
Survivin in lung cancer: a potential target for therapy and prevention—a narrative review
Introduction
Dysregulated apoptosis results in prolonged cell survival in various cancers, autoimmune disorders, and certain viral illnesses (1). Inhibitors of apoptosis proteins (IAPs) are a family of molecules that play a role in cell death, prolong cell viability, and may contribute to cancer cell viability (2). Evidence suggests that identifying and targeting genes encoding such proteins could open a new avenue for cancer treatment. A recently identified gene of interest is survivin, which is generally absent in normal mature adult cells but expressed in transformed cell lines. This makes it a potential target for cancer therapy (3). IAPs are a group of heterogenous proteins expressed in various human malignancies including lung, colon, brain, pancreas, prostate, breast, and lymphatic cancers. Of the various IAPs, survivin is the most extensively studied in lung cancer. Increased expression of survivin in lung cancer tissue samples is reported to be associated with poor prognosis and resistance to chemotherapy (4).
Lung cancer is the most common cause of cancer mortality worldwide and is responsible for one-quarter of all cancer-related deaths (5). Non-small cell lung cancer (NSCLC), including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, accounts for over 85% of lung cancers. Although surgical resection is the curative treatment for lung cancers, 70% of patients present at an advanced stage (stages III and IV) when inoperable (6). Identification of novel molecular markers which characterize lung cancer biology can help find new directions for early diagnosis and treatment at a molecular level. Since the early 2000s, researchers have focused on the diagnostic and prognostic significance of several molecular markers, which lead to the development of targeted therapies centered on endothelial-derived growth factor (EGFR) and BRAF mutations, ALK, ROS1 and RET rearrangements, NTRK and anti-PD-L1 fusions (7). Survivin is one such potential biomarker. It is essential for mitosis, can inhibit apoptosis, is differentially expressed in tumor and normal adult cells, and acts both by intrinsic and extrinsic apoptosis pathways (8). Here, we review the role of survivin in lung cancer, starting from the structure and expression of survivin, polymorphisms, and its role in therapy. We present this article in accordance with the Narrative Review reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-621/rc).
Methods
The PubMed medical literature database and the United States National Library of Medicine search engine at the National Institutes of Health were used on 24 August 2023 to identify published research studies. Literature published in English till 2023 that included all study designs that were possibly relevant to this review were included. Only full manuscripts were considered. Reviews of existing literature, abstracts, preprints, and letters to editors were excluded. The search string, designed to cover the primary themes of the review and optimized for high sensitivity, was: “((((((airway [Title/Abstract]) OR (lung [Title/Abstract])) OR (pulm[Title/Abstract])) OR (bronch[Title/Abstract])) OR (nslc[Title/Abstract])) AND (((cancer[Title/Abstract]) OR (carcino[Title/Abstract])) OR (oncol[Title/Abstract]))) AND (survivin[Title/Abstract])”. This yielded 728 results. After screening the title and abstracts, 168 titles were shortlisted, and full text studied. The discussions are added to relevant sections. The strategy is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 8/24/2023 |
| Databases and other sources searched | PubMed; United States National Library of Medicine search engine at the National Institutes of Health |
| Search terms used | “((((((airway [Title/Abstract]) OR (lung [Title/Abstract])) OR (pulm[Title/Abstract])) OR (bronch[Title/Abstract])) OR (nslc[Title/Abstract])) AND (((cancer[Title/Abstract]) OR (carcino[Title/Abstract])) OR (oncol[Title/Abstract]))) AND (survivin[Title/Abstract])” |
| Timeframe | 1997–2023 |
| Exclusion criteria | Excluded: narrative reviews, non-English articles, full article unavailable online |
| Selection process | Primary author went through the articles and decided the relevance |
Survivin-structure and function
All the proteins in the IAP family are characterized by a domain of 70 amino acids known as baculoviral IAP repeat (BIR). Survivin is the best studied and the smallest member of the IAP family, with a weight of 16.5kda. It contains a single BIR and lacks a carboxy-terminal RING finger unlike other proteins of the family (Figure 1A) (9). It is encoded by the BIRC5 gene on chromosome chr17q25 (3). It undergoes multiple constitutional changes, which influence its various functions such as its stability, role in mitosis, role in apoptosis, intracellular localization, and proliferation signaling. Survivin is expressed maximally in the G2/M phase and rapidly declines thereafter (10). This implies the important role of survivin in cell-cycle regulation, affecting cell proliferation and cell stability (Figure 1B). Survivin plays a major role in cell division at multiple steps as a part of a complex called the chromosomal passenger complex (11). In proliferating cells, after phosphorylation by aurora B, survivin binds to the centromeres in the G2 phase. It communicates with the spindle checkpoint tension sensor BubR1 (BUB1B) to ensure that chromosomes are properly aligned (12). This association lasts until the metaphase-anaphase transition. Thereafter, survivin plays a role in cytokinesis by delineating the cleavage planes (13). It also influences events like mitotic spindle assembly, control of the mitotic checkpoint, and chromosomal condensation (14). By promoting microtubule stability, survivin plays a role in acquiring resistance to anti-cancer agents.
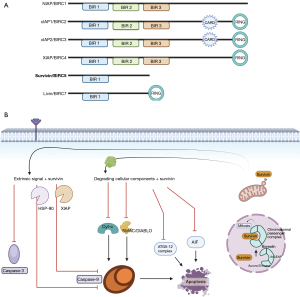
In a non-dividing cell, survivin is localized to the nucleus and/or cytoplasm and rarely in the mitochondria. Although no correlation has been established regarding the significance of the nuclear-to-cytoplasmic ratio of survivin (15), it is observed that there is a progressive increase in the cytoplasmic proportion over time in cancer cells (16). Mitochondrial survivin is specifically seen in cancer cells and is thought to play an active role. This pool increases during stress, is released into the cytosol in response to apoptotic stimuli, and has enhanced anti-apoptotic activity (17).
