
The presence of micropapillary and/or solid subtypes is an independent prognostic factor for patients undergoing curative resection for stage I lung adenocarcinoma with ground-glass opacity
Highlight box
Key findings
• The presence of minimal micropapillary and/or solid (MP/S) subtypes is substantially associated with a poorer prognosis for early-stage lung adenocarcinoma (ADCs) with ground-glass opacity (GGO).
What is known and what is new?
• Lung ADC typically exhibits a heterogeneous mixture of various histologic subtypes, each possessing distinct clinicopathologic and genomic characteristics. MP/S subtypes, even when occupying only a small proportion of the entire tumor, have been reported to be significant indicators of poor prognosis. Furthermore, the presence of a GGO component, as observed on computed tomography scans, has been established as a significant prognostic factor in early-stage lung ADCs.
• In this study, GGO-featured lung ADCs with MP/S components were demonstrated to represent a distinct “grey zone” within early-stage non-small cell lung cancer, wherein an increasing proportion of high-grade patterns corresponded to a progressively negative post-operative prognosis.
What is the implication, and what should change now?
• Considering the prognostic significance of minimal MP/S components in GGO-featured lung ADCs, further investigations are warranted to ascertain whether this particular patient population can be safely treated through sublobar resection. Additionally, it is crucial to explore the implications of adjuvant systemic therapy in these cases.
Introduction
In 2011, the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) introduced an updated histological classification identifying five distinct predominant patterns of invasive adenocarcinoma (ADC) (1). Over the past decade, many studies have reported significant correlation between the presence of high-grade patterns (solid, micropapillary, and complex glandular) and inferior survival outcomes of patients with resected lung ADCs (2-7), even if those high-grade components only occupy a minor portion of the entire tumor (2,4,8-14). Although high-grade patterns were demonstrated to correlate with many other histological features [nuclear grading, necrosis, mitotic activity, spread through air space (STAS), etc.] (15,16), which also independently predict prognosis, inclusion of those morphological features into the IASLC grading model did not translate into better prognostication (17). However, prior studies have mainly investigated the prognostic role of high-grade subtypes in solid tumors. Whether the presence of micropapillary and/or solid (MP/S) components in lung ADCs with radiologically ground-glass opacity (GGO) components is prognostic remains poorly understood. This specific group of tumors is known to exhibit less aggressive biological behavior and a more favorable prognosis compared to pure solid tumors at the same stage (18-20).
Conceptually, a radiological GGO component is typically associated with lepidic (Lep) growth, while the solid part corresponds to histologically invasive components. However, the majority of lung ADCs exhibits mixed subtypes, and there are no definitive boundaries in terms of tumor biology between tumors with and without GGO. Building upon evidence from previous studies, we propose a hypothesis that the prognostic value of MP/S patterns extends beyond pure solid tumors and may have been underestimated in tumors featuring GGOs.
Therefore, the objective of the present study was to investigate whether non-predominant or even a minimal proportion of MP/S subtypes would have a prognostic impact on patients who underwent surgical resection for GGOs. We represent this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-736/rc).
Methods
Study participants
In this retrospective cohort study, we retrospectively screened 1,499 patients who underwent surgical resection for pathological stage I GGOs at the National Cancer Center (Beijing, China) between January 2014 and December 2016. To be included in our study sample, eligible patients were required to have undergone thin-section computed tomography (TS-CT) scans within 30 days prior to surgery. The exclusion criteria were as follows: (I) ADC in situ or minimally invasive ADC; (II) pure-solid tumors without GGO components; (III) ADC with a maximum tumor diameter (MTD) over 3 cm; (IV) patients with previous treatment for lung cancer; (V) patients who did not undergo curative pulmonary resection and mediastinal lymph node sampling or dissection; and (VI) patients with other malignant tumors. Patients with multiple primary lung cancers were also excluded, unless the secondary nodules were concurrently resected and confirmed to be non-invasive lesions (atypical adenomatous hyperplasia or ADC in situ). Ultimately, a total of 1,004 patients were included in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the National Cancer Center (NCC3692), and the requirement for written informed consent was waived due to the retrospective nature of the study.
Radiologic evaluation
Preoperative contrast-enhanced TS-CT scans were conducted using 64-detector-row CT scanners (Lightspeed Ultra, GE Healthcare, Chicago, IL, USA; Toshiba, Tokyo, Japan) for all patients included in the study. Two independent investigators reviewed all original diagnostic reports and CT images with a section thickness of 1.2 mm. The total tumor diameter and solid size were measured based on the longest diameter of each tumor and the maximal size of its solid portion in the axial, coronal, and sagittal planes. The measurements were performed using a window level of −500 Hounsfield units and a window width of 1,400 Hounsfield units (lung window). The consolidation-to-tumor ratio (CTR) was calculated as the ratio of the solid size to the total tumor diameter. In cases where there were discrepancies between the original diagnostic reports and the interpretations of the two investigators, a consensus was reached to resolve the discrepancies.
