Robot-assisted minimally invasive bronchial resection with primary anastomosis for schwannoma arising from left main bronchus: a case report
Highlight box
Key findings
• The first sleeve resection for bronchial schwannoma using Da Vinci surgical robotic system.
What is known and what is new?
• Tracheobronchial schwannomas are extremely rare. The main treatment option is surgery. Endoscopic intervention can also be selected.
• Surgical resection is the first choice for tracheobronchial schwannoma, which may offer a better prognosis than endoscopic intervention.
• The application of Da Vinci surgical robotic system benefited the process of the bronchial resection and primary anastomosis of this surgery.
What is the implication, and what should change now?
• There should be more case reports and clinical trials to reveal clinical details of tracheobronchial schwannoma in the future, helping with the guidelines production to achieve more systematic diagnosis and treatment.
Introduction
Schwannomas are among the most common benign encapsulated nerve sheath tumors, arising from Schwann cells (1), while tracheobronchial schwannomas are sporadic and account for lower than 0.2% in all pulmonary tumors (2). In large part because of the rarity and insufficient reported clinical details, tracheobronchial tumors are hard to diagnose. The delay in diagnosis of these tumors can range from months to years. Complete resection of the tumor combined with reconstruction of the trachea or bronchus is the main treatment for tracheobronchial schwannoma, preventing the recurrence (2,3).
An increasing number of thoracic surgery cases were performed on the robotic platform in recent years. This new technique offers precise and flexible manipulation of multiple instruments, a three-dimensional visual field, and seated ergonomics (4), improving the surgeons’ overall control of the operations to complete high technique required surgical procedures.
In this report, we described a left main bronchial schwannoma case in an adult female, which was treated by robot-assisted minimally invasive bronchial resection and primary anastomosis. All procedures were performed using the Da Vinci Si robotic surgical system. We present this case in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-819/rc).
Case presentation
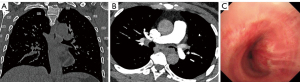
A 48-year-old female felt shortness of breath for more than 1 year. There was no relevant medical, family, or psycho-social history for the patient. She denied all the relevant past interventions. Chest computed tomography (CT) and bronchoscopy examination revealed a new growth of sessile nodule in the left main bronchus, which was 1.5 cm in longest diameter (Figure 1). The distance from the nodule to the tracheal carina and the left secondary carina were about 2.0 cm each. The other bronchi were all unobstructed. It was considered a neurogenic tumor, schwannoma, by transbronchial biopsy, using single-use pulmonary biopsy forceps.
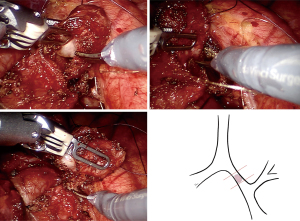
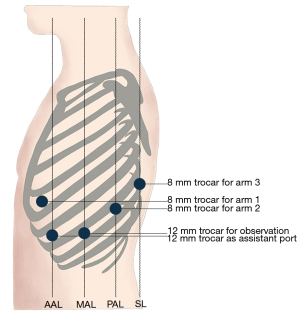
The shape and size of the nodule led to high risk of hemorrhage and bronchial perforation brought by endoscopic intervention. The patient’s physical condition could tolerate the surgery under general anesthesia. Given these considerations, we believed in the necessity and feasibility of surgery. After adequate communication with the patient, we performed robot-assisted minimally invasive bronchial resection with primary anastomosis of the left main bronchus for her (Video 1). Equipment and personnel were positioned in a manner similar to that of other robot-assisted thoracic surgery (RATS) procedures. The patient was placed in the right-lateral decubitus position with single-lung ventilation. Five trocars were placed. Three 8.0 mm trocars were placed in the fifth intercostal space between the anterior axillary line and midclavicular line for arm one, eighth intercostal space in the posterior axillary line for arm two, eighth intercostal space in the scapular line for arm three. One 12.0 mm trocar for observation was placed in the eighth intercostal space in the midaxillary line. One 12.0 mm trocar as assistant port was placed in the seventh intercostal space in the anterior axillary line. Our trocar placement (Figure 2) allows flexible movement of robot arms and reduces damage to the intercostal nerve of patients.
