
Novel immunotherapy combinations in neoadjuvant non-small cell lung cancer (NSCLC): a better chance at cure?
In the past decade, immunotherapy has emerged as a new pillar of cancer therapy, complementing surgery, radiation, chemotherapy and targeted therapy. Broadly, immunotherapy refers to any therapeutic agent that creates or modifies host immune responses (1). It most frequently involves the use of targeted antibodies or small molecules that act on immune regulatory pathways or cytokine signaling but may also involve the adoptive transfer of isolated and/or genetically modified tumor-targeting immune cells.
The most drugged immunotherapy pathway is the programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) axis. PD-L1 is a cell surface protein primarily expressed by tumor and stromal cells on many cancer types. It interacts with PD-1, which is expressed by immune cells. In healthy hosts, PD-1/PD-L1 interactions dampen T cell responses. Thus, blockade of this pathway can ‘unleash’ T cells to attack tumor and stromal cells (2,3). There are now at least six FDA-approved antibodies that act on this receptor-ligand pair, including durvalumab which is a human monoclonal antibody that is specific for PD-L1 and blocks its interaction with PD-1 (4).
The treatment of advanced non-small cell lung cancer (NSCLC) has been revolutionized by immunotherapies targeting the PD-1/PD-L1 axis, which enable a significant subset of metastatic patients to achieve long-term survival (5). Due to the effectiveness of immunotherapy in advanced-stage disease, there has been recent interest in testing whether immunotherapy can improve outcomes in early-stage resectable NSCLC. The positive results from the IMpower010 and KEYNOTE-091 studies led to FDA approvals for atezolizumab and pembrolizumab, respectively, in the adjuvant setting (6,7). Encouraging earlier phase data led to the phase III CheckMate 816 clinical trial, in which patients with stage IB–IIIA disease were randomized to three cycles of nivolumab and chemotherapy versus chemotherapy alone prior to surgical resection (8,9). Event-free survival (EFS) and major pathologic response (MPR) rates were significantly higher in patients receiving neoadjuvant nivolumab, leading to the FDA approval of nivolumab plus platinum chemotherapy in March 2022 (10). Subsequently, the perioperative trials KEYNOTE-671 and AEGEAN demonstrated positive results of incorporating checkpoint inhibitors plus chemotherapy in the neoadjuvant setting, followed by an additional 1 year of adjuvant checkpoint inhibitor therapy (11,12). On October 16, 2023, the FDA approved pembrolizumab with platinum chemotherapy as neoadjuvant treatment and with continuation of pembrolizumab as post-surgical adjuvant treatment.
There is now considerable interest in identifying new immune checkpoints and formulating more effective combination immunotherapies to build upon these recent successes. Novel drugs targeting the immune targets CD73, NKG2A, and STAT3 are being explored to augment immune responses. CD73 is a cell surface molecule that is expressed by regulatory T and intratumoral natural killer (NK) cells, promotes their production of immunosuppressive adenosine, and whose blockade impedes tumor growth in pre-clinical models (13,14). Oleclumab is a CD73-specific monoclonal antibody under clinical development. NKG2A is an inhibitory immune receptor expressed by both T and NK cells and can be targeted with monalizumab, a humanized anti-NKG2A antibody (15). Recent research indicates blockade of this receptor improves antitumor response in mouse models (16). The JAK-STAT signaling pathway is well studied in the context of immune cell cytokine signaling, but STAT3 signaling has also been implicated in lung cancer tumorigenesis due to its engagement downstream of epidermal growth factor receptor (EGFR) signaling and IL-6 production (17). Danvatirsen is an antisense oligonucleotide targeting STAT3 that has recently been shown to have activity against lymphoma and lung cancer (18).
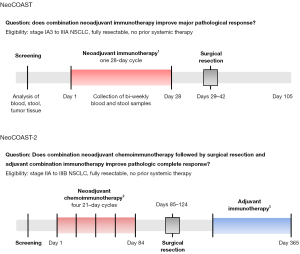
The NeoCOAST study was a phase II study that asked whether combining durvalumab with either CD73, NKG2A, and STAT3 targeted therapies could improve outcomes in the neoadjuvant setting (19). Eighty-four individuals with untreated, resectable stage IA3 to IIIA NSCLC from seventeen centers across North America and Europe were randomized to receive a single 28-day cycle of durvalumab monotherapy versus a combination of durvalumab with either oleclumab, monalizumab, or danvatirsen (Figure 1). Individuals were then taken to surgery for resection within two weeks of completing neoadjuvant therapy, and blood and stool samples were collected and analyzed over time. As a surrogate for outcome, the trial monitored for MPR at the time of resection, which has been proposed to serve as a correlate for survival in the neoadjuvant setting (20).