Survivin is expressed in cells during fetal development (18), in proliferating cells (19) and is thought to be largely undetectable in mature adult cells. Recent evidence suggests that it is expressed in activated T lymphocytes, erythroblasts, and self-renewing stem cells (20-22). Expression in these cell types is regulated by multiple factors including miRNAs and various downstream signaling pathways, during transcription and via post-translational modifications. The promoter region of the survivin gene contains a cell cycle-dependent element (CDE)/cell cycle genes homology region element, indicating that survivin may be a gene regulated by the cell cycle. Thus, survivin expression is influenced by the various phases of the cell cycle (23).
A variety of miRNAs have also been identified to regulate survivin expression via binding to the survivin mRNA, thereby resulting in the alteration of survivin protein translation or leading to its degradation. Out of the multiple miRNAs, molecular mechanisms of miR-34a and miR-203 have been extensively studied. While miRNA-203 directly interacts with the survivin gene and contributes to cancer progression and metastasis, miRNA-34 was shown to reduce survivin via both direct and indirect regulation (24). Table 2 presents multiple other miRNAs which regulate survivin in other malignancies (Figure 2).
Table 2
| MicroRNA | Type of cancer | Mode of action | Reference |
|---|---|---|---|
| miR-34a | Multiple cancers | Direct and via E2F3 | (23) |
| miR-542-3p | Non-small cell lung cancer | Direct | (23) |
| miR-708 | Human renal cell carcinomas | Direct | (23) |
| miRNA125a, miR-125b and miR-205 | Breast cancer | Via STAT3 pathway | (25) |
| miR-203 | Laryngeal cancer, hepatocellular carcinoma, lung cancer, and pancreatic cancer | Direct and via NF-κB | (23) |
| miRNA-218 | Nasopharyngeal cancer, osteosarcoma | Affects multiple oncogenes | (23) |
| miRNA-150 | Burkitt’s | Via p53 | (23) |
| miRNA-16 | Colorectal cancer | Via p53 | (26) |
Post-translational regulation occurs through protein modifications like phosphorylation and polyubiquitination, affecting survivin levels (23). Activation of EGFR can increase survivin protein levels in a phosphatidylinositol 3-kinase (PI3K)-dependent pathway (27). On stimulation by insulin-like growth factor 1 (IGF-1), survivin expression is enhanced through a mechanism mediated by the mammalian target of rapamycin (mTOR) pathway (28). The PI3K/Akt (protein kinase B) signaling-dependent transcription has a role in the expression of genes such as survivin and vascular endothelial growth factor (VEGF), a critical regulator of tumor angiogenesis (29). Signal transducer and activator of transcription 3 (STAT3), a proto-oncogene, on activation, mediates cancer progression through a pathway involving survivin, and STAT3 inhibition leads to apoptosis due to reduced survivin (30).
Survivin and carcinogenesis
As discussed previously, survivin, unlike other IAPs, is exclusively expressed in proliferating cells, making it a key biomarker of cancer proliferation and tumor cell death. As noted above, it is upregulated in many mammalian cancers. More recently, survivin expression has been observed in several preneoplastic lesions, including Bowen’s disease, hypertrophic actinic keratosis (31), polyps of the colon (32), cervical squamous intraepithelial lesions (33), and precancerous oral lesions (34). Kawasaki et al. showed increased survivin immunoreactivity along the adenoma-carcinoma sequence providing evidence for the role of survivin in tumorigenesis (32). Puccio et al. studied the expression of survivin in non-dysplastic Barrett’s esophagus, concluding that survivin levels progressively increase in the transition from metaplasia to dysplasia to adenocarcinoma (35). Similarly, Nakanishi et al. studied levels of survivin in various premalignant lesions of lung cancer low-grade atypical adenomatous hyperplasia (AAH), high-grade AAH, and adenocarcinoma (36). It was found that an increasing proportion of patients expressed survivin as the grade of the lesions increased. These observations underscore survivin’s significance as an early-stage biomarker in multiple human cancers, contributing to carcinogenesis.
Mechanistically, survivin plays a major role in tumorigenesis by regulating programmed cell death at the molecular level. The mechanism of apoptosis can be an intrinsic pathway for intra-cellular stress stimuli or an extrinsic pathway for tumor necrosis factor-mediated cell lysis, both of which are mediated by caspases (37,38). After initiation by cytochrome C and initiator caspases, through a cascade of reactions, caspase 3 and caspase 7 (executioner caspases) bring about cell death (38). The current consensus is that survivin binds to complexes such as XIAP and hepatitis B virus X-interacting protein (HBXIP, also known as LAMTOR5) to interact with caspases or to enhance the effect of other IAPs (39). Other actions include the prevention of the release of apoptotic protease activating factor-1 (APAF-1) from mitochondria and the sequestration of IAP inhibitor—the second mitochondrial-derived activator of caspases (SMAC) (40,41). Survivin may also act independently of caspases by interfering with mitochondrial apoptosis-inducing factor, which acts through DNA fragmentation (41). Furthermore, the survivin-XIAP complex enhances NF-κB and upregulates the genes responsible for cell invasion and metastasis (42). Apart from these mechanisms, results from two of the early studies testing survivin-based vaccines showed significantly reduced tumor volumes, number of blood vessels, and delay in metastases in in vivo studies showing that survivin participates in tumor angiogenesis and helps in tumor growth and metastasis (10,43).