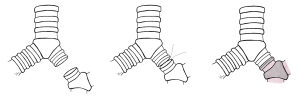
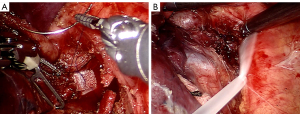
The tumor was completely removed by sleeve bronchial resection (Figure 3) followed by end-to-end anastomosis with running suture, using 3/0 V-Loc suture (Figure 4). The anastomoses were reinforced by a pericardium patch and reserved subcarinal tissue (Figure 5). Fiberoptic bronchoscopy check was performed intra-operatively and a day after the operation, which proofed the well anastomosis, and confirmed no active bleeding.
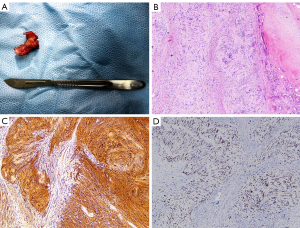
The resected tumor measured 1.5 cm × 1.5 cm × 1.0 cm (Figure 6A). Histologically, the tumor was well-circumscribed, but without fibrous capsule. It contained areas composed of fascicles of Schwann cells that have a spindle cell morphology (Antoni A pattern) and areas with more loosely textured and microcystic areas (Antoni B pattern). The Schwann cells presented with faintly eosinophilic cytoplasm, ovoid or spindle nuclei, and no sign of mitotic figures (Figure 6B). The immunohistochemistry and special staining showed positive staining for S-100 (Figure 6C) and SOX-10 (Figure 6D). Negative staining for CD117, DOG-1, CD34, smooth muscle actin (SMA), desmin, AE1/AE3, STAT-6, and p16. The protein expression of H3K27Me3 was detected. There was a 2% positive staining for MIB-1 (Ki67). These results came to a definitive diagnosis of schwannoma. Surgical margins were reported clear on final histopathology.
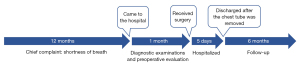
The patient tolerated the operation without any complications. She was discharged at 5 days postoperatively, after removing the chest tube. At present, 6 months after the intervention, the patient continues to be asymptomatic, with a normal functional status, and without any sign of local recurrence. Figure 7 gives the specific dates and times of important components of the case.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript, the accompanying image, and the video. A copy of the written consent is available for review by the editorial office of this journal.
Patient perspective
Over the past year, I often felt shortness of breath, especially in the morning. This symptom affected my daily life a lot, so I went to the hospital. I was finally referred to the Department of Thoracic Surgery for further diagnosis and treatment. I received a chest CT examination first, and new growth of nodule was found in my left main bronchus. Then the bronchoscopy examination revealed that the nodule was a sessile tumor, which was considered a schwannoma, by transbronchial biopsy.
From the diagnosis, everything went very quickly. My doctors told me that the tumor was difficult to be removed completely by endoscopic intervention without risk of hemorrhage and bronchial perforation because of its size and shape, so the surgery was necessary. This surgery was a high technique required one, and the Da Vinci Si robotic surgical system could help the surgeons to complete it with ease. I went through comprehensive preoperative evaluation, making sure that I could tolerate the surgery under general anesthesia. Then the surgery took place. Thanks to the successful operation, I recovered quickly without severe pain. The chest tube was removed 5 days after the surgery, then I was discharged.
It was 6 months after the surgery, when my doctors contacted me for written consent. The result of the chest CT examination found no sign of local recurrence. I am now living a normal life. I am very satisfied for the accurate and timely diagnosis, the comprehensive preoperative evaluation, and the appropriate and skilled surgical techniques to end a year of breath shortness.
Discussion
Tracheobronchial tumors are extremely infrequent, accounting for about 0.4% of all tumors (5). The benign tracheobronchial tumors only account for 0.5% of tracheobronchial tumors (6). Tracheobronchial schwannoma is even uncommon. Its share of benign tracheobronchial tumors is only 2.2% (6).
The first reported case of tracheobronchial schwannoma was in 1951 with recurrent pneumonia as chief complaint. The left-main-bronchus-located tumor was established by multi-stage tracheoscopic resection (7). Following reports revealed that tracheobronchial schwannomas can be located in any area of the airway without characteristic symptoms, or even asymptomatically (3), resulting in delayed diagnosis (5). Consequently, it is important to conduct further evaluation with chest CT and bronchoscopy examination, if a tracheobronchial tumor is suspected (8). The positron emission tomography (PET)-CT is an optional choice to rule out malignant lesions, when necessary (8). In this case, the patient received a chest CT and bronchoscopy examination. The malignant lesions were excluded by transbronchial biopsy, therefore PET-CT was not adopted.
