
Outcomes following minimally invasive approaches vs. open extended lobectomy for non-small cell lung cancer: a propensity-matched analysis of the National Cancer Database
Highlight box
Key findings
• Minimally invasive surgery (MIS) for extended lobectomy in non-small cell lung cancer (NSCLC) is safe and feasible. It can offer shorter hospital stays than traditional open surgery, without lowering 5-year survival rates.
What is known, and what is new?
• Video-assisted thoracoscopic surgery is superior to open lobectomy, with MIS for extended lobectomy being previously limited and challenging.
• According to the National Cancer Database, a national database in the United States, this study is one of the first to use propensity score matching to report outcomes between open and MIS extended lobectomy. The MIS approach to extended lobectomy can be adopted in various hospital settings not just academic centers.
What is the implication, and what should change now?
• The study findings support a potential shift toward broader adoption of MIS for extended lobectomy in NSCLC, emphasizing the need for enhanced surgeon training, patient selection, and further research to validate and refine this approach.
Introduction
Lung cancer remains one of the leading causes of cancer death worldwide (1). Surgery is widely accepted as an essential treatment option for this disease, offering the best potential for cure in patients with lesions amenable to resection. As the technology in the surgical field advances, the approach to lung resection surgery is shifting to offering minimally invasive techniques as the standard approach (2-7).
In many extensive database studies, video-assisted thoracoscopic surgery (VATS) approaches to lobectomy are superior to open approaches. In a propensity-matched analysis based on the Society of Thoracic Surgeons (STS) database, Paul et al. found the thoracoscopic approach to lobectomy was associated with a lower incidence of postoperative complications and an overall shorter length of stay (LOS) compared to the open approach (2). This has been validated several times in other databases, including the Surveillance, Epidemiology, and End Results (SEER)-Medicare database as well as the National Cancer Database (NCDB) (3,4). These studies have paved the way for a paradigm shift that has led to the VATS approach for classic lobectomy becoming the standard of care for patients with resectable lung cancer confined to one anatomic lobe.
Unfortunately, some patients require additional dissection to achieve an R0 resection for the best oncologic results depending on disease progression and location (8-10). The recent literature attests to a broader adoption of the minimally invasive approach for extended lung resections, including chest wall resections, bronchoplastic and arterial sleeve resections, and pneumonectomy (11-18). Although the results of these single-institution studies have been impressive, it is unclear whether these same results can be achieved in the broader surgical community.
There have been no prospective clinical trials comparing open vs. minimally invasive surgery (MIS) for extended lobectomy. In the NCDB, extended lobectomy is defined to include surgery codes 45 [lobectomy or bilobectomy extended, not otherwise specified (NOS)], 46 (lobectomy or bilobectomy extended, with chest wall), 47 (with pericardium), and 48 (with diaphragm). Using the NCDB, we analyzed the national outcomes of the minimally invasive approach to extended lobectomy with the primary outcome of 5-year overall survival following surgery. We also evaluated safety and feasibility after surgery. We present this article in accordance with the STROBE reporting checklist (19) (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-37/rc).
Methods
Cohort selection
We performed a retrospective analysis of the NCDB, a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. We identified patients diagnosed with non-small cell lung cancer (NSCLC) undergoing extended lobectomy between 2010 and 2014. We chose 2010 as the starting year because this was when the NCDB began to include the approach data. We chose 2014 as the cutoff year because this was the latest dataset with follow-up information when we started our study. Extended lobectomy was defined as the Facility Oncology Registry Data Standards manual site-specific surgery codes 45 (lobectomy or bilobectomy extended, NOS), 46 (lobectomy or bilobectomy extended, with chest wall), 47 (lobectomy or bilobectomy extended, with pericardium), and 48 (lobectomy or bilobectomy extended, with diaphragm). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
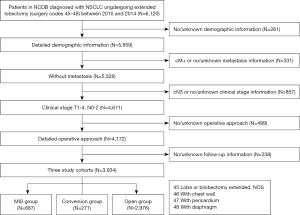
The inclusion criteria of this study were as follows: according to the intention-to-treat analysis, patients diagnosed with NSCLC undergoing extended lobectomy via either MIS or thoracotomy between January 2010 and December 2014 were analyzed, including converted patients. The MIS group included VATS and robot-assisted thoracoscopic surgery (RATS). The exclusion criteria consisted of patients with missing demographic information, metastatic disease, no/unknown metastasis information, cN3 cases, no/unknown clinical-stage information, and missing or no/unknown operation approach information and follow-up information (Figure 1).