Survivin knockdown studies undertaken to prove the biological causation also consolidate the hypothesis about its effect on cancer cell growth. Combined knockdown of anti-apoptotic proteins Livin, XIAP, and survivin reduced human bladder cancer cell proliferation, due to disinhibition of caspases (44). Another study where survivin was knocked down by vector-based shRNA showed suppressed tumor growth in vitro and reduced tumorigenesis in mice (45). A549/DDP lung cancer cells with survivin knockdown showed reduced proliferation and increased sensitivity to cisplatin (46).
Survivin in lung cancer
Consistent with the role of survivin in other cancers, the expression of survivin is higher in lung cancer compared to normal lung (47). Compared to normal lung epithelium, the promoter region of survivin in lung cancer cells is activated (48). This is true in almost all types of lung cancer. However, survivin expression depends on multiple factors like pathological type, metastasis, smoking, and others. Hirano et al. studied the association between smoking, ki67, and nuclear survivin levels revealing that smokers with lung cancer have elevated levels compared to non-smokers (49). Mohamed et al. showed that nuclear survivin can be an independent prognostic marker by comparing the overall survival in lung cancer patients. Such a correlation between survival and nuclear survivin is not found in patients of small cell lung cancer (50). However, the nearly ubiquitous expression of survivin in lung cancer makes it an attractive target for various diagnostic purposes and novel therapies. A retrospective study of 102 patients with NSCLC showed elevated survivin in 53% of patients and there was a correlation with the size of the tumor, stage of tumor, and a negative correlation with survival. This clinical study showed that survivin is a good biomarker for malignancy and suggested that it can potentially be a therapeutic target due to its selective expression (51). In contrast, no correlation was found between survival and survivin expression in several other studies that looked at survivin protein and mRNA overexpression (52). In a study of circulating cancer cells, 63 of 143 patients expressed survivin positivity and correlated with cancer stage, poorer survival, and nodal status (53). In another study, where 210 NSCLC tissues were examined, survivin expression was detected in 53.3%. Out of these, 67% of patients had VEGF-positivity. This study not only established the reduced survival in patients with increased survivin expression but confirmed its correlation with tumor vascularity and a higher chance of early metastasis (54). Apart from being correlated with prognosis, survivin expression has been associated with response to chemotherapy. A prospective study with 47 patients undergoing chemotherapy showed a significantly increased expression of survivin in patients with cisplatin resistance (55). Similarly, survivin is noted to have increased expression in patients with a diminished response to tyrosine kinase inhibitors and vincristine (56). YM155, a small molecule survivin suppressor, is shown to sensitize NSCLC cells to tyrosine kinase inhibitors when given in combination (57). Transfection of carboplatin-resistant lung cancer cell lines with microRNA, miR-205, and miR-218 induced survivin knockdown and resulted in the downstream cascade leading to apoptosis (58). YM155 also has an interesting role in enhancing the effect in response to radiation, through dedifferentiation of non-stem cancer cells to cancer stem cells (59).
Survivin as a therapeutic target
Presented below is a comprehensive list of therapeutic agents investigated in lung cancer treatment, focusing on their impact on survivin regulation and survivin-associated downstream pathways. These agents are classified into two groups: one that directly influences survivin expression (Table 3), while the other group affects downstream complexes in survivin immune pathways, thereby influencing chemotherapy resistance (Table 4). It’s important to note that while this list is exhaustive, most of these agents are currently in either preclinical or phase I/II of clinical trials. Ongoing clinical trials currently underway are tabulated in Table 5.
Table 3
| Class of drugs | Therapeutic agent | Action | Reference |
|---|---|---|---|
| Transcription inhibitors | YM155 | Single-agent activity in refractory, advanced NSCLC without significant toxicity profile | Giaccone et al., 2009, (60) |
| FL118 | Activity against cancer stem cells, option in resistant and metastatic cancer | Wang et al., 2017, (61) | |
| Protein-protein interaction blockers | |||
| SMAC mimetics | UC-112 analogs | Potential in multi-drug resistant tumors | Wang et al., 2018, (62) |
| Heat shock protein 90-survivin inhibitors | Shepherdin | Extensive suppression of survivin and Akt in vivo experiments | Plescia et al., 2005, (63) |
| Survivin homodimerization inhibitors | Abbott 8, LQZ-7, LQZ-7F | Showed small molecular binding sites which can be targeted to inhibit dimerization and promote degradation | Wendt et al., 2007; Qi et al., 2016, (64,65) |
| Mitosis-related protein inhibitors | Indinavir | Interacts with aurora-b and affects the survivin-XIAP complex | Martínez-García et al., 2019, (66) |
NSCLC, non-small cell lung cancer; SMAC, second mitochondrial-derived activator of caspases.