Definite diagnosis can only be made by pathological study and immunohistochemistry staining. Schwannomas are well-circumscribed encapsulated nerve sheath tumors, arising from Schwann cells (1). It features positive staining for S-100 (1). As described, SOX-10 is superior to S-100 in the diagnosis of schwannoma (9,10). The Ki-67, a tumor cell proliferation marker, has been reported in determining malignant potential for peripheral nerve sheath tumors (11), which often exhibits under 5% nuclear staining for benign tumors (12). Positive staining for S-100 and SOX-10, 2% positive staining for Ki-67, helped with the definite diagnosis.
As an extremely uncommon tumor, tracheobronchial schwannoma lacks guidelines for diagnosis and treatment. The main treatment option is surgery. Endoscopic intervention can also be selected. With a low malignant potential, the goal of the treatment is complete resection of the tumor to relieve obstructive symptoms. To summarize the treatment options for tracheobronchial schwannoma, we searched the cases with the combination of terms via PubMed, and identified 56 cases in the full-text available reports written in English (Table 1). Among those surgery-applied cases, 13 trachea-located schwannomas were treated with tracheotomy (14,18,21-23,36-38,43,47,53,54,59), 14 bronchus-located schwannomas received surgeries including pneumonectomy, lobectomy, segmentectomy, or bronchial resection (3,16,19,24-27,35,39,40,42,45,46,52). Others received endoscopic interventions as primary treatments or follow-up (7,13,15,17,20,28-34,41,44,48-51,55-58). Notably, recurrence was observed in six endoscopic intervention cases, but not reported in the surgery group. The difference in mortality between the two treatments was not found. In addition, the shape of the tumor might be an essential factor for the choice of the treatment, for the sessile schwannoma might increase the risk of endoscopic intervention-related complications. Thus, we believe surgical resection is the first choice for tracheobronchial schwannoma, which offers a favorable prognosis. The surgical procedure depends on the size and location of the tumor (5). In this case, the performed surgery ensured complete resection of the tumor, and the two ends of the bronchus provided enough length for reconstruction. Moreover, patients’ physical status must be assessed carefully for the tolerance of surgery. The risk of all kinds of surgical-related complications should also be considered.
Table 1
| Citations | Gender | Age (years) | Tumor location (originated) | Number | Max-D of tumor (mm) | Shape | Primary treatment | Surgical approach | Follow-up | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| Straus GD, 1951 (7) | M | 28 | Left main bronchus | Single | 12 | – | Endoscopic intervention | – | 6 weeks | N |
| Feldhaus RJ, 1989 (13) | M | 74 | Right main bronchus | Single | 20 | – | Endoscopic intervention | – | 1 year | N |
| Stack PS, 1990 (14) | M | 35 | Trachea | Single | 30 | – | Tracheotomy | Cervical incision | 3 weeks | N |
| Rusch VW, 1994 (15) | M | 45 | Trachea | Single | 20 | Pedunculated | Endoscopic intervention | – | 5.5 years | N |
| Nesbitt JC, 1996 (16) | F | 64 | Right main bronchus | Single | 30 | – | Pneumonectomy | – | 3 years | N |
| Weiner DJ, 1998 (17) | M | 16 | Trachea | Single | 20 | – | Endoscopic intervention | – | – | N |
| Dorfman J, 2000 (18) | M | 33 | Trachea | Single | 22 | Pedunculated | Tracheotomy | Cervical incision | 3 months | N |
| Chen SR, 2003 (19) | M | 18 | Right lower lobe bronchus | Single | 15 | Sessile | Lobectomy | – | 2 months | N |
| Kasahara K, 2003 (20) | M | 76 | Ridge of right middle and lower bronchus | Single | 2–3 | – | Endoscopic intervention | – | 1 year | N |