Patients’ demographics and clinicopathological characteristics were compared between the two groups (open vs. MIS). Postoperative LOS, unplanned readmission, 30-day mortality, and 90-day mortality were measured as the primary perioperative outcomes. The following was analyzed to measure oncological outcomes: examined lymph nodes, positive lymph nodes, R0 rates, and 1-, 3-, and 5-year overall survival.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation (SD), and the Student’s t-test or Mann-Whitney test was used for comparison. Comparison of categorical variables was performed with the Chi-squared test or Fisher exact test when appropriate. Survival curves were plotted with the Kaplan-Meier formula. The log-rank test was used to compare survival between different groups. As the baseline characteristics in the two groups were not balanced, propensity score matching (PSM) analysis was performed with SPSS 23 software (IBM Corp., Armonk, NY, USA). Propensity scores were estimated using a logistic regression model. PSM was performed in a 1:2 ratio according to gender, age, race, insurance, income, education, reporting facility, Charlson Comorbidity Index, clinical T stage, clinical N stage, surgery codes, histological classification, neoadjuvant therapy, and tumor location, with a SD of less than 0.20 logit of the propensity score. Patients who underwent MIS were ordered and sequentially matched to the nearest unmatched patients who underwent thoracotomy. Surgical and postoperative outcomes were then compared between the matched groups. Multivariable analysis was performed with a Cox proportional model and the enter method. We selected demographic and clinicopathological characteristics, as well as surgical information, as our variables of Cox proportional hazard model. Entry limits were a P value <0.2.
Subgroup analyses were performed, including comparisons between VATS and RATS, conversion and open procedures, and MIS and open surgeries in community hospital settings. Statistical significance was defined as P<0.05 throughout the study.
Results
Cohort characteristics
Between January 2010 and December 2014, 6,120 patients were diagnosed with NSCLC and underwent extended lobectomy according to our inclusion criteria. Based on our inclusion and exclusion criteria, 958 extended lobectomies were attempted with a conversion to open thoracotomy occurring in 28.3% [271] of patients. Therefore, for our analysis, 687 patients were included in the MIS group, 271 in the conversion group, and 2,976 in the open group (Figure 1). Figure 2 shows the annual number of patients who underwent extended lobectomy. The MIS rate steadily increased, and the conversion rate essentially decreased. The postoperative LOS, unplanned readmission rates, 30-day mortality, and 90-day mortality mainly decreased in the MIS group year by year. The patients’ demographics and clinical characteristics are listed in Table S1. Most of the clinicopathologic factors were not comparable between the two groups. MIS patients were slightly older (mean age 67.46±9.74 vs. 66.29±9.74 years), and a higher proportion of MIS patients were female (51.5% vs. 46.5%, P=0.018). The average tumor size of the open group was larger than that of the MIS group (39.87±25.38 vs. 49.00±51.02 mm, P<0.001). Therefore, more MIS group patients were diagnosed with clinical T1 stage (39.4% vs. 28.6%, P<0.001). Table 1 shows the perioperative outcomes before PSM in the overall cohort.