With the limitation of the small sample size of this study, it is notable that pathological assessment of resection specimens demonstrated that combination immunotherapy with durvalumab and either oleclumab, monalizumab, or danvatirsen improved the rates of MPR as compared to durvalumab alone. Specifically, the rates of MPR were 11.1% (3/27 patients) in the durvalumab monotherapy arm, 19.0% (4/21 patients) in the durvalumab + oleclumab arm, 30.0% (6/20 patients) in the durvalumab + monalizumab arm, and 31.3% (5/16 patients) in the durvalumab + danvatirsen arm.
There were slightly higher rates of adverse events in the combination groups. Treatment-emergent adverse events (TRAEs) occurred in 9 (34.6%), 12 (57.1%), 10 (50.0%), and 7 (43.8%) patients in the durvalumab monotherapy, durvalumab + oleclumab, durvalumab + monalizumab, and durvalumab + danvatirsen arms, respectively. Serious TRAEs only occurred in 3 patients—1 in the durvalumab monotherapy arm (immune-mediated arthritis), 1 in the durvalumab + oleclumab arm (diabetic ketoacidosis) and 1 in the durvalumab + danvatirsen arm (procedural hemorrhage). Any claims regarding safety are limited by the short duration of neoadjuvant treatment (28 days), small group sizes (~20 patients), and short interval follow-up of 105 days. Notably, the COAST trial evaluated oleclumab and monalizumab as consolidation therapy after concurrent chemoradiation for patients with unresectable stage III NSCLC and also did not reveal major safety concerns regarding these agents (21). Nonetheless, additional patients and longer-term follow-up are needed to robustly assess the safety profiles of oleclumab, monalizumab, and danvatirsen in combination with durvalumab (22).
A major strength of the study was the collection of blood, stool, and tissue specimens across time that enabled detailed correlative analyses. Through this work, the authors found that combination immunotherapy with durvalumab and either oleclumab or monalizumab increased the frequency and effector phenotypes of NK and CD8+ T cells within resected tumors compared with pre-treatment samples. Further superficial transcriptomic analyses suggested that the cellular infiltrates may be more activated with cytotoxic capabilities. Microbiome analyses were also performed but were limited by the small sample sizes. Nonetheless, the breadth of these correlative analyses provides a framework by which future early-stage clinical trials can address important biological questions in the field.
A major unresolved question in the field is whether pre-treatment PD-L1 expression or other biomarkers can predict which patients will benefit from neoadjuvant and/or adjuvant immunotherapy. In the KEYNOTE-671 and CheckMate 816 trials, patients with high PD-L1 (≥50%) benefited most from neoadjuvant immunotherapy, though patients with lower levels of PD-L1 expression also variably benefited (10,11). In the NeoCOAST trial, MPRs were more frequent in patients with PD-L1 ≥1% expression versus <1% expression in both the durvalumab + oleclumab and durvalumab + monalizumab arms, but interestingly not in the durvalumab monotherapy or durvalumab + danvatirsen arms. We suspect this is due to technical limitations and small sample sizes because none of the patients who achieved a MPR in these latter arms were evaluable for baseline PD-L1. Thus, future studies are needed to better define which patients may benefit from neoadjuvant immunotherapies.
Another key remaining question from many of the perioperative immunotherapy studies in NSCLC is whether there is an overall benefit, or if certain patients may benefit, from continuing immunotherapy as adjuvant treatment after neoadjuvant therapy and surgical resection. For instance, patients who achieve a complete pathologic response after neoadjuvant treatment may not need additional adjuvant therapy, particularly those who do not have evidence of circulating tumor DNA after surgery. The optimal number of cycles that should be administered to patients both neoadjuvantly and adjuvantly is also unknown. The neoSCORE trial was a small, randomized phase II study that compared two versus three cycles of neoadjuvant immunotherapy in patients with resectable NSCLC (23). While there were more pathologic responses amongst patients who received three cycles of neoadjuvant immunotherapy, the difference did not meet statistical significance. Interestingly, the NeoCOAST trial administered only one cycle of neoadjuvant treatment and achieved MPR rates that are comparable to MPR rates noted in CheckMate 816, KEYNOTE-671, and AEGEAN studies in which 3–4 cycles of neoadjuvant treatment were administered (10-12). Additional carefully designed trials are needed to answer these open questions.