Table 4
| Pathway | Therapeutic agent | Action | Reference |
|---|---|---|---|
| PI3K/Akt | LY294002 | Both anti-apoptotic and cell survival inhibiting, p53 independent heat sensitizer | Peng et al., 2006, (67) |
| Dihydromyricetin | A compound from Ampelopsis grossedentata with EGFR-related anti-tumor activity | Yu et al., 2021, (68) | |
| SP101 | Survivin suppression in tumor cells and xenograft along with efficacy against gefitinib-resistant cell lines | Kim et al., 2015, (69) | |
| Simvastatin | Synergistic effect with LY294002 and sulindac | Hwang et al., 2011, (70) | |
| Src homology phosphotyrosyl phosphatase 2 (SHP2) | Response in cisplatin-resistant cell lines mediated by survivin | Tang et al., 2018, (71) | |
| Deguelin | In vitro experiments show downregulation of survivin mediated by Akt | Jin et al., 2007, (72) | |
| Metformin | Downregulates survivin in in vitro lung cancer cells | Luo et al., 2019, (73) | |
| Chaperonin-containing TCP-1 (CCT) | Increases chemoresistance and metastasis by regulating survivin | Chang et al., 2020, (74) | |
| JAK/STAT | Arctigenin | Enhances chemosensitivity to cisplatin through this pathway | Wang et al., 2014, (75) |
| Ritonavir | Causes G0/G1 arrest and apoptosis by regulating survivin. It has synergistic actions with several chemotherapy agents | Srirangam et al., 2011, (76) | |
| T21 | Natural-based compound regulating survivin, tested on human cell lines from resected tumor tissue | Martínez-García et al., 2019, (77) | |
| mTOR | Docetaxel | Docetaxel increases cytotoxicity and has synergistic activity with mTOR inhibitors | Niu et al., 2011, (78) |
| CDK2/4 | Fascaplysin | Downregulates survivin and HIF-1α, resulting in suppression of tumor growth in xenograft tumor tissues | Oh et al., 2017, (79) |
| P38 MAPK | COX 2 inhibitors | Induces IL-6 which regulates survivin expression via cascade | Dalwadi et al., 2005, (80) |
| P53 signaling | Matrine | Causes mitochondrial apoptosis in cisplatin-resistant tumors | Liao et al., 2017, (81) |
| Phoyunnanin E | Plant-based protein induces apoptosis in H460 lung cancer cells by downregulating survivin | Phiboonchaiyanan et al., 2018, (82) |
PI3K, phosphatidylinositol 3-kinase; JAK-STAT, Janus kinase-signal transducer and activator of transcription; mTOR, mammalian target of rapamycin; CDK2/4, cyclin dependent kinase 2/4; MAPK, mitogen-activated protein kinases; EGFR, endothelial-derived growth factor; HIF-1α, hypoxia-inducible factor 1-alpha.
Table 5
| Study title | ID | Investigator/sponsor | Website address |
|---|---|---|---|
| Study of an Immunotherapeutic, DPX-Survivac, in Combination With Low Dose Cyclophosphamide & Pembrolizumab, in Subjects With Selected Advanced & Recurrent Solid Tumors | NCT03836352 active, ongoing | ImmunoVaccine Technologies, Inc. (IMV Inc.) | https://clinicaltrials.gov/study/NCT03836352 |
| Survivin Long Peptide Vaccine in Treating Patients with Metastatic Neuroendocrine Tumors | NCT03879694 active, ongoing | Roswell Park Cancer Institute-Renuka V. Iyer | https://clinicaltrials.gov/study/NCT03879694 |
| A Phase I/II Study of Paclitaxel, Carboplatin and YM155 (Survivin Suppressor) in Subjects with Solid Tumors (Phase I) and Advanced Non-Small Cell Lung Carcinoma (Phase II) | NCT01100931 completed | National Institutes of Health Clinical Center | https://clinicaltrials.gov/study/NCT01100931 |
| Survivin and Fibulin-3 in Benign and Malignant Respiratory Diseases | NCT04413292 completed | Mohammed H. Hassan, South Valley University |
https://clinicaltrials.gov/study/NCT04413292 |
Direct survivin inhibitors
A multicenter phase II trial with YM155, a small molecule survivin suppressor was tried as a single agent, in lung cancer patients after treatment failure. Their results showed slightly improved efficacy with respect to objective tumor response rate and overall survival, with a good safety profile (60). A camptothecin-derived product, FL118 (such as irinotecan), showed a decrease in downstream markers of the survivin signaling pathway when studied in vitro in human lung cancer cell lines. This resulted in the inhibition of cell growth, reduced invasion capacity, and resistance-associated proteins (61). SMAC is an apoptosis mediator released from mitochondria that releases caspase-9 after binding to IAPs. Overexpression of survivin can sequester SMAC, leading to the inhibition of its pro-apoptotic function. SMAC mimetics such as UC-112 and its analogs act as competitive binders to survivin, thereby facilitating the liberation of caspases to execute apoptosis (62). Shepherdin, a protein that disrupts the guarding chaperone protein heat shock protein-90 (HSP-90) from survivin, affects the stability and function. Administration of Shepherdin induced tumor cell death in both in vitro and in vivo experiments with human cell lines in mice (63). An in vitro study identified a compound, LQZ-7F, which targets survivin dimerization cores resulting in survivin degradation and reduced tumor growth, as noted in human cancer cell lines and xenograft mouse models (64).
Indirect survivin inhibitors
A novel tyrosine kinase inhibiting compound, named SP101, reduced survivin expression and tumor growth in gefitinib-resistant human lung cancer cells xenografted into mice (69). An experiment conducted on human lung cancer cell lines (A549 cells) showed survivin downregulation and apoptosis prevention in the presence of sulindac and simvastatin, mediated by the Akt signaling pathway. This study also showed suppression of Akt or PI3K inhibitor, LY294002 acts synergistically with sulindac and simvastatin resulting in enhanced apoptosis (70). Src homology phosphotyrosyl phosphatase 2 (SHP-2) is an important domain in cytokine growth and migration, with a role in lung cancer cell resistance to cisplatin. Tang et al. studied the protective effect of it in H446 cell lines and noted that, the cells that overexpressed SHP-2 also led to significant survivin upregulation along with Akt and pAkt (71).