Histopathologic evaluation
The pathologic diagnosis of the lung cancer cases included in this study was determined according to the 2015 World Health Organization (WHO) criteria for lung cancer (21). Only invasive non-mucinous ADC was considered for inclusion. Detailed information, such as the pathologic stage (p-stage) based on the seventh edition of the tumor-node-metastasis (TNM) classification (22), the presence of certain histologic subtypes (as defined in the IASLC/ATS/ERS classification of lung ADC) (1), and the proportion of each histologic subtype (estimated semi-quantitatively in 5% increments), was collected from pathology reports. In cases where the MP/S components occupied less than 5% of the tumor, the percentage was recorded as 1%. Additionally, information regarding the presence of visceral-pleural invasion or lymphovascular invasion (LVI), as well as mutation data, was also collected. With respect to gene testing, DNA extraction from paraffin-embedded tissue was performed using the Autostation FFPE DNA One-Step Kit (ACCB Biotech, Beijing, China), while qualitative assay of human epidermal growth factor receptor (EGFR) mutations was detected using Human EGFR Gene 18-21 Exon Mutation Assay Kit (ACCB Biotech). Stratagene Mx3000P and ABl7500 (Applied Biosystems, Waltham, MA, USA) were used to perform amplification refractory mutation system-polymerase chain reaction (ARMS-PCR). No EGFR mutations and EGFR nonsense mutations (T790M) were defined as wild-type, while effective EGFR mutations were defined as mutant type.
Follow-up for tumor recurrence and survival
All eligible patients were followed up starting from the day of pulmonary resection. Physical examination, lung cancer biomarker testing, and chest CT scans were performed every 6 months during the initial 2 years, and annually thereafter. Doctors had the option to perform other imaging examinations or blood testing for circulating tumor DNA (ctDNA) on people with suspicious clinical symptoms. Follow-up imaging examinations, including chest and abdominal CT scans, brain magnetic resonance imaging (MRI), bone scintigraphy, and positron emission tomography (PET)-CT, were independently reviewed by two investigators to assess any tumor relapse. In cases where there were discrepancies between the interpretations of the two investigators, discussions were held to reach a consensus. Locoregional recurrence was defined as tumor relapse occurring at the resection margins, at the ipsilateral pleura, or within lymph nodes stations 1–14. Distant recurrence was defined as tumor relapse in the contralateral lung or at extrathoracic sites. The dates of the patient’s initial surgical resection, the first diagnosis of recurrent disease, and death (obtained from electronic medical records or through a telephone interview) were recorded to calculate recurrence-free survival (RFS) and overall survival (OS). Otherwise, patients were censored at the time of the last follow-up (if available).
Statistical analysis
Statistical analyses were performed using R software (version 4.2.2, https://www.r-project.org/). Patient demographic and clinicopathological characteristics were reported as medians with interquartile ranges (IQRs), means with standard deviations (SDs), or frequencies with percentages. To compare these characteristics between the MP/S positive (MP/S+) group and the MP/S negative (MP/S−) group, we utilized the Mann-Whitney U test for continuous variables and either the chi-squared test or Fisher’s exact test for categorical variables.
The primary endpoint was RFS (duration from surgery to any tumor relapse or death). The second endpoint was OS (duration from surgery to death). Survival statistics, including OS and RFS, were estimated using the Kaplan-Meier method and evaluated using the log-rank test. The Cox regression hazard model was employed to determine the independent prognostic value. The significant clinicopathological factors identified in the univariable model would be included in the multivariable model. Factors demonstrating a significant hazard ratio (HR) (P<0.05) with a 95% confidence interval (CI) entirely below or above 1.00 would be considered to have an independent prognostic impact.
To flexibly model the association of the MP/S subtype percentage, a restricted cubic spine (RCS) with four knots at the 5th, 35th, 65th, and 95th centiles was applied. The potential non-linearity was assessed using the likelihood ratio test. Significant covariates identified through the aforementioned Cox regression analysis were adjusted for in the model. All significance tests were two-sided, and a P value less than 0.05 was considered statistically significant.
Results
Table 1 presents the clinicopathological characteristics of patients diagnosed with MP/S+ and MP/S− tumors. Among the total of 1,004 eligible individuals enrolled in this study, with an average age of 58 years, nearly two-thirds (n=656, 65.3%) were of the female gender, and the majority (n=786, 78.3%) were non-smokers. The median total tumor size and solid size in CT, and the median pathological tumor size were 17.0, 5.0, and 15.0 mm, respectively. In terms of the pathologic predominant subtypes, there were 302 cases (30.1%) classified as Lep-predominant, 699 cases (69.6%) classified as acinar/papillary-predominant, and 3 cases (0.3%) classified as MP/S-predominant. Neither in the MP/S+ group nor in the MP/S− group, were there any patients with ADC exhibiting a complex glandular pattern.