| M | 86 | Left lingular lobe bronchus | Single | 2–3 | – | Endoscopic intervention | – | Few weeks | N | |
| Nio M, 2005 (21) | F | 9 | Trachea | Single | 15 | Pedunculated | Tracheotomy | – | 4 months | N |
| Righini CA, 2005 (22) | F | 51 | Trachea | Single | 15 | – | Tracheotomy | Cervical incision | 3 years | N |
| Dincer SI, 2006 (23) | M | 49 | Trachea | Single | 17.4 | – | Tracheotomy | Thoracotomy | 7 months | N |
| Shigematsu H, 2007 (24) | M | 41 | Left lingular lobe bronchus | Single | – | – | Segmentectomy | – | – | N |
| Nakamura R, 2009 (25) | F | 48 | Left main bronchus | Single | 37 | Sessile | Bronchial resection | Thoracotomy | 3 years | N |
| Stouffer CW, 2010 (26) | F | 18 | Left upper lobe bronchus | Single | – | – | Lobectomy | Thoracotomy | 6 months | N |
| Tansel T, 2010 (27) | F | 8 | Left main bronchus | Single | 15 | – | Pneumonectomy | Thoracotomy | 4 years | N |
| Dumoulin E, 2012 (28) | M | 64 | Carina right middle lobe bronchus right lower lobe bronchus | Multiple | – | – | Endoscopic intervention | – | 3 months | N |
| Lee BR, 2012 (29) | F | 44 | Left main bronchus | Single | 19 | – | Endoscopic intervention | – | 4 months | N |
| Melendez J, 2012 (30) | M | 63 | Trachea | Single | – | Sessile | Endoscopic intervention | – | 6 months | N |
| Thomas R, 2012 (31) | F | 37 | Trachea | Single | – | Pedunculated | Endoscopic intervention | – | – | Y |
| Dalar L, 2014 (32) | M | 42 | Trachea | Single | – | – | Endoscopic intervention | – | – | N |
| Kushima H, 2014 (33) | F | 71 | Left lower lobe bronchus | Single | – | Sessile | Follow-up | – | 10 years | N |
| Isaac BT, 2015 (34) | M | 24 | Trachea | Single | – | – | Endoscopic intervention | – | 6 months | N |
| Oliveira RC, 2016 (35) | F | 66 | Left upper lobe bronchus | Single | 37.5 | – | Lobectomy | – | 3 weeks | N |
| Hamouri S, 2017 (36) | M | 60 | Trachea | Single | <20 | Sessile | Tracheotomy | Cervical incision | 1 year | N |
| Han DP, 2017 (37) | F | 45 | Trachea | Single | 15 | Sessile | Tracheotomy | Cervical incision | – | N |
| Ally M, 2018 (38) | M | 54 | Trachea | Single | – | – | Tracheotomy | Cervical incision | 1 year | N |
| Komatsu M, 2018 (39) | F | 58 | Left main bronchus | Single | 12 | – | Surgery | VATS | – | N |
| Liao H, 2019 (40) | F | 42 | Left lower lobe bronchus | Single | 43 | – | Lobectomy | – | 1 year | N |
| Chen H, 2019 (41) | F | 23 | Trachea | Single | 16 | Sessile | Endoscopic intervention | – | 6 months | Y |
| Ishibashi H, 2019 (42) | M | 64 | Right lower lobe bronchus | Single | 20 | – | Lobectomy | Thoracotomy | 7 years | N |
| Chávez-Fernández DA, 2020 (43) | F | 31 | Trachea | Single | 14 | – | Tracheotomy | Cervical incision | 14 months | N |
| Zhang L, 2020 (44) | M | 11 | Trachea | Single | 10 | – | Endoscopic intervention | – | 4 weeks | N |
| Zhou D, 2020 (45) | F | 56 | Right upper lobe bronchus | Single | 70 | – | Lobectomy | Thoracotomy | – | – |
| Imen T, 2021 (46) | M | 60 | Right upper lobe bronchus | Single | 60 | – | Lobectomy | – | 4 weeks | N |
| Esch M, 2021 (47) | F | 65 | Trachea | Single | 30 | – | Tracheotomy | Cervical incision | 8 years | N |
| Jin B, 2021 (48) | F | 59 | Trachea | Single | 15 | Pedunculated | Endoscopic intervention | – | 7 years | N |
| M | 32 | Left main bronchus | Single | 20 | Pedunculated | Endoscopic intervention | – | – | Y | |
| M | 57 | Trachea | Single | 40 | Sessile | Endoscopic intervention | – | 12 years | Y | |
| M | 56 | Left main bronchus | Single | 10 | Pedunculated | Endoscopic intervention | – | 6 years | N | |
| M | 63 | Trachea | Multiple | 20 | Sessile | Endoscopic intervention | – | 3 years | N | |