Table 1
| Results | Unmatched cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| MIS (n=687) | Open (n=2,976) | P value | MIS (n=683) | Open (n=1,317) | P value | ||
| R0 | 625 (91.0) | 2,575 (86.5) | 0.001 | 621 (90.9) | 1,163 (88.3) | 0.081 | |
| LN number | 12.59±9.62 | 11.17±8.79 | 0.001 | 12.18±9.74 | 10.61±8.55 | 0.001 | |
| Positive LN number | 0.66±1.76 | 0.69±1.86 | 0.673 | 0.66±1.78 | 0.64±1.76 | 0.462 | |
| Pathological T stage | <0.001 | 0.472 | |||||
| T0 | 15 (2.2) | 54 (1.8) | 15 (2.2) | 18 (1.4) | |||
| T1 | 196 (28.5) | 690 (23.2) | 193 (28.3) | 389 (29.5) | |||
| T2 | 233 (33.9) | 864 (29.0) | 233 (34.1) | 414 (31.4) | |||
| T3 | 183 (26.6) | 1,060 (35.6) | 183 (26.8) | 369 (28.0) | |||
| T4 | 49 (7.1) | 185 (6.2) | 48 (7.0) | 74 (5.6) | |||
| Tx | 11 (1.6) | 123 (4.1) | 11 (1.6) | 53 (4.0) | |||
| Pathological N stage | 0.833 | 0.706 | |||||
| N0 | 487 (70.9) | 2,143 (72.0) | 484 (70.9) | 952 (72.3) | |||
| N1 | 121 (17.6) | 442 (14.9) | 120 (17.6) | 200 (15.2) | |||
| N2 | 55 (8.0) | 246 (8.3) | 55 (8.1) | 97 (7.4) | |||
| N3 | 1 (0.1) | 1 (<0.1) | 1 (0.1) | 0 (0.0) | |||
| Nx | 23 (3.3) | 144 (4.8) | 23 (3.4) | 68 (5.2) | |||
| Postoperative LOS (days) | 7.14±7.01 | 8.75±7.90 | <0.001 | 7.15±7.02 | 8.40±7.74 | <0.001 | |
| Readmission | 39 (5.7) | 170 (5.7) | 0.971 | 38 (5.6) | 72 (5.5) | 0.920 | |
| 30-day mortality | 22 (3.2) | 104 (3.5) | 0.816 | 22 (3.2) | 44 (3.3) | 0.887 | |
| 90-day mortality | 36 (5.2) | 211 (7.1) | 0.091 | 36 (5.3) | 87 (6.6) | 0.280 | |
| Adjuvant radiotherapy | 66 (9.6) | 373 (12.5) | 0.037 | 65 (9.5) | 150 (11.4) | 0.223 | |
| Adjuvant chemotherapy | 200 (29.1) | 908 (30.5) | 0.490 | 199 (29.1) | 353 (26.8) | 0.269 | |
Data are presented as n (%) or mean ± SD. MIS, minimally invasive surgery; PSM, propensity score matching; R0, no residual tumor; LN, lymph node; LOS, length of stay; SD, standard deviation.
PSM analysis of the MIS and open groups
After 1:2 PSM was completed, 683 MIS and 1,317 open-surgery patients were included for further analysis. The baseline was well-balanced between the two groups (Table S1). MIS was significantly associated with a greater number of dissected lymph nodes (12.18±9.74 vs. 10.61±8.55, P=0.001) and shorter postoperative LOS (7.15±7.02 vs. 8.40±7.74 days, P<0.001). The R0 rates, unplanned readmission rates, 30-day mortality, and 90-day mortality were similar between the MIS and open surgery groups (Table 1).
We also compared the perioperative results and demographic and oncological characteristics between the RATS and the VATS groups. The details of the demographic and oncological characteristics of the RATS and the VATS group before and after PSM are shown in Table S2. There was no significant difference in lymph nodes examined, R0 rates, LOS, unplanned readmission rates, 30-day mortality, or 90-day mortality between the RATS and VATS groups before and after PSM (Table 2). It should be noted that RATS was not performed as often before 2014 as it is presently.