Table 1
| Characteristics | Total (n=1,004) | MP/S− (n=918) | MP/S+ (n=86) | P value (MP/S+vs. MP/S−) |
|---|---|---|---|---|
| Sex | 0.11 | |||
| Female | 656 (65.3) | 607 (66.1) | 49 (57.0) | |
| Male | 348 (34.7) | 311 (33.9) | 37 (43.0) | |
| Age (years) | 58 (9.1) | 57.62 (9.1) | 60.64 (8.6) | <0.01 |
| Smoking history | 0.06 | |||
| Never | 786 (78.3) | 726 (79.1) | 60 (69.8) | |
| Ever | 218 (21.7) | 192 (20.9) | 26 (30.2) | |
| FEV1 (% predicted) | 88.0 (77.0, 98.0) | 88.0 (77.0, 98.0) | 87.0 (73.1, 97.7) | 0.22 |
| DLco (% predicted) | 94.0 (78.0, 113.8) | 94.0 (78.4, 114.0) | 89.9 (74.9, 107.5) | 0.18 |
| Total tumor size in CT (mm) | 17.0 (13.0, 22.0) | 1.70 (13.0, 22.0) | 21.0 (16.0, 25.0) | <0.001 |
| Solid size (mm) | 5.0 (0.0, 10.0) | 4.0 (0.0, 9.0) | 13.0 (8.0, 19.0) | <0.001 |
| CTR | <0.001 | |||
| ≤0.5 | 738 (73.5) | 707 (77.0) | 31 (36.0) | |
| >0.5 to <1 | 266 (26.5) | 211 (23.0) | 55 (64.0) | |
| CEA (ng/mL) | 1.9 (1.2, 2.8) | 1.85 (1.2, 2.8) | 2.18 (1.6, 3.4) | 0.02 |
| Surgical procedure | 0.01 | |||
| Lobectomy | 845 (84.2) | 764 (83.2) | 81 (94.2) | |
| Segmentectomy | 159 (15.8) | 154 (16.8) | 5 (5.8) | |
| Tumor size (mm) | 15.0 (10.0, 18.0) | 14.0 (10.0, 18.0) | 18.0 (15.0, 22.0) | <0.001 |
| Invasive tumor size (mm) | 10.0 (7.0, 15.0) | 10.0 (6.0,14.0) | 18.0 (15.0, 22.0) | <0.001 |
| Predominant pattern | <0.001 | |||
| Lep | 302 (30.1) | 299 (32.6) | 3 (3.5) | |
| Acinar/papillary | 699 (69.6) | 619 (67.4) | 80 (93.0) | |
| MP/S | 3 (0.3) | 0 (0.0) | 3 (3.49) | |
| VPI (present) | 171 (17.0) | 140 (15.3) | 31 (36.0) | <0.001 |
| LVI (present) | 25 (2.5) | 14 (1.5) | 11 (12.8) | <0.001 |
| EGFR status | n=499 | n=455 | n=44 | 0.99 |
| Wild | 142 (28.5) | 130 (28.6) | 12 (27.3) | |
| Mutation | 357 (71.5) | 325 (71.4) | 32 (72.7) |
Data are presented as n (%), mean (SD), or median (IQR). −, negative; +, positive. p-stage, pathologic stage; ADC, adenocarcinoma; MP/S, micropapillary and/or solid; FEV1, forced expiratory volume in 1 second; DLco, diffusing capacity of lungs for carbon monoxide; CT, computed tomography; CTR, consolidation-to-tumor ratio; CEA, carcinoembryonic antigen; Lep, lepidic; VPI, visceral pleural invasion; LVI, lymphovascular invasion; EGFR, epidermal growth factor receptor; SD, standard deviation; IQR, interquartile range.
Clinicopathologic characteristics between MP/S+ and MP/S− tumors
Among the entire dataset, 86 patients (8.6%) were identified as having MP/S+ tumors, while the remaining 918 patients (91.4%) had MP/S− subtypes (Table 1). Within the MP/S+ group, there were 28 cases exclusively characterized by micropapillary subtypes, 43 cases exclusively characterized by solid subtypes, and 15 cases with a combination of both subtypes. The mean proportion of MP/S subtypes of tumors in these three subgroups were 12%, 8%, and 23%, respectively. The clinicopathological characteristics of these two patient groups exhibited notable disparities, except for gender distribution, pulmonary function [represented by forced expiratory volume in 1 second (FEV1) (% predicted) and diffusing capacity of lungs for carbon monoxide (DLco) (% predicted)], and the EGFR mutation status. Patients with MP/S+ tumors tended to be older, underwent lobectomy more frequently (94.2% vs. 83.2%, P=0.01), and exhibited higher carcinoembryonic antigen (CEA) levels compared to those with MP/S− tumors. Although statistical significance was not achieved (P=0.06), patients with MP/S+ tumors were more inclined to have a history of smoking (30.2% vs. 20.9%). MP/S+ tumors were significantly larger in terms of both total tumor size and solid size as measured by CT scans. Furthermore, they were more likely to possess a CTR greater than 0.5 to 1 (64.0% vs. 23.0%, P<0.001) compared to MP/S− tumors. Additionally, MP/S+ tumors had a higher likelihood of harboring visceral pleural invasion (VPI) (36.0% vs. 15.3%, P<0.001) and LVI (12.8% vs. 1.5%, P<0.001).