| M | 37 | Left main bronchus | Single | 15 | Sessile | Endoscopic intervention | – | 3 years | Y | |
| F | 26 | Right main bronchus | Single | 20 | Sessile | Endoscopic intervention | – | 2 years | N | |
| Aoyama Y, 2022 (3) | M | 37 | Right middle lobe bronchus | Single | 17 | – | Bilobectomy | VATS | – | N |
| Jahromi MG, 2022 (49) | F | 7 | Right main bronchus | Single | 18 | – | Endoscopic intervention | – | – | N |
| Nishi Y, 2022 (50) | F | 89 | Trachea | Single | – | – | Endoscopic intervention | – | 3 years | Y |
| Shen YS, 2022 (51) | F | 61 | Trachea | Single | 15 | – | Endoscopic intervention | – | 2 months | N |
| Shimada T, 2022 (52) | F | 79 | Right main bronchus | Single | 25 | Sessile | Surgery | – | 3 years | N |
| Afsin E, 2022 (53) | M | 21 | Trachea | Single | 28 | – | Tracheotomy | Cervical incision | – | N |
| Xia C, 2022 (54) | M | 54 | Trachea | Single | 27 | Sessile | Tracheotomy | Thoracotomy | 6 months | N |
| Lina G, 2023 (55) | F | 60 | Right main bronchus | Single | 40 | – | Endoscopic intervention | – | 10 days | N |
| Alkhars HF, 2023 (56) | M | 37 | Trachea | Single | 22 | – | Endoscopic intervention | – | 2 weeks | N |
| Burton KA, 2023 (57) | F | 71 | Left lower lobe secondary carina | Single | 2 | – | Follow-up | – | – | N |
| Botero JD, 2023 (58) | F | 57 | Trachea | Single | – | Sessile | Endoscopic intervention | – | 3 months | N |
| Karam C, 2023 (59) | F | 19 | Trachea | Single | 17 | – | Tracheotomy | – | – | N |
†, the details in the full-text available reports written in English searched with the combination of terms (“schwannoma”[Title] OR “neurilemmoma”[Title] OR “neurilemoma”[Title] OR “neurinoma”) AND (“bronchus”[Title] OR “trachea”[Title] OR “tracheobronchial”[Title] OR “endobronchial”[Title] OR “bronchial”[Title] OR “endotracheal”[Title] OR “tracheal”[Title]) via PubMed. “–” indicates not mentioned. Max-D, maximal diameter; M, male; N, no; F, female; Y, yes; VATS, video-assisted thoracic surgery.
The bronchial anastomosis and reconstruction of this surgery required high technique for surgeon. The high-definition three-dimensional video of Da Vinci robotic system provided the surgeons with a clear picture of anatomic structures, helping to reduce the visual fatigue of surgeons during the operation (60). Furthermore, with tremor suppression and better maneuverability of instruments (61), it contributed to the precise sutures and knotting in a narrow anatomical space during the process of the primary anastomosis of this surgery, indisputably. However, like other surgical technology, the Da Vinci robotic system also has limitations. First of all, robotic surgery is still a new technology and lacks enough long-term follow-up studies to well establish its uses and efficacy. And the multiple incisions may increase patients’ injury, which will be optimized by single-port robotic surgery with Da Vinci SP robotic surgical system. Other disadvantages are the size and cost of this system. We believe all the disadvantages will be remedied with the development of technology.
Conclusions
In conclusion, primary tracheobronchial schwannomas are extremely rare. Surgical resection is the first choice for these tumors, which may offer a better prognosis than endoscopic intervention. We reported the first sleeve resection for bronchial schwannoma using Da Vinci surgical robotic system. The application of Da Vinci Si robotic surgical system benefited the process of this surgery, undoubtedly. Hopefully, more case reports and clinical trials will shed light on the clinical details of tracheobronchial schwannoma, and help with the guidelines production to achieve more systematic diagnosis and treatment.