Table 2
| Results | Unmatched cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| RATS (n=154) | VATS (n=533) | P value | RATS (n=149) | VATS (n=286) | P value | ||
| R0 | 139 (90.3) | 486 (91.2) | 0.750 | 134 (89.9) | 261 (91.3) | 0.727 | |
| LN number | 11.53±8.48 | 12.27±9.58 | 0.382 | 11.46±8.56 | 11.67±8.96 | 0.812 | |
| Positive LN number | 0.60±1.39 | 0.63±1.82 | 0.826 | 0.58±1.36 | 0.64±1.87 | 0.762 | |
| Postoperative LOS (days) | 6.70±5.43 | 7.26±7.40 | 0.383 | 6.74±5.47 | 7.63±8.36 | 0.774 | |
| Readmission | 7 (4.5) | 32 (6.0) | 0.559 | 7 (4.7) | 23 (8.0) | 0.234 | |
| 30-day mortality | 5 (3.2) | 17 (3.2) | 0.972 | 5 (3.4) | 12 (4.2) | 0.798 | |
| 90-day mortality | 7 (4.5) | 29 (5.4) | 0.838 | 7 (4.7) | 21 (7.3) | 0.410 | |
| Adjuvant radiotherapy | 9 (5.8) | 57 (10.7) | 0.087 | 9 (6.0) | 31 (10.8) | 0.117 | |
| Adjuvant chemotherapy | 46 (29.8) | 154 (28.9) | 0.841 | 46 (30.9) | 73 (25.5) | 0.258 | |
Data are presented as n (%) or mean ± SD. RATS, robot-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery; PSM, propensity score matching; R0, no residual tumor; LN, lymph node; LOS, length of stay; SD, standard deviation.
Figure 3A,3B show the overall survival curves for extended lobectomy in an unmatched and matched cohort of the open and MIS groups. The MIS group achieved slightly better survival than the open group in the unmatched cohorts (P=0.041). After PSM was conducted, the two groups had no significant difference in overall survival (Figure 3B; P=0.683). We also performed univariable and multivariable analyses and found that the surgical approach [MIS vs. open: hazard ratio (HR), 0.967; 95% confidence interval (CI): 0.832–1.123; P=0.657] was not associated with the survival of patients undergoing extended lobectomy (Table S3).

PSM analysis of the conversion to thoracotomy and open groups
Perioperative outcomes and demographic and oncological characteristics were compared between the conversion and open groups. The details of demographic and oncological characteristics of the conversion and the open group before and after PSM are shown in Table S4. The R0 rates, postoperative LOS, unplanned readmission rates, 30-day mortality, and 90-day mortality were similar between the conversion and open groups after PSM (Table 3). The conversion group had more examined lymph nodes than the open surgery group (13.91±12.36 vs. 11.34±8.98, P=0.034). The overall survival was similar between the two groups (Figure 3C).
Table 3
| Results | Unmatched cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| Conversion (n=271) | Open (n=2,976) | P value | Conversion (n=271) | Open (n=542) | P value | ||
| R0 | 242 (89.3) | 2,575 (86.5) | 0.224 | 242 (89.3) | 458 (84.5) | 0.068 | |
| LN number | 13.91±12.36 | 11.17±8.79 | <0.001 | 13.91±12.36 | 11.34±8.98 | 0.034 | |
| Positive LN number | 0.76±1.70 | 0.69±1.86 | 0.136 | 0.76±1.70 | 0.74±2.00 | 0.624 | |
| Postoperative LOS (days) | 9.49±8.00 | 8.75±7.90 | <0.001 | 9.49±8.00 | 8.73±7.88 | 0.197 | |
| Readmission | 22 (8.1) | 170 (5.7) | 0.107 | 22 (8.1) | 32 (5.9) | 0.235 | |
| 30-day mortality | 10 (3.7) | 104 (3.5) | 0.863 | 10 (3.7) | 19 (3.5) | 0.894 | |
| 90-day mortality | 27 (10.0) | 211 (7.1) | 0.088 | 27 (10.0) | 38 (7.0) | 0.143 | |
| Adjuvant radiotherapy | 24 (8.8) | 373 (12.5) | 0.081 | 24 (8.8) | 66 (12.2) | 0.192 | |
| Adjuvant chemotherapy | 85 (31.3) | 908 (30.5) | 0.783 | 85 (31.3) | 174 (32.1) | 0.873 | |
Data are presented as n (%) or mean ± SD. PSM, propensity score matching; R0, no residual tumor; LN, lymph node; LOS, length of stay; SD, standard deviation.