A new phase II study, termed NeoCOAST-2 (NCT05061550) is evaluating novel combination immunotherapies in conjunction with chemotherapy administered both neoadjuvantly and adjuvantly (Figure 1) (24). The study’s endpoints are safety, feasibility, and pathologic complete response. As new combination immunotherapies enter trials for NSCLC, we must continually monitor for added toxicities that can result from multiple overlapping immunotherapeutic agents or excessive cycles. Moving forward, it will be important to try to individualize treatment decisions for patients based on response to neoadjuvant treatment, with a goal to avoid overtreating patients who are likely cured after neoadjuvant treatment and surgical resection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-735/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-735/coif). A.I.S. is an inventor of patents related to cancer immunotherapy, some of which have been licensed to Lyell Immunopharma. M.D. has participated in advisory boards for Advarra, Astra Zeneca, Bristol Myer Squibb, Catalyst Pharmaceuticals, Gilead, Guardant, Janssen, Novocure, Regeneron, Genzyme and Sanofi; has provided consulting services for Eurofins, Abbvie, and Janssen; has received institutional grant funding from Merck, Genentech, CellSight, Novartis, Varian, and Verily; is the President of the Association of Northern California Oncologists; and has received travel funds and honoraria for speaking at various meetings related to cancer immunotherapy (Plexus, IDEO, Springer, Medical Educator Consortium, Dedham Group, DAVA Oncology, MJH Healthcare Holdings, ANCO, Aptitude Health, Med Learning Group, Curio, and Triptych Health). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-9. [Crossref] [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [Crossref] [PubMed]
- Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 2020;10:727-42.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Garassino MC, Gadgeel S, Speranza G, et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol 2023;41:1992-8. [Crossref] [PubMed]
- O'Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022;23:1274-86. [Crossref] [PubMed]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. Erratum in: N Engl J Med 2018;379:2185. [Crossref] [PubMed]
- Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:491-503. [Crossref] [PubMed]
- Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:1672-84. [Crossref] [PubMed]
- Chen S, Wainwright DA, Wu JD, et al. CD73: an emerging checkpoint for cancer immunotherapy. Immunotherapy 2019;11:983-97. [Crossref] [PubMed]
- Neo SY, Yang Y, Record J, et al. CD73 immune checkpoint defines regulatory NK cells within the tumor microenvironment. J Clin Invest 2020;130:1185-98. [Crossref] [PubMed]
- van Hall T, André P, Horowitz A, et al. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J Immunother Cancer 2019;7:263. [Crossref] [PubMed]
- André P, Denis C, Soulas C, et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018;175:1731-1743.e13. [Crossref] [PubMed]
- Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 2007;117:3846-56. [Crossref] [PubMed]
- Hong D, Kurzrock R, Kim Y, et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med 2015;7:314ra185. [Crossref] [PubMed]
- Cascone T, Kar G, Spicer JD, et al. Neoadjuvant Durvalumab Alone or Combined with Novel Immuno-Oncology Agents in Resectable Lung Cancer: The Phase II NeoCOAST Platform Trial. Cancer Discov 2023;13:2394-411. [Crossref] [PubMed]
- Waser NA, Adam A, Schweikert B, et al. Pathologic response as early endpoint for survival following neoadjuvant therapy (NEO-AT) in resectable non-small cell lung cancer (rNSCLC): Systematic literature review and meta-analysis. Ann Oncol 2020;31:S806.
- Herbst RS, Majem M, Barlesi F, et al. COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:3383-93.
- Nishina T, Fujita T, Yoshizuka N, et al. Safety, tolerability, pharmacokinetics and preliminary antitumour activity of an antisense oligonucleotide targeting STAT3 (danvatirsen) as monotherapy and in combination with durvalumab in Japanese patients with advanced solid malignancies: a phase 1 study. BMJ Open 2022;12:e055718. [Crossref] [PubMed]
- Shao M, Yao J, Wang Y, et al. Two vs three cycles of neoadjuvant sintilimab plus chemotherapy for resectable non-small-cell lung cancer: neoSCORE trial. Signal Transduct Target Ther 2023;8:146. [Crossref] [PubMed]
- Guisier F, Bennouna J, Spira AI, et al. NeoCOAST-2: A phase 2 study of neoadjuvant durvalumab plus novel immunotherapies (IO) and chemotherapy (CT) or MEDI5752 (volrustomig) plus CT, followed by surgery and adjuvant durvalumab plus novel IO or volrustomig alone in patients with resectable non-small-cell lung cancer (NSCLC). J Clin Oncol 2023;41:TPS860.