Jin and colleagues postulated that deguelin, a natural plant-derived compound, has the potential to suppress survivin expression and trigger apoptosis in both premalignant and malignant cells. This effect is primarily achieved through modulation of the mTOR pathway, with Akt and adenosine monophosphate-activated protein kinase (AMPK) regulation serving as key mediators (72). Amid recent reports of anti-cancer activity, the antidiabetic drug metformin also showed a decrease in survivin expression in A549 & H460 cell lines in vitro. This is attributed to AMPK activation along with suppression of protein kinase A (PKA) and glycogen synthase kinase 3β (GSK-3β) activation (73). Using the same pathway is an 8-subunit chaperonin, Chaperonin-containing TCP-1 (CCT) promoted chemoresistance and enhanced metastasis, as seen in the in vitro study conducted on human lung cancer cell line CL1-5 (74).
Therapeutic agents with the potential to disrupt survivin-mediated apoptotic processes may be strategically integrated into chemotherapy protocols, thereby augmenting the sensitivity of these treatment regimens. Arctigenin, a dibenzyl butyrolactone lignan, enhanced cisplatin-mediated apoptosis in NSCLC H460 cells., by significantly downregulating survivin and inducing G1/G0 cell cycle arrest (75). Anti-retroviral agent Ritonavir, with some evidence of anti-cancer activity, is noted to be active across a wide range of lung adenocarcinoma cell lines, by STAT3-mediated survivin suppression. It is also shown to have a synergistic effect with standard lung cancer regimen gemcitabine + cisplatin (76). Likewise, mTOR inhibitors enhance the effectiveness of docetaxel, by synergistically reducing survivin levels and increasing apoptosis in human lung cancer cell lines A549 and SPC-A-1 (78). Fascaplysin, which is thought to be a CDK4 inhibitor, reduced survivin protein expression but not mRNA, in a time and dose-dependent manner, thereby increasing the cell viability and inhibiting angiogenesis (79).
Not all the agents identified above could progress to clinical trials because these therapeutic agents and the trials had their limitations. The phase II trial looking at the effect of YM155, although proved the safety profile, showed only a marginally improved disease control rate compared to the standard chemotherapy (60). The sample was too small to examine the correlation between survivin downregulation and clinical response. Most of the indirect survivin inhibitors act by affecting one of the many survivin signaling pathways, due to which the pre-clinical observations are not translated into effective clinical responses. This occurs when the targeted pathway is bypassed by another compensatory mechanism (83). This validates the idea of using combination agents with synergistic effects, which could also help in reducing dose, reducing systemic toxicity, and enhancing patient benefit.
Survivin as an immunotherapy target
Studies were undertaken to investigate whether the survivin protein expressed by tumor cells exhibits immunogenicity comparable to the products of proto-oncogenes and tumor suppressor genes. A high prevalence of anti-survivin antibodies was noted in sera from the patients of lung cancer (84). Schmitz et al. showed that dendritic cells loaded with survivin-specific peptides in vitro generated widespread cytotoxic T-cell responses against multiple tumor cell lines (85). Therefore, survivin’s potential immunogenicity to provoke both T-cell mediated, and humoral immunity could serve as a foundation for eliciting therapeutic anti-tumor immunity for cancer patients. In such an attempt, a DNA-based vaccine was developed by fusion of soluble PD-1, tumor antigens, survivin, and MUC1 which produced anti-tumor immune CD8+ T cell responses (86). This marked the beginning of the exploration for clinical trials aimed at inducing anti-tumor immune responses as a potential therapy for lung cancer. Later, Xiang et al. developed a DNA vaccine with chemokine CCL21 co-expression and proved that this vaccine could suppress angiogenesis and result in the eradication of lung tumors (43). Similar vaccines were tried on several tumors and promising results were seen in oral, colorectal, breast, and melanoma. A survivin long peptide vaccine, SurVaxM was studied in patients with malignant glioma, and immunogenicity, safety, and tolerability were demonstrated (87).
The current survivin-based therapies are primarily directed towards mitigating multi-drug resistance and advanced metastatic cancers, with reasonable success in in vitro and in vivo studies. An avenue that is still open to investigate would be mediators of survivin regulatory pathways useful for chemo-immunoprevention. Given its dual role in regulating tumor growth and modulating immune responses, survivin stands out as a highly promising target for investigation. Exploring this avenue will require high-standard clinical studies that show efficacy in not merely decelerating the cancer progression but also laying the path for cancer prevention.
Future directions
Further exploration and advancement of survivin-targeted therapies through rigorous clinical trials are essential. Simultaneous studies on combinational therapies and newer drug delivery systems need to be planned for enhanced therapeutic efficacy.
Continuation of research into survivin-based immunotherapies, including vaccines and immune checkpoint inhibitors, can harness the body’s immune response to effectively target lung cancer cells with limited systemic toxicity.
Further understanding of the molecular and cellular mechanisms of survivin regulation could uncover new therapeutic avenues and potential drug regimens for immuno-prevention before the onset of carcinogenesis or slow progression for premalignant lesions of lung cancer.