Comparison of the MIS and open groups in the community/comprehensive hospital setting
The details of demographic and oncological characteristics of the community cohort before and after PSM are shown in Table S5. The MIS group had a shorter postoperative LOS than the open group in the matched cohort in the community and comprehensive community hospitals (6.89±5.85 vs. 8.43±6.39 days, P<0.001). The R0 rates were higher in the MIS group after PSM (90.2% vs. 85.5%, P=0.047). The unplanned readmission rate within 30 days of discharge, number of lymph nodes examined, number of positive lymph nodes, 30-day mortality, and 90-day mortality were similar between the MIS and open surgery groups in both the unmatched and matched cohorts (Table 4). There was no significant difference in overall survival between the two groups after PSM (Figure 3D).
Table 4
| Results | Unmatched cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| MIS (n=305) | Open (n=1,683) | P value | MIS (n=305) | Open (n=605) | P value | ||
| R0 | 275 (90.2) | 1,450 (86.2) | 0.066 | 275 (90.2) | 517 (85.5) | 0.047 | |
| LN number | 10.15±8.47 | 9.26±7.45 | 0.288 | 10.15±8.47 | 9.10±7.39 | 0.257 | |
| Positive LN number | 0.59±1.70 | 0.62±1.61 | 0.836 | 0.59±1.70 | 0.70±1.64 | 0.337 | |
| Postoperative LOS (days) | 6.89±5.85 | 8.84±7.37 | <0.001 | 6.89±5.85 | 8.43±6.39 | <0.001 | |
| Readmission | 19 (6.2) | 90 (5.3) | 0.497 | 19 (6.2) | 24 (4.0) | 0.138 | |
| 30-day mortality | 16 (5.2) | 68 (4.0) | 0.352 | 16 (5.2) | 22 (3.6) | 0.292 | |
| 90-day mortality | 21 (6.9) | 136 (8.1) | 0.564 | 21 (6.9) | 37 (6.1) | 0.668 | |
| Adjuvant radiotherapy | 33 (10.8) | 216 (12.8) | 0.349 | 33 (10.8) | 76 (12.6) | 0.517 | |
| Adjuvant chemotherapy | 86 (28.2) | 530 (31.5) | 0.282 | 86 (28.2) | 190 (31.4) | 0.359 | |
Data are presented as n (%) or mean ± SD. PSM, propensity score matching; MIS, minimally invasive surgery; R0, no residual tumor; LN, lymph node; LOS, length of stay; SD, standard deviation.
Discussion
This is one of the first PSM studies to report outcomes between open and MIS extended lobectomy based on a national database in the United States. In a cohort of over 6,000 patients who underwent extended lobectomy, a minimally invasive approach was used in roughly 11.2% of these cases. The PSM analysis showed no difference in R0 resection rates, readmission rates, 30-day mortality, and 90-day mortality. Patients who underwent a MIS approach had more lymph nodes harvested and a decreased overall postoperative LOS. Similar results were observed in the separate PSM analysis of patients undergoing MIS extended lobectomy for NSCLC in the community/comprehensive community cancer program setting.
An initial criticism of MIS approaches for any cancer operation is the potential to compromise the overall oncologic results. Our study showed that the MIS approach to extended lobectomies did not compromise oncological outcomes in terms of R0 resection rates. Some studies have reported that MIS could provide similar or even better lymph node evaluation than the open approach in simple lobectomy (20-22). In our study, there was no difference in the number of positive lymph nodes between groups, and the total number of lymph nodes were examined. Additionally, our study showed no difference in overall survival after MIS or open extended lobectomies. These results show that adopting MIS approaches to an oncologic extended lobectomy can produce at least equivalent oncologic outcomes to the standard open thoracotomy approach. Furthermore, the nearly identical results in the matched community hospital cohort suggest that the MIS approach to extended lobectomy can be adopted in many hospital settings and should be open to more than academic centers.