Acknowledgments
Funding: This study was supported by t
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-819/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-819/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-819/coif). All authors report that this study was supported by the National Natural Science Foundation of China (Nos. 82372855 and 82072557), the Interdisciplinary Program of Shanghai Jiao Tong University (No. YG2023ZD04), the Novel Interdisciplinary Research Project from Shanghai Municipal Health Commission (No. 2022JC023), the Shanghai Municipal Education Commission - Gaofeng Clinical Medicine Grant (No. 20172005, the 2nd round of disbursement), and the National Key Research and Development Program of China (No. 2021YFC2500900). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript, the accompanying image, and the video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hilton DA, Hanemann CO. Schwannomas and their pathogenesis. Brain Pathol 2014;24:205-20. [Crossref] [PubMed]
- Grillo HC, Mathisen DJ. Primary tracheal tumors: treatment and results. Ann Thorac Surg 1990;49:69-77. [Crossref] [PubMed]
- Aoyama Y, Miyamoto A, Fujii T, et al. Primary bronchial schwannoma: A case report. Medicine (Baltimore) 2022;101:e31062. [Crossref] [PubMed]
- Geraci TC, Scheinerman J, Chen D, et al. Beyond the learning curve: a review of complex cases in robotic thoracic surgery. J Thorac Dis 2021;13:6129-40. [Crossref] [PubMed]
- Macchiarini P. Primary tracheal tumours. Lancet Oncol 2006;7:83-91. [Crossref] [PubMed]
- Shah H, Garbe L, Nussbaum E, et al. Benign tumors of the tracheobronchial tree. Endoscopic characteristics and role of laser resection. Chest 1995;107:1744-51. [Crossref] [PubMed]
- Straus GD, Guckien JL. Schwannoma of the tracheobronchial tree. A case report. Ann Otol Rhinol Laryngol 1951;60:242-6. [Crossref] [PubMed]
- Park CM, Goo JM, Lee HJ, et al. Tumors in the tracheobronchial tree: CT and FDG PET features. Radiographics 2009;29:55-71. [Crossref] [PubMed]
- Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol 2008;32:1291-8. [Crossref] [PubMed]
- Ng J, Celebre A, Munoz DG, et al. Sox10 is superior to S100 in the diagnosis of meningioma. Appl Immunohistochem Mol Morphol 2015;23:215-9. [Crossref] [PubMed]
- Watanabe T, Oda Y, Tamiya S, et al. Malignant peripheral nerve sheath tumours: high Ki67 labelling index is the significant prognostic indicator. Histopathology 2001;39:187-97. [Crossref] [PubMed]
- Kindblom LG, Ahldén M, Meis-Kindblom JM, et al. Immunohistochemical and molecular analysis of p53, MDM2, proliferating cell nuclear antigen and Ki67 in benign and malignant peripheral nerve sheath tumours. Virchows Arch 1995;427:19-26. [Crossref] [PubMed]
- Feldhaus RJ, Anene C, Bogard P. A rare endobronchial neurilemmoma (Schwannoma). Chest 1989;95:461-2. [Crossref] [PubMed]
- Stack PS, Steckler RM. Tracheal neurilemmoma: case report and review of the literature. Head Neck 1990;12:436-9. [Crossref] [PubMed]
- Rusch VW, Schmidt RA. Tracheal schwannoma: management by endoscopic laser resection. Thorax 1994;49:85-6. [Crossref] [PubMed]
- Nesbitt JC, Vega DM, Burke T, et al. Cellular schwannoma of the bronchus. Ultrastruct Pathol 1996;20:349-54. [Crossref] [PubMed]
- Weiner DJ, Weatherly RA, DiPietro MA, et al. Tracheal schwannoma presenting as status asthmaticus in a sixteen-year-old boy: airway considerations and removal with the CO2 laser. Pediatr Pulmonol 1998;25:393-7. [Crossref] [PubMed]
- Dorfman J, Jamison BM, Morin JE. Primary tracheal schwannoma. Ann Thorac Surg 2000;69:280-1. [Crossref] [PubMed]