Conclusions
Survivin plays a distinct role in cell division and programmed cell death. The transcription, interactions, and signaling have been extensively studied to be used in the management of multiple cancers. Survivin is selectively upregulated in malignant cells, making it a potential target for therapy and a molecular marker for early diagnosis. Several tumor-specific transcriptional and post-translational therapeutic modalities with good safety profiles have been described. This knowledge of survivin-regulated pathways can be harnessed to revolutionize the fields of lung cancer prevention and treatment of advanced-stage cancers. Further phase II and III trials need to be planned with the objective of targeted therapy based on individual tumor histological findings, laying special emphasis on vaccines, immunotherapy, and gene targeting, for highly selective tumor-specific action, and limited systemic side effects, to increase survival in patients of lung cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-621/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-621/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-621/coif). S.Y. serves as an unpaid editorial board member of Translational Lung Cancer Research from October 2023 to September 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995;267:1456-62. [Crossref] [PubMed]
- Hrdinka M, Yabal M. Inhibitor of apoptosis proteins in human health and disease. Genes Immun 2019;20:641-50. [Crossref] [PubMed]
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997;3:917-21. [Crossref] [PubMed]
- Karczmarek-Borowska B, Filip A, Wojcierowski J, et al. Survivin antiapoptotic gene expression as a prognostic factor in non-small cell lung cancer: in situ hybridization study. Folia Histochem Cytobiol 2005;43:237-42.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Osmani L, Askin F, Gabrielson E, et al. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol 2018;52:103-9. [Crossref] [PubMed]
- Ortega MA, Navarro F, Pekarek L, et al. Exploring histopathological and serum biomarkers in lung adenocarcinoma: Clinical applications and translational opportunities Int J Oncol 2022;61:154. (Review). [Crossref] [PubMed]
- Garg H, Suri P, Gupta JC, et al. Survivin: a unique target for tumor therapy. Cancer Cell Int 2016;16:49. [Crossref] [PubMed]
- Chantalat L, Skoufias DA, Kleman JP, et al. Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alpha-helical extensions. Mol Cell 2000;6:183-9.
- Hofmann UB, Voigt H, Andersen MH, et al. Identification and characterization of survivin-derived H-2Kb-restricted CTL epitopes. Eur J Immunol 2009;39:1419-24. [Crossref] [PubMed]
- Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Curr Opin Cell Biol 2006;18:616-22. [Crossref] [PubMed]
- Wheatley SP, Barrett RM, Andrews PD, et al. Phosphorylation by aurora-B negatively regulates survivin function during mitosis. Cell Cycle 2007;6:1220-30. [Crossref] [PubMed]
- Rajagopalan S, Balasubramanian MK. Schizosaccharomyces pombe Bir1p, a nuclear protein that localizes to kinetochores and the spindle midzone, is essential for chromosome condensation and spindle elongation during mitosis. Genetics 2002;160:445-56. [Crossref] [PubMed]
- Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol 2006;18:609-15. [Crossref] [PubMed]
- Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res 2007;67:5999-6002. [Crossref] [PubMed]
- Temme A, Diestelkoetter-Bachert P, Schmitz M, et al. Increased p21(ras) activity in human fibroblasts transduced with survivin enhances cell proliferation. Biochem Biophys Res Commun 2005;327:765-73. [Crossref] [PubMed]
- Dohi T, Beltrami E, Wall NR, et al. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest 2004;114:1117-27. [Crossref] [PubMed]
- Uren AG, Wong L, Pakusch M, et al. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol 2000;10:1319-28. [Crossref] [PubMed]
- Li F, Ambrosini G, Chu EY, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998;396:580-4. [Crossref] [PubMed]
- Keerthivasan G, Liu H, Gump JM, et al. A novel role for survivin in erythroblast enucleation. Haematologica 2012;97:1471-9. [Crossref] [PubMed]
- Leung CG, Xu Y, Mularski B, et al. Requirements for survivin in terminal differentiation of erythroid cells and maintenance of hematopoietic stem and progenitor cells. J Exp Med 2007;204:1603-11. [Crossref] [PubMed]
- Martini E, Schneider E, Neufert C, et al. Survivin is a guardian of the intestinal stem cell niche and its expression is regulated by TGF-β. Cell Cycle 2016;15:2875-81. [Crossref] [PubMed]
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer 2003;3:46-54. [Crossref] [PubMed]
- Huang J, Lyu H, Wang J, et al. MicroRNA regulation and therapeutic targeting of survivin in cancer. Am J Cancer Res 2015;5:20-31.
- Shishavan NS, Sasani ST, Salehi Z, et al. Downregulation of miR-125a-5p Leads to STAT3 Increased Expression in Breast Cancer Patients. Microrna 2022;11:263-70. [Crossref] [PubMed]
- Ma Q, Wang X, Li Z, et al. microRNA-16 represses colorectal cancer cell growth in vitro by regulating the p53/survivin signaling pathway. Oncol Rep 2013;29:1652-8. [Crossref] [PubMed]
- Asanuma H, Torigoe T, Kamiguchi K, et al. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res 2005;65:11018-25. [Crossref] [PubMed]
- Vaira V, Lee CW, Goel HL, et al. Regulation of survivin expression by IGF-1/mTOR signaling. Oncogene 2007;26:2678-84. [Crossref] [PubMed]
- Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci 2011;4:51. [Crossref] [PubMed]
- Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 2003;101:1535-42. [Crossref] [PubMed]
- Grossman D, McNiff JM, Li F, et al. Expression of the apoptosis inhibitor, survivin, in nonmelanoma skin cancer and gene targeting in a keratinocyte cell line. Lab Invest 1999;79:1121-6.