According to the literature, the conversion rates of MIS extended lobectomies are significantly higher than those of standard MIS lobectomy. Yang et al. reported a 17.5% rate of conversion to open surgery in a VATS cohort and 10.3% in a robotic lobectomy cohort in the same NCDB (4). Our study’s conversion rate was 28.3% for patients undergoing MIS extended lobectomies. Although the NCDB does not readily provide the cause of these conversions, this may be due to the learning curve. Many studies on standard VATS and robotic lobectomies have reported a continual decrease in conversion rates with experience (23,24).
Similarly, with the improvement of experience and technique, we observed a marked reduction in conversion rates and increased use of MIS for extended lobectomy in this study. However, other factors may ultimately affect conversion rates, such as disease location, lymph node calcification, pleural adhesions, and surgeon experience (25). Additional studies will be necessary to identify this elevated conversion rate associated with MIS-extended lobectomies.
Preoperative imaging is often inaccurate in determining advanced T stage. Extended lobectomy for advanced T stage tumor remains an essential option if a complete resection can be accomplished (26-28). Although the conversion rate was high in extended lobectomy, the number of T3 and T4 cases increased yearly in the MIS group indicating that the surgeons were more likely to attempt the MIS approach for more challenging cases as experience was gained. Moreover, the perioperative results were also generally improved year on year. Our study found that the number of lymph nodes examined increased, and the postoperative LOS, readmission rates, 30-day mortality, and 90-day mortality decreased from 2010 to 2014. These promising results support the MIS approach as feasible in extended lobectomy and worth attempting.
Consistent with our results, previous studies have demonstrated that postoperative morbidity and mortality rates are similar between conversion cases and thoracotomy (29,30). The R0 rates, postoperative LOS, unplanned readmission rates, 30-day mortality, and 90-day mortality were similar between the conversion and open groups. The overall survival was also similar between the two groups, indicating that MIS is safe and that the conversion to thoracotomy during the operation does not compromise treatment outcome.
Limitations
Due to the nature and design of this study, some limitations should be noted. First, due to the retrospective study design, confounding and selection biases might have been introduced. Moreover, there was quite a large number of T1 or T2 stage patients who required extended lobectomy. The exact reasons for this are still being determined, and the details could not be obtained. The centrally located small tumors may still require bronchial sleeve resection. Second, because the analysis was performed on a nationally collected database, there needs to be more granularity in the data recorded on both the patient and institutional levels. Similarly, many specific and important intraoperative and postoperative complications regarding the surgery, therefore, could not be analyzed. For example, whether MIS requires more operative time than the open operation remains to be seen. Third, the analysis of relapse-free survival could not be assessed, as these data are not recorded in the NCDB. Nonetheless, this study represents one of the first reports on minimally invasive approaches to extended lobectomy using a national US database.
Conclusions
Minimally invasive approaches to extended lobectomy are challenging and only used in a small minority of patients. However, minimally invasive extended lobectomy is a safe and feasible option for NSCLC. As conversion does not compromise the outcomes and as the perioperative results appeared to improve year on year, MIS can at least be attempted first in certain patients by experienced surgical teams. Perioperative management for open conversion is essential if extended lobectomy is anticipated preoperatively. MIS extended lobectomy can achieve similar oncologic results and is associated with similar or even better perioperative outcomes if not converted to open surgery. However, prospective studies are needed to confirm this conclusion.
Acknowledgments
We want to thank Yuan Liu from the Statistics Center of Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, for helping with the statistical analysis of the data in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-37/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-37/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-37/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459-544. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Treasure T, et al. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ 2014;349:g5575. [Crossref] [PubMed]
- Yang CF, Sun Z, Speicher PJ, et al. Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann Thorac Surg 2016;101:1037-42. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Lim E, Batchelor TJP, Dunning J, et al. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid 2022;1:EVIDoa2100016.