Survival analysis of the MP/S+ and MP/S− tumors
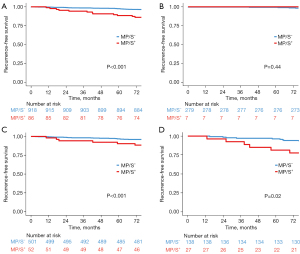
Figure 1 illustrates the RFS curves for patients with MP/S+ and MP/S− tumors in a median of 7.3 years follow-up. In the entire cohort, a statistically significant difference in RFS was observed between lung ADCs with and without MP/S components (Figure 1A; 5-year RFS, 88.3% vs. 97.4%; P<0.001). In the subsequent subgroup analysis, although there was no between-group difference in RFS for tumors measuring less than or equal to 1.0 cm (Figure 1B; P=0.44), significantly inferior RFS of MP/S+ lung ADCs were observed in tumors measuring greater than 1.0 to 2.0 cm and greater than 2.0 to 3.0 cm (Figure 1C,1D; P<0.001 and P=0.02, respectively). However, for tumors measuring less than or equal to 1.0 cm, there was no significant difference in RFS (Figure 1B; P=0.44). OS also exhibited a significant difference between MP/S+ and MP/S− tumors (Figure S1; 5-year OS, 94.1% vs. 98.6%; P=0.008).
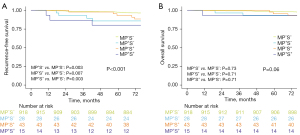
To further assess the prognostic impact of different high-grade patterns, tumors within the MP/S+ group were further categorized into MP+S−, MP−S+, and MP+S+ subgroups based on the presence of MP/S patterns. As depicted in Figure 2, tumors in these subgroups demonstrated significantly worse RFS (but comparable OS) compared to those in the MP/S− group, regardless of the specific pattern they contained. However, no significant differences in RFS or OS were observed between any two MP/S+ subgroups.
Prognostic impact of the histologic Lep component
The entire dataset was categorized into four subgroups: MP/S−Lep+, MP/S−Lep−, MP/S+Lep+, and MP/S+Lep−, based on the presence of Lep and MP/S patterns. The RFS curves and between-group comparisons are depicted in Figure 3A. The 5-year RFS rates for the MP/S−Lep+, MP/S−Lep−, MP/S+Lep+, and MP/S+Lep− subgroups were 98.0%, 95.3%, 93.4%, and 83.2%, respectively (P<0.001). In MP/S− tumors, the presence of the Lep component was significantly associated with better RFS (5-year RFS, 98.0% vs. 95.3%; P=0.01). Similarly, in MP/S+ tumors, the Lep component exhibited a prognostic advantage in terms of RFS (5-year RFS, 93.4% vs. 83.2%; P=0.10), although it did not reach statistical significance.
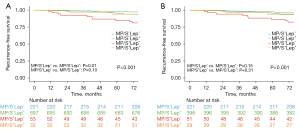
Further analyses were conducted by excluding Lep-predominant tumors, which were known to have an excellent prognosis, as well as MP/S-predominant tumors. In the dataset comprising 699 patients with acinar-predominant and papillary-predominant tumors, the 5-year RFS rates for the MP/S−Lep+, MP/S−Lep−, MP/S+Lep+, and MP/S+Lep− subgroups were 97.4%, 95.2%, 92.7%, and 86.1%, respectively (P<0.001). However, the Lep component did not demonstrate prognostic significance in either MP/S− (MP/S−Lep+vs. MP/S−Lep−, P=0.15) or MP/S+ tumors (MP/S+Lep+vs. MP/S+Lep−, P=0.31) (Figure 3B).
Univariate and multivariate Cox regression analysis for RFS
In the univariate analysis, several factors were found to be associated with worse RFS (Table 2). These factors included patient age, solid size, CTR, tumor size, invasive tumor size, the presence of Lep component, MP/S+ tumors (compared to MP/S− tumors), and the presence of pleural invasion. Acinar/papillary-predominant ADCs were found to have significantly worse RFS compared to Lep-predominant ADCs (95% CI: 1.38–3.54; P=0.001). However, MP/S ADCs did not show a significant association with RFS (95% CI: 0.56–31.79; P=0.16).