- Kawasaki H, Toyoda M, Shinohara H, et al. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer 2001;91:2026-32. [Crossref] [PubMed]
- Frost M, Jarboe EA, Orlicky D, et al. Immunohistochemical localization of survivin in benign cervical mucosa, cervical dysplasia, and invasive squamous cell carcinoma. Am J Clin Pathol 2002;117:738-44. [Crossref] [PubMed]
- Lo Muzio L, Pannone G, Leonardi R, et al. Survivin, a potential early predictor of tumor progression in the oral mucosa. J Dent Res 2003;82:923-8. [Crossref] [PubMed]
- Puccio I, Khan S, Butt A, et al. Immunohistochemical assessment of Survivin and Bcl3 expression as potential biomarkers for NF-κB activation in the Barrett metaplasia-dysplasia-adenocarcinoma sequence. Int J Exp Pathol 2018;99:10-4. [Crossref] [PubMed]
- Nakanishi K, Kawai T, Kumaki F, et al. Survivin expression in atypical adenomatous hyperplasia of the lung. Am J Clin Pathol 2003;120:712-9. [Crossref] [PubMed]
- Zhang C, Zhang F. Iron homeostasis and tumorigenesis: molecular mechanisms and therapeutic opportunities. Protein Cell 2015;6:88-100. [Crossref] [PubMed]
- Ghavami S, Hashemi M, Ande SR, et al. Apoptosis and cancer: mutations within caspase genes. J Med Genet 2009;46:497-510. [Crossref] [PubMed]
- Marusawa H, Matsuzawa S, Welsh K, et al. HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO J 2003;22:2729-40. [Crossref] [PubMed]
- Altieri DC. New wirings in the survivin networks. Oncogene 2008;27:6276-84. [Crossref] [PubMed]
- Altieri DC. Survivin and IAP proteins in cell-death mechanisms. Biochem J 2010;430:199-205. [Crossref] [PubMed]
- Knight BB, Oprea-Ilies GM, Nagalingam A, et al. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocr Relat Cancer 2011;18:413-28. [Crossref] [PubMed]
- Xiang R, Mizutani N, Luo Y, et al. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res 2005;65:553-61.
- Yang D, Song X, Zhang J, et al. Therapeutic potential of siRNA-mediated combined knockdown of the IAP genes (Livin, XIAP, and Survivin) on human bladder cancer T24 cells. Acta Biochim Biophys Sin (Shanghai) 2010;42:137-44. [Crossref] [PubMed]
- Zhang R, Ma L, Zheng M, et al. Survivin knockdown by short hairpin RNA abrogates the growth of human hepatocellular carcinoma xenografts in nude mice. Cancer Gene Ther 2010;17:275-88. [Crossref] [PubMed]
- Liu JL, Wang Y, Jiang J, et al. Inhibition of survivin expression and mechanisms of reversing drug-resistance of human lung adenocarcinoma cells by siRNA. Chin Med J (Engl) 2010;123:2901-7.
- Monzó M, Rosell R, Felip E, et al. A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J Clin Oncol 1999;17:2100-4. [Crossref] [PubMed]
- Xu Y, Fang F, Ludewig G, et al. A mutation found in the promoter region of the human survivin gene is correlated to overexpression of survivin in cancer cells. DNA Cell Biol 2004;23:419-29. [Crossref] [PubMed]
- Hirano H, Maeda H, Yamaguchi T, et al. Survivin expression in lung cancer: Association with smoking, histological types and pathological stages. Oncol Lett 2015;10:1456-62. [Crossref] [PubMed]
- Mohamed S, Yasufuku K, Nakajima T, et al. Nuclear survivin in pN2 nonsmall cell lung cancer: prognostic and clinical implications. Eur Respir J 2009;33:127-33. [Crossref] [PubMed]
- Kren L, Brazdil J, Hermanova M, et al. Prognostic significance of anti-apoptosis proteins survivin and bcl-2 in non-small cell lung carcinomas: a clinicopathologic study of 102 cases. Appl Immunohistochem Mol Morphol 2004;12:44-9. [Crossref] [PubMed]
- Falleni M, Pellegrini C, Marchetti A, et al. Survivin gene expression in early-stage non-small cell lung cancer. J Pathol 2003;200:620-6. [Crossref] [PubMed]
- Yie SM, Lou B, Ye SR, et al. Clinical significance of detecting survivin-expressing circulating cancer cells in patients with non-small cell lung cancer. Lung Cancer 2009;63:284-90. [Crossref] [PubMed]
- Chen P, Zhu J, Liu DY, et al. Over-expression of survivin and VEGF in small-cell lung cancer may predict the poorer prognosis. Med Oncol 2014;31:775. [Crossref] [PubMed]
- Cho HJ, Kim HR, Park YS, et al. Prognostic value of survivin expression in stage III non-small cell lung cancer patients treated with platinum-based therapy. Surg Oncol 2015;24:329-34. [Crossref] [PubMed]
- Zhou C, Zhu Y, Lu B, et al. Survivin expression modulates the sensitivity of A549 lung cancer cells resistance to vincristine. Oncol Lett 2018;16:5466-72. [Crossref] [PubMed]
- Shimizu T, Nishio K, Sakai K, et al. Phase I safety and pharmacokinetic study of YM155, a potent selective survivin inhibitor, in combination with erlotinib in patients with EGFR TKI refractory advanced non-small cell lung cancer. Cancer Chemother Pharmacol 2020;86:211-9. [Crossref] [PubMed]
- Zarogoulidis P, Petanidis S, Kioseoglou E, et al. MiR-205 and miR-218 expression is associated with carboplatin chemoresistance and regulation of apoptosis via Mcl-1 and Survivin in lung cancer cells. Cell Signal 2015;27:1576-88. [Crossref] [PubMed]
- Rhodes A, Hillen T. Mathematical Modeling of the Role of Survivin on Dedifferentiation and Radioresistance in Cancer. Bull Math Biol 2016;78:1162-88. [Crossref] [PubMed]
- Giaccone G, Zatloukal P, Roubec J, et al. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol 2009;27:4481-6. [Crossref] [PubMed]
- Wang J, Liu Z, Zhang D, et al. FL118, a novel survivin inhibitor, wins the battle against drug-resistant and metastatic lung cancers through inhibition of cancer stem cell-like properties. Am J Transl Res 2017;9:3676-86.