- Reardon ES, Schrump DS. Extended resections of non-small cell lung cancers invading the aorta, pulmonary artery, left atrium, or esophagus: can they be justified? Thorac Surg Clin 2014;24:457-64. [Crossref] [PubMed]
- Loi M, Mazzella A, Desideri I, et al. Chest wall resection and reconstruction for lung cancer: surgical techniques and example of integrated multimodality approach. J Thorac Dis 2020;12:22-30. [Crossref] [PubMed]
- Lanuti M. Surgical Management of Lung Cancer Involving the Chest Wall. Thorac Surg Clin 2017;27:195-9. [Crossref] [PubMed]
- Caso R, Watson TJ, Khaitan PG, et al. Outcomes of minimally invasive sleeve resection. J Thorac Dis 2018;10:6653-9. [Crossref] [PubMed]
- Dal Agnol G, Oliveira R, Ugalde PA. Video-assisted thoracoscopic surgery lobectomy with chest wall resection. J Thorac Dis 2018;10:S2656-63. [Crossref] [PubMed]
- Pan X, Gu C, Wang R, et al. Initial Experience of Robotic Sleeve Resection for Lung Cancer Patients. Ann Thorac Surg 2016;102:1892-7. [Crossref] [PubMed]
- Liang Z, Chen J, He Z, et al. Video-assisted thoracoscopic pneumonectomy: the anterior approach. J Thorac Dis 2013;5:855-61. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Hennon MW, Dexter EU, Huang M, et al. Does Thoracoscopic Surgery Decrease the Morbidity of Combined Lung and Chest Wall Resection? Ann Thorac Surg 2015;99:1929-34; discussion 1934-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [Crossref] [PubMed]
- Towe CW, Servais EL, Grau-Sepulveda M, et al. Impact of Chest Wall Resection on Mortality After Lung Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2022;114:2023-31. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Crossref] [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 960-1. [Crossref] [PubMed]
- Fabbri G, Femia F, Lampridis S, et al. Long-Term Oncologic Outcomes in Robot-Assisted and Video-Assisted Lobectomies for Non-Small Cell Lung Cancer. J Clin Med 2023;12:6609. [Crossref] [PubMed]
- Adams RD, Bolton WD, Stephenson JE, et al. Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann Thorac Surg 2014;97:1893-8; discussion 1899-900. [Crossref] [PubMed]
- Puri V, Patel A, Majumder K, et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015;149:55-61, 62.e1.
- Tong C, Li T, Huang C, et al. Risk Factors and Impact of Conversion to Thoracotomy From 20,565 Cases of Thoracoscopic Lung Surgery. Ann Thorac Surg 2020;109:1522-9. [Crossref] [PubMed]
- DiPerna CA, Wood DE. Surgical management of T3 and T4 lung cancer. Clin Cancer Res 2005;11:5038s-44s. [Crossref] [PubMed]
- Yamanashi K, Menju T, Hamaji M, et al. Prognostic factors related to postoperative survival in the newly classified clinical T4 lung cancer. Eur J Cardiothorac Surg 2020;57:754-61. [Crossref] [PubMed]
- Li Q, Zhang P, Wang Y, et al. T4 extension alone is more predictive of better survival than a tumour size >7 cm for resected T4N0-1M0 non-small-cell lung cancer†. Eur J Cardiothorac Surg 2019;55:682-90. [Crossref] [PubMed]
- Fourdrain A, De Dominicis F, Iquille J, et al. Intraoperative conversion during video-assisted thoracoscopy does not constitute a treatment failure†. Eur J Cardiothorac Surg 2019;55:660-5. [Crossref] [PubMed]
- Bongiolatti S, Gonfiotti A, Viggiano D, et al. Risk factors and impact of conversion from VATS to open lobectomy: analysis from a national database. Surg Endosc 2019;33:3953-62. [Crossref] [PubMed]