Table 2
| Variables | Univariate analysis | Multivariate analysis I | Multivariate analysis II | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||
| Age (years) | 1.05 | 1.02–1.07 | <0.001 | 1.03 | 1.01–1.05 | 0.003 | 1.03 | 1.01–1.05 | 0.004 | ||
| Male (vs. female) | 0.96 | 0.66–1.39 | 0.81 | ||||||||
| Smoking (ever vs. never) | 0.76 | 0.90–1.96 | 0.16 | ||||||||
| Solid size (mm) | 2.04 | 1.67–2.50 | <0.001 | 1.18 | 0.66–2.11 | 0.57 | 1.14 | 0.64–2.04 | 0.65 | ||
| CTR | 5.57 | 3.30–9.39 | <0.001 | 2.21 | 0.65–7.55 | 0.20 | 2.35 | 0.69–7.99 | 0.17 | ||
| Segmentectomy (vs. lobectomy) | 0.67 | 0.37–1.22 | 0.16 | ||||||||
| Tumor size (mm) | 1.83 | 1.37–2.45 | 0.001 | 0.80 | 0.31–2.02 | 0.63 | 0.83 | 0.33–2.08 | 0.70 | ||
| Invasive tumor size (mm) | 2.07 | 1.60–2.67 | <0.001 | 1.30 | 0.49–3.48 | 0.60 | 1.24 | 0.47–3.28 | 0.66 | ||
| CEA (ng/mL) | 1.00 | 0.99–1.01 | 0.95 | ||||||||
| EGFR (mutation vs. wild) | 0.97 | 0.55–1.74 | 0.93 | ||||||||
| Lep component (presence) | 0.46 | 0.33–0.66 | <0.001 | 0.82 | 0.48–1.42 | 0.48 | 0.81 | 0.47–1.40 | 0.46 | ||
| MP/S+ (vs. MP/S−) | 2.85 | 1.85–4.40 | <0.001 | 1.52 | 0.92–2.51 | 0.10 | |||||
| Proportion of MP/S (≥5% vs. <5%) | 3.32 | 2.13–5.19 | <0.001 | 1.77 | 1.07–2.94 | 0.03 | |||||
| Predominant pattern | |||||||||||
| Lep | Ref. | ||||||||||
| Acinar/papillary | 2.85 | 1.38–3.54 | 0.001 | ||||||||
| MP/S | 3.32 | 0.56–31.79 | 0.16 | ||||||||
| Pleural invasion (presence) | 2.07 | 1.42–3.03 | <0.001 | 1.26 | 0.83–1.91 | 0.28 | 1.23 | 0.81–1.88 | 0.32 | ||
| LVI (presence) | 2.17 | 0.96–4.94 | 0.06 | 0.93 | 0.39–2.20 | 0.86 | 0.98 | 0.42–2.29 | 0.95 | ||
−, negative; +, positive. RFS, recurrence-free survival; p-stage, pathologic stage; ADC, adenocarcinoma; HR, hazard ratio; CI, confidence interval; CTR, consolidation-to-tumor ratio; CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor; Lep, lepidic; MP/S, micropapillary and/or solid; ref., reference; LVI, lymphovascular invasion.
Factors with a P value less than 0.1 were included in the multivariate model. However, in the multivariate analysis I, all evaluated factors, except for patient age, were no longer significantly prognostic. The researchers observed that MP/S+ tumors were observed to have a marginal significance (HR =1.52; 95% CI: 0.92–2.51; P=0.10). Therefore, we decided to conduct another multivariable regression analysis (multivariate analysis II), replacing the variable “MP/S+ tumors (vs. MP/S− tumors)” with the variable “proportion of MP/S (≥5% vs. <5%)”. In this alternative analysis, the proportion of MP/S component ≥5% (HR =1.77; 95% CI: 1.07–2.94; P=0.03) and patient age (HR =1.03; 95% CI: 1.01–1.05; P=0.004) were identified as independent predictors of worse RFS. The proportion of MP/S, when included as a continuous variable in the multivariate analysis, was also shown to be a significant prognostic factor (Table S1). The same findings were observed in the dataset of patients with acinar-predominant and papillary-predominant tumors, as shown in Table S2.
Correlation between the proportion of MP/S components and RFS
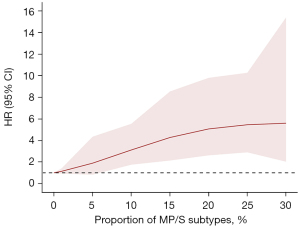
We utilized RCS to visualize the correlation between the proportion of MP/S components and RFS. The RCS plot, with adjustment for patient age and pathological invasive size, showed a non-linear relationship (P for non-linearity: <0.001) between the HR of RFS and the proportion of MP/S components (Figure 4). The plot revealed that the HR of RFS continued to increase as the proportion of MP/S components increased. As the proportion of MP/S components increased from 0% to 15%, the HR for RFS rapidly grew from 1.0 to 4.3 (95% CI: 2.1–8.5). When the MP/S component proportion reached 20%, the HR for RFS increased to 5.1 (95% CI: 2.6–9.8). Once the MP/S component exceeded 20%, the HR growth for RFS significantly slowed down, eventually reaching 5.6 (95% CI: 2.0–15.4) at 30%.
Discussion
Both the favorable prognosis of patients with GGO ADC and the prognostic significance of histologic subtyping are nothing new. However, there is limited understanding of the long-term outcomes of resected GGO ADCs containing high-risk histologic patterns, specifically MP/S components. In this study, we observed that there were significant differences in the clinicopathological characteristics between stage I lung ADC with and without MP/S components. Patients with MP/S− tumors had excellent 5-year RFS and 5-year OS rates, both above 97%. However, the long-term outcomes of patients with MP/S+ tumors were unsatisfactory. On multivariable analysis, MP/S ≥5% was identified as the only tumor-related, independent predictor of worse RFS. This study also noted that the proportion of MP/S subtypes was positively (but non-linearly) correlated with a poorer RFS. The RCS model is a powerful tool to describe for describing the dose-response association between a continuous variable (such as the proportion of MP/S components) and a time-to-event outcome (such as RFS), especially when non-linear dose-response associations are expected (23,24). This approach was chosen over subjective categorization of the variable (i.e., categorizing MP/S+ tumors based on varying ranges of subtype proportions). Therefore, this study suggested that even the presence of a small proportion of MP/S patterns should be considered significant when predicting prognosis of node-negative GGO ADC.