- Wang Q, Arnst KE, Xue Y, et al. Synthesis and biological evaluation of indole-based UC-112 analogs as potent and selective survivin inhibitors. Eur J Med Chem 2018;149:211-24. [Crossref] [PubMed]
- Plescia J, Salz W, Xia F, et al. Rational design of shepherdin, a novel anticancer agent. Cancer Cell 2005;7:457-68. [Crossref] [PubMed]
- Qi J, Dong Z, Liu J, et al. Effective Targeting of the Survivin Dimerization Interface with Small-Molecule Inhibitors. Cancer Res 2016;76:453-62. [Crossref] [PubMed]
- Wendt MD, Sun C, Kunzer A, et al. Discovery of a novel small molecule binding site of human survivin. Bioorg Med Chem Lett 2007;17:3122-9. [Crossref] [PubMed]
- Martínez-García D, Manero-Rupérez N, Quesada R, et al. Therapeutic strategies involving survivin inhibition in cancer. Med Res Rev 2019;39:887-909. [Crossref] [PubMed]
- Peng XH, Karna P, Cao Z, et al. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem 2006;281:25903-14. [Crossref] [PubMed]
- Yu ZW, Zhang N, Jiang CY, et al. Exploring the genes involved in biosynthesis of dihydroquercetin and dihydromyricetin in Ampelopsis grossedentata. Sci Rep 2021;11:15596. [Crossref] [PubMed]
- Kim YS, Seol CH, Jung JW, et al. Synergistic Effect of Sulindac and Simvastatin on Apoptosis in Lung Cancer A549 Cells through AKT-Dependent Downregulation of Survivin. Cancer Res Treat 2015;47:90-100. [Crossref] [PubMed]
- Hwang KE, Na KS, Park DS, et al. Apoptotic induction by simvastatin in human lung cancer A549 cells via Akt signaling dependent down-regulation of survivin. Invest New Drugs 2011;29:945-52. [Crossref] [PubMed]
- Tang C, Luo H, Luo D, et al. Src homology phosphotyrosyl phosphatase 2 mediates cisplatin-related drug resistance by inhibiting apoptosis and activating the Ras/PI3K/Akt1/survivin pathway in lung cancer cells. Oncol Rep 2018;39:611-8. [Crossref] [PubMed]
- Jin Q, Feng L, Behrens C, et al. Implication of AMP-activated protein kinase and Akt-regulated survivin in lung cancer chemopreventive activities of deguelin. Cancer Res 2007;67:11630-9. [Crossref] [PubMed]
- Luo Z, Chen W, Wu W, et al. Metformin promotes survivin degradation through AMPK/PKA/GSK-3β-axis in non-small cell lung cancer. J Cell Biochem 2019;120:11890-9. [Crossref] [PubMed]
- Chang YX, Lin YF, Chen CL, et al. Chaperonin-Containing TCP-1 Promotes Cancer Chemoresistance and Metastasis through the AKT-GSK3β-β-Catenin and XIAP-Survivin Pathways. Cancers (Basel) 2020;12:3865. [Crossref] [PubMed]
- Wang HQ, Jin JJ, Wang J. Arctigenin enhances chemosensitivity to cisplatin in human nonsmall lung cancer H460 cells through downregulation of survivin expression. J Biochem Mol Toxicol 2014;28:39-45. [Crossref] [PubMed]
- Srirangam A, Milani M, Mitra R, et al. The human immunodeficiency virus protease inhibitor ritonavir inhibits lung cancer cells, in part, by inhibition of survivin. J Thorac Oncol 2011;6:661-70. [Crossref] [PubMed]
- Martínez-García D, Pérez-Hernández M, Korrodi-Gregório L, et al. The Natural-Based Antitumor Compound T21 Decreases Survivin Levels through Potent STAT3 Inhibition in Lung Cancer Models. Biomolecules 2019;9:361. [Crossref] [PubMed]
- Niu H, Wang J, Li H, et al. Rapamycin potentiates cytotoxicity by docetaxel possibly through downregulation of Survivin in lung cancer cells. J Exp Clin Cancer Res 2011;30:28. [Crossref] [PubMed]
- Oh TI, Lee YM, Nam TJ, et al. Fascaplysin Exerts Anti-Cancer Effects through the Downregulation of Survivin and HIF-1α and Inhibition of VEGFR2 and TRKA. Int J Mol Sci 2017;18:2074. [Crossref] [PubMed]
- Dalwadi H, Krysan K, Heuze-Vourc'h N, et al. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res 2005;11:7674-82. [Crossref] [PubMed]
- Liao XZ, Tao LT, Liu JH, et al. Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer Cell Int 2017;17:124. [Crossref] [PubMed]
- Phiboonchaiyanan PP, Petpiroon N, Sritularak B, et al. Phoyunnanin E Induces Apoptosis of Non-small Cell Lung Cancer Cells via p53 Activation and Down-regulation of Survivin. Anticancer Res 2018;38:6281-90. [Crossref] [PubMed]
- Fulda S. Promises and Challenges of Smac Mimetics as Cancer Therapeutics. Clin Cancer Res 2015;21:5030-6. [Crossref] [PubMed]
- Rohayem J, Diestelkoetter P, Weigle B, et al. Antibody response to the tumor-associated inhibitor of apoptosis protein survivin in cancer patients. Cancer Res 2000;60:1815-7.
- Schmitz M, Diestelkoetter P, Weigle B, et al. Generation of survivin-specific CD8+ T effector cells by dendritic cells pulsed with protein or selected peptides. Cancer Res 2000;60:4845-9.
- Liu C, Lu Z, Xie Y, et al. Soluble PD-1-based vaccine targeting MUC1 VNTR and survivin improves anti-tumor effect. Immunol Lett 2018;200:33-42. [Crossref] [PubMed]
- Fenstermaker RA, Ciesielski MJ, Qiu J, et al. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol Immunother 2016;65:1339-52. [Crossref] [PubMed]



