations and fusions, including ROS1 fusions, BRAF V600E mutations, ERBB2 (HER2), and KRAS mutations (when part of a comprehensive panel), NTRK1-3 and RET fusions, and MET amplification. By expanding testing beyond traditional EGFR and ALK mutations, healthcare providers can identify patients who would most likely benefit from TTs and provide personalized treatment options (38,40,41). In this context, next-generation sequencing, which is capable of determining specific genetic mutations or alterations that may drive the growth and spread of cancer, could be helpful in the management of NSCLC and may be used to develop TTs tailored to each patient’s unique genetic profile.
Although the emergence of TTs and ICIs as treatment options for NSCLC has led to improved clinical outcomes in patients receiving conventional chemotherapy-based therapies, the dramatic increase in treatment-related costs for both patients and national health insurance is a potential issue. Based on the results of our study, a significantly higher medical cost was observed after ICI therapy. Therefore, extensive profiling of TTs and ICIs is essential to improve the selection and sequencing of treatments, reduce the likelihood of ineffective therapies, and enhance patient outcomes.
Conclusions
Expanding the national insurance coverage to ICIs as second-line drugs has improved clinical outcomes, including OS and PFS, in patients with advanced NSCLC. However, the results of this study indicate that the annual medical cost per patient was significantly higher in patients treated with ICIs than in those treated with TT. In contrast, the retention rates of ICIs were lower than those of TT.
Acknowledgments
This study used the National Health Information Database (NHIS-2022-1-735), made by NHIS. The authors alone are responsible for the content and writing of the paper.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-686/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-686/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-686/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-686/coif). S.C.L. reports that this work was supported by the NHIS Ilsan Hospital grant (NHIMC-2022-PR-004). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the NHIS Ilsan Hospital (NHIMC 2022-10-015), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. International Agency for Research on Cancer. Lyon, France. 2020.
- Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol 2023;20:624-39. [Crossref] [PubMed]
- Kang MJ, Won YJ, Lee JJ, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2019. Cancer Res Treat 2022;54:330-44. [Crossref] [PubMed]
- Kang MJ, Jung KW, Bang SH, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2020. Cancer Res Treat 2023;55:385-99. [Crossref] [PubMed]
- Lee J, Kim Y, Kim HY, et al. Feasibility of implementing a national lung cancer screening program: Interim results from the Korean Lung Cancer Screening Project (K-LUCAS). Transl Lung Cancer Res 2021;10:723-36. [Crossref] [PubMed]
- Lee JG, Kim HC, Choi CM. Recent Trends of Lung Cancer in Korea. Tuberc Respir Dis (Seoul) 2021;84:89-95. [Crossref] [PubMed]
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med 2021;27:1345-56. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- D'Addario G, Pintilie M, Leighl NB, et al. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol 2005;23:2926-36. [Crossref] [PubMed]
- Abdel-Rahman O, Morris D. Immune checkpoint inhibitors and non-small-cell lung cancer management: 2018 update. Immunotherapy 2019;11:149-53. [Crossref] [PubMed]
- Tamiya A. Long-term survival of patients with advanced non-small cell lung cancer treated using immune checkpoint inhibitors. Respir Investig 2024;62:85-9. [Crossref] [PubMed]
- Callahan MK, Postow MA, Wolchok JD, Targeting T. Cell Co-receptors for Cancer Therapy. Immunity 2016;44:1069-78. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Song P, Zhang J, Shang C, et al. Real-world evidenceand clinical observations of the treatment of advanced non-small cell lung cancer with PD-1/PD-L1 inhibitors. Sci Rep 2019;9:4278. [Crossref] [PubMed]
- Youn B, Trikalinos NA, Mor V, et al. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non-small cell lung cancer. Cancer 2020;126:978-85. [Crossref] [PubMed]
- Muchnik E, Loh KP, Strawderman M, et al. Immune Checkpoint Inhibitors in Real-World Treatment of Older Adults with Non-Small Cell Lung Cancer. J Am Geriatr Soc 2019;67:905-12. [Crossref] [PubMed]
- Bannay A, Chaignot C, Blotière PO, et al. The Best Use of the Charlson Comorbidity Index With Electronic Health Care Database to Predict Mortality. Med Care 2016;54:188-94. [Crossref] [PubMed]
- Stavem K, Hoel H, Skjaker SA, et al. Charlson comorbidity index derived from chart review or administrative data: agreement and prediction of mortality in intensive care patients. Clin Epidemiol 2017;9:311-20. [Crossref] [PubMed]
- Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13:S38-44. [Crossref] [PubMed]
- Kontopantelis E, Doran T, Springate DA, et al. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 2015;350:h2750. [Crossref] [PubMed]
- Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017;46:348-55. [Crossref] [PubMed]
- Herbst RS, Garon EB, Kim DW, et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J Thorac Oncol 2021;16:1718-32. [Crossref] [PubMed]
- Lefebvre C, Martin E, Hendriks LEL, et al. Immune checkpoint inhibitors versus second line chemotherapy for patients with lung cancer refractory to first line chemotherapy. Respir Med Res 2020;78:100788. [Crossref] [PubMed]
- Rothschild SI, Nachbur R, Herzog N, et al. Second-line therapy improves overall survival in primary refractory non-small cell lung cancer (NSCLC) patients. ESMO Open 2021;6:100013. [Crossref] [PubMed]
- Pasello G, Pavan A, Attili I, et al. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat Rev 2020;87:102031. [Crossref] [PubMed]
- Singh BP, Britton SL, Prins P, et al. Molecular profiling (MP) for malignancies: Knowledge gaps and variable practice patterns among United States oncologists (Onc). J Clin Oncol 2019;37:10510.
- Matter-Walstra K, Schwenkglenks M, Aebi S, et al. A Cost-Effectiveness Analysis of Nivolumab versus Docetaxel for Advanced Nonsquamous NSCLC Including PD-L1 Testing. J Thorac Oncol 2016;11:1846-55. [Crossref] [PubMed]
- Khozin S, Abernethy AP, Nussbaum NC, et al. Rates of PD-L1 expression testing in US community-based oncology practices (USCPs) for patients with metastatic non-small cell lung cancer (mNSCLC) receiving nivolumab (N) or pembrolizumab (P). J Clin Oncol 2017;35:11596.
- Khozin S, Miksad RA, Adami J, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer 2019;125:4019-32. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Yu DP, Cheng X, Liu ZD, et al. Comparative beneficiary effects of immunotherapy against chemotherapy in patients with advanced NSCLC: Meta-analysis and systematic review. Oncol Lett 2017;14:1568-80. [Crossref] [PubMed]
- Tan PS, Aguiar P Jr, Haaland B, et al. Comparative effectiveness of immune-checkpoint inhibitors for previously treated advanced non-small cell lung cancer - A systematic review and network meta-analysis of 3024 participants. Lung Cancer 2018;115:84-8. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Korytowsky B, Radtchenko J, Nwokeji ED, et al. Understanding total cost of care in advanced non-small cell lung cancer pre- and postapproval of immuno-oncology therapies. Am J Manag Care 2018;24:S439-47.
- Pennell NA, Arcila ME, Gandara DR, et al. Biomarker Testing for Patients With Advanced Non-Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am Soc Clin Oncol Educ Book 2019;39:531-42. [Crossref] [PubMed]
- Hanna NH, Schneider BJ, Temin S, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol 2020;38:1608-32. [Crossref] [PubMed]
- Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin 2015;25:185-97. [Crossref] [PubMed]
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]