Early-stage lung ADC, specifically stage I or stage IA ADC, represents a heterogeneous assemblage of tumors that demonstrate varying oncologic behavior. At the very inception of oncogenesis and tumor progression, pre-invasive and Lep-predominant lesions emerge, providing a substantial period of observation before decisive interventions are taken. On the other end of the tumor spectrum, we encounter sizable, hypermetabolic tumors that typically exhibit a distinct pure-solid appearance on CT scans and possess aggressive pathological features. Previous investigations have underscored the prognostic significance of GGO, even when it constitutes a minor component (25-29). The prevalence of tumor upstaging, along with other aggressive pathological findings such as LVI and STAS, can exhibit notable disparities between c-stage IA solid tumors and solid-predominant tumors with a small GGO component (30). Given the clear differentiation between pure-solid lung cancers and those with GGO, some proponents argue that measuring the solid component should be limited to pure-solid tumors rather than part-solid tumors (25,28). Our findings align with this viewpoint, as neither the size of the solid component nor the CTR in GGO-featured tumors demonstrated an association with reduced RFS in the multivariate analysis. However, the prognostic value of these radiologic factors was likely overshadowed by another factor, namely the presence of a MP/S component, rather than being completely negated. Therefore, careful consideration is warranted when contemplating the extent of pulmonary resection and lymph node examination for part-solid tumors with a large solid size (such as cT1c) or a high CTR.
There have been many investigations into the clinical significance of minor MP/S patterns in lung ADCs both before and after the IASLC/ATS/ERS classification. Different studies have defined MP/S+ tumors based on varying criteria. Some considered MP/S+ tumors when MP/S patterns occupied ≥1% of the entire tumor (4,8-10,13,14) while others required a proportion ≥5% for the presence of MP/S components (2,11,12,31,32). Given the low prevalence of MP/S subtypes among GGO-featured tumors, we adopted the former criteria introduced by Tsutsumida et al. (8). Their study demonstrated a strong correlation between micropapillary pattern-positive tumors (ratio ≥1%) and lymph node metastasis, as well as a significantly lower disease-free survival (DFS) rate. Subsequent studies indicated that not only the presence of a minimal MP pattern but also a minimal S pattern served as valuable predictors of poor prognosis (9,13,14). Our findings align with these studies, suggesting that both minor MP and S patterns are associated with inferior RFS. In the two multivariate analyses conducted in our study, the presence of MP/S (≥1%) was very close to but not statistically significant in predicting RFS. However, the proportion of MP/S ≥5% demonstrated statistical significance. Interestingly, the incremental prognostic value of MP/S patterns, as visualized by the RCS curves, becomes apparent only when the proportion exceeds 5%. Therefore, we believe that a cutoff point of 5%, in line with the proposed increment for histologic subtyping, would be more reasonable for distinguishing MP/S+ and MP/S− tumors in early-stage ADCs with GGO.
Matsuoka (10), Liu (32), and Hou (31) have conducted separate evaluations of the prognostic value of MP/S components in acinar- and papillary-predominant invasive lung ADCs. They found that minimal MP/S components were negative predictors of postoperative survival. Our analysis of the intermediate-grade-predominant tumor subgroup aligns with their findings. Various studies have also explored the interaction between the Lep component and other growth patterns, but the results have been inconclusive due to significant heterogeneity in the study samples and statistical designs (31,33-36). According to Mäkinen et al. (34), a non-predominant Lep pattern was identified as an independent predictor for favorable disease-specific survival (DSS). They defined the “intermediate” ADC group based on combinations of a minimal Lep pattern and different predominant patterns, such as acinar or papillary predominance without a Lep pattern and MP/S predominance with a Lep pattern. A study focusing on p-stage I acinar/papillary-predominant tumors highlighted that MP/S+ tumors lacking a Lep pattern were the only independent predictor of RFS, although the presence of a Lep component was associated with superior RFS in both MP/S+ and MP/S− tumors (31). Another study involving p-stage IA ADCs found that the Lep component did not exhibit clear prognostic influence (35). Similarly, in our study, the presence of a Lep pattern in GGO-featured tumors did not add significant prognostic value, especially when it coexisted with MP/S patterns. The relatively limited number of MP/S+ patients in our study may have complicated the interpretation of these findings. Nevertheless, caution should be exercised when dealing with MP/S+ tumors, regardless of the presence or absence of the Lep component.
To our best knowledge, this is first study to assess the clinical significance of minimal MP/S components in surgically resected lung ADCs with GGO, using a sufficiently large sample size. Another notable strength of this study lies in the exclusion of patients who underwent wedge resection or did not undergo mediastinal lymph node sampling, thereby minimizing potential biases in the analysis of patient prognosis.
This study possesses several limitations. Firstly, although our overall sample size is substantial, we encompassed a relatively modest number of MP/S+ tumors. Secondly, the data were retrospectively gathered from a solitary institution, rendering our findings potentially reflective solely of the clinical practices within our institution during a specific timeframe. Objective and precise assessment of the proportion of MP/S component under microscopic examination is challenging, which may to some extent affected the credibility of the RCS model. Particularly, evaluating minor proportions involved a high degree of subjectivity, as the personal inclination of pathologists could greatly influence the determination of whether the MP/S proportion was 1% or 5%. Thirdly, we lack insights into the prognostic implications of diverse patterns of postoperative treatment and their potential biases on our survival analysis. Previous studies have demonstrated the potential benefits of postoperative chemotherapy for lung ADCs with minimal MP/S components (14,37,38). We did consider a similar analysis; however, it was rendered infeasible due to the limited sample size (n=19) and the wide array of medication regimens. Further investigations are warranted to determine whether this distinct population would derive advantages from adjuvant chemotherapy or targeted therapy. Fourthly, we noticed that the mean proportion of high-grade subtypes differed between tumors with only a MP/S subtype and tumors with both subtypes. The RCS curves clearly demonstrated the incremental prognostic value of MP/S components, but we do not know whether the coexistence of the two subtypes (a marker of higher MP/S components) affects this effect. Lastly, our study exclusively encompassed patients undergoing curative resection, specifically at least segmentectomy accompanied by mediastinal lymph node sampling, to mitigate unwarranted selection bias. Merely five MP/S+ cases underwent segmentectomy, thereby precluding us from determining the oncological feasibility of sublobar resection for ground-glass nodules (GGNs) exhibiting minimal MP/S patterns. Further evidence pertaining to this subject matter is imperative.
Conclusions
In summary, our study has revealed a distinct “grey zone” within a homogeneous group of early-stage lung ADCs. Specifically, we have identified that GGO-featured ADCs containing MP/S patterns demonstrate aggressive oncological characteristics and are linked to unfavorable prognoses. The presence and proportion of minimal MP/S components have significant implications for the staging and grading of lung ADCs presenting with GGO components.
Acknowledgments
The authors thank all staff in the Department of Thoracic Surgery of Cancer Hospital, Chinese Academy of Medical Sciences for their support during this study.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-736/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-736/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-736/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-736/coif). B.Q. serves as an Associate Editor-in-Chief of Translational Lung Cancer Research from September 2023 to August 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the National Cancer Center (NCC3692), and the requirement for written informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Watanabe K, Sakamaki K, Ito H, et al. Impact of the micropapillary component on the timing of recurrence in patients with resected lung adenocarcinoma. Eur J Cardiothorac Surg 2020;58:1010-8. [Crossref] [PubMed]
- Bertoglio P, Aprile V, Ventura L, et al. Impact of High-Grade Patterns in Early-Stage Lung Adenocarcinoma: A Multicentric Analysis. Lung 2022;200:649-60. [Crossref] [PubMed]
- Jeon YJ, Lee J, Shin S, et al. Prognostic impact of micropapillary and solid histological subtype on patients undergoing curative resection for stage I lung adenocarcinoma according to the extent of pulmonary resection and lymph node assessment. Lung Cancer 2022;168:21-9. [Crossref] [PubMed]
- Wang Y, Yang X, Liu B, et al. Percentage of Newly Proposed High-Grade Patterns Is Associated with Prognosis of Pathological T1-2N0M0 Lung Adenocarcinoma. Ann Surg Oncol 2022; Epub ahead of print. [Crossref]
- Li Y, Byun AJ, Choe JK, et al. Micropapillary and Solid Histologic Patterns in N1 and N2 Lymph Node Metastases Are Independent Factors of Poor Prognosis in Patients With Stages II to III Lung Adenocarcinoma. J Thorac Oncol 2023;18:608-19. [Crossref] [PubMed]
- Mikubo M, Tamagawa S, Kondo Y, et al. Micropapillary and solid components as high-grade patterns in IASLC grading system of lung adenocarcinoma: Clinical implications and management. Lung Cancer 2024;187:107445. [Crossref] [PubMed]
- Tsutsumida H, Nomoto M, Goto M, et al. A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein A expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Mod Pathol 2007;20:638-47. [Crossref] [PubMed]
- Cha MJ, Lee HY, Lee KS, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg 2014;147:921-928.e2. [Crossref] [PubMed]
- Matsuoka Y, Yurugi Y, Takagi Y, et al. Prognostic Significance of Solid and Micropapillary Components in Invasive Lung Adenocarcinomas Measuring ≤3 cm. Anticancer Res 2016;36:4923-30. [Crossref] [PubMed]
- Zhao Y, Wang R, Shen X, et al. Minor Components of Micropapillary and Solid Subtypes in Lung Adenocarcinoma are Predictors of Lymph Node Metastasis and Poor Prognosis. Ann Surg Oncol 2016;23:2099-105. [Crossref] [PubMed]
- Yoshida Y, Nitadori JI, Shinozaki-Ushiku A, et al. Micropapillary histological subtype in lung adenocarcinoma of 2 cm or less: impact on recurrence and clinical predictors. Gen Thorac Cardiovasc Surg 2017;65:273-9. [Crossref] [PubMed]
- Choi SH, Jeong JY, Lee SY, et al. Clinical implication of minimal presence of solid or micropapillary subtype in early-stage lung adenocarcinoma. Thorac Cancer 2021;12:235-44. [Crossref] [PubMed]
- Chen C, Chen ZJ, Li WJ, et al. Impact of minimal solid and micropapillary components on invasive lung adenocarcinoma recurrence. Ann Diagn Pathol 2022;59:151945. [Crossref] [PubMed]
- Mäkinen JM, Laitakari K, Johnson S, et al. Histological features of malignancy correlate with growth patterns and patient outcome in lung adenocarcinoma. Histopathology 2017;71:425-36. [Crossref] [PubMed]
- Ahn B, Yoon S, Kim D, et al. Clinicopathologic and genomic features of high-grade pattern and their subclasses in lung adenocarcinoma. Lung Cancer 2022;170:176-84. [Crossref] [PubMed]
- Moreira AL, Ocampo PSS, Xia Y, et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal From the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol 2020;15:1599-610. [Crossref] [PubMed]
- Hattori A, Hirayama S, Matsunaga T, et al. Distinct Clinicopathologic Characteristics and Prognosis Based on the Presence of Ground Glass Opacity Component in Clinical Stage IA Lung Adenocarcinoma. J Thorac Oncol 2019;14:265-75. [Crossref] [PubMed]
- Watanabe Y, Hattori A, Nojiri S, et al. Clinical impact of a small component of ground-glass opacity in solid-dominant clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2022;163:791-801.e4. [Crossref] [PubMed]
- Hattori A, Suzuki K, Takamochi K, et al. Prognostic impact of a ground-glass opacity component in clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;161:1469-80. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol 2009;62:511-7.e1. [Crossref] [PubMed]
- Rutherford MJ, Crowther MJ, Lambert PC. The use of restricted cubic splines to approximate complex hazard functions in the analysis of time-to-event data: a simulation study. J Stat Comput Simul 2015;85:777-93.
- Hattori A, Matsunaga T, Takamochi K, et al. Prognostic impact of a ground glass opacity component in the clinical T classification of non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:2102-2110.e1. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Importance of Ground Glass Opacity Component in Clinical Stage IA Radiologic Invasive Lung Cancer. Ann Thorac Surg 2017;104:313-20. [Crossref] [PubMed]
- Berry MF, Gao R, Kunder CA, et al. Presence of Even a Small Ground-Glass Component in Lung Adenocarcinoma Predicts Better Survival. Clin Lung Cancer 2018;19:e47-51. [Crossref] [PubMed]
- Hamada A, Suda K, Fujino T, et al. Presence of a Ground-Glass Opacity Component Is the True Prognostic Determinant in Clinical Stage I NSCLC. JTO Clin Res Rep 2022;3:100321. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Fukui M, et al. Prognostic influence of a ground-glass opacity component in hypermetabolic lung adenocarcinoma. Eur J Cardiothorac Surg 2022;61:249-56. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Fukui M, et al. Prognostic Impact of Very Small Ground-Glass Opacity Component in Stage IA Solid Predominant Non-small Cell Lung Cancer. Semin Thorac Cardiovasc Surg 2022; [Crossref]
- Hou Y, Song W, Chen M, et al. The presence of lepidic and micropapillary/solid pathological patterns as minor components has prognostic value in patients with intermediate-grade invasive lung adenocarcinoma. Transl Lung Cancer Res 2022;11:64-74. [Crossref] [PubMed]
- Liu W, Zhang Q, Zhang T, et al. Minor histological components predict the recurrence of patients with resected stage I acinar- or papillary-predominant lung adenocarcinoma. Front Oncol 2022;12:1090544. [Crossref] [PubMed]
- Jeon HW, Kim YD, Sim SB, et al. Prognostic impact according to the proportion of the lepidic subtype in stage IA acinar-predominant lung adenocarcinoma. Thorac Cancer 2021;12:2072-7. [Crossref] [PubMed]
- Mäkinen JM, Laitakari K, Johnson S, et al. Nonpredominant lepidic pattern correlates with better outcome in invasive lung adenocarcinoma. Lung Cancer 2015;90:568-74. [Crossref] [PubMed]
- Okubo Y, Kashima J, Teishikata T, et al. Prognostic Impact of the Histologic Lepidic Component in Pathologic Stage IA Adenocarcinoma. J Thorac Oncol 2022;17:67-75. [Crossref] [PubMed]
- Park BJ, Woo W, Cha YJ, et al. Proposal of a revised International Association for the Study of Lung Cancer grading system in pulmonary non-mucinous adenocarcinoma: The importance of the lepidic proportion. Lung Cancer 2023;175:1-8. [Crossref] [PubMed]
- Qian F, Yang W, Wang R, et al. Prognostic significance and adjuvant chemotherapy survival benefits of a solid or micropapillary pattern in patients with resected stage IB lung adenocarcinoma. J Thorac Cardiovasc Surg 2018;155:1227-1235.e2. [Crossref] [PubMed]
- Wang C, Yang J, Lu M. Micropapillary Predominant Lung Adenocarcinoma in Stage IA Benefits from Adjuvant Chemotherapy. Ann Surg Oncol 2020;27:2051-60. [Crossref] [PubMed]