- Chen SR, Chen MH, Ho DM, et al. Massive hemoptysis caused by endobronchial schwannoma in a patient with neurofibromatosis 2. Am J Med Sci 2003;325:299-302. [Crossref] [PubMed]
- Kasahara K, Fukuoka K, Konishi M, et al. Two cases of endobronchial neurilemmoma and review of the literature in Japan. Intern Med 2003;42:1215-8. [Crossref] [PubMed]
- Nio M, Sano N, Kotera A, et al. Primary tracheal schwannoma (neurilemoma) in a 9-year-old girl. J Pediatr Surg 2005;40:E5-7. [Crossref] [PubMed]
- Righini CA, Lequeux T, Laverierre MH, et al. Primary tracheal schwannoma: one case report and a literature review. Eur Arch Otorhinolaryngol 2005;262:157-60. [Crossref] [PubMed]
- Dincer SI, Demir A, Kara HV, et al. Primary tracheal schwannoma: a case report. Acta Chir Belg 2006;106:254-6. [Crossref] [PubMed]
- Shigematsu H, Aoe M, Date H. Schwannoma occurring from the lingular bronchus. Eur J Cardiothorac Surg 2007;32:537. [Crossref] [PubMed]
- Nakamura R, Ishikawa S, Sakai M, et al. Increased fluorodeoxyglucose-uptake in positron emission tomography with an endobronchial schwannoma occluding the left main stem bronchus. J Thorac Oncol 2009;4:1183-4. [Crossref] [PubMed]
- Stouffer CW, Allan RW, Shillingford MS, et al. Endobronchial schwannoma presenting with bronchial obstruction. Interact Cardiovasc Thorac Surg 2010;10:133-4. [Crossref] [PubMed]
- Tansel T, Toker A, Yilmazbayhan D, et al. Primary endobronchial schwannoma. J Pediatr Surg 2010;45:2241-3. [Crossref] [PubMed]
- Dumoulin E, Gui X, Stather DR, et al. Endobronchial schwannoma. J Bronchology Interv Pulmonol 2012;19:75-7. [Crossref] [PubMed]
- Lee BR, Choi YD, Kim YI, et al. Endobronchial schwannoma treated by rigid bronchoscopy with argon plasma coagulation. Tuberc Respir Dis (Seoul) 2012;73:174-7. [Crossref] [PubMed]
- Melendez J, Cornwell L, Green L, et al. Treatment of large subglottic tracheal schwannoma with microdebrider bronchoscopy. J Thorac Cardiovasc Surg 2012;144:510-2. [Crossref] [PubMed]
- Thomas R, Christopher DJ, Thangakunam B, et al. Tracheal schwannoma as a mimic of bronchial asthma. Singapore Med J 2012;53:e95-6.
- Dalar L, Sökücü SN, Ünver N, et al. Initial bronchoscopic treatment of tracheal schwannoma: a rarely seen tumour. West Indian Med J 2014;63:209-11. [Crossref] [PubMed]
- Kushima H, Ishii H, Okada F, et al. The case of primary endobronchial neurinoma. BMJ Case Rep 2014;2014:bcr2014205844. [Crossref] [PubMed]
- Isaac BT, Christopher DJ, Thangakunam B, et al. Tracheal schwannoma: Completely resected with therapeutic bronchoscopic techniques. Lung India 2015;32:271-3. [Crossref] [PubMed]
- Oliveira RC, Nogueira T, Sousa V, et al. Bronchial schwannoma: a singular lesion as a cause of obstructive pneumonia. BMJ Case Rep 2016;2016:bcr2016217300. [Crossref] [PubMed]
- Hamouri S, Novotny NM. Primary tracheal schwannoma a review of a rare entity: current understanding of management and followup. J Cardiothorac Surg 2017;12:105. [Crossref] [PubMed]
- Han DP, Xiang J, Ye ZQ, et al. Primary tracheal schwannoma treated by surgical resection: a case report. J Thorac Dis 2017;9:E249-52. [Crossref] [PubMed]
- Ally M, Kinshuck AJ, Rouhani M, et al. The surgical management of recurrent tracheal schwannoma. AME Case Rep 2018;2:16. [Crossref] [PubMed]
- Komatsu M, Hachiya T, Takahashi H, et al. Left Main Bronchial Stenosis due to Schwannoma. Intern Med 2018;57:1947-8. [Crossref] [PubMed]
- Liao H, Song W, Chen N, et al. Left lower lobe sleeve resection for endobronchial schwannoma. Ann Transl Med 2019;7:50. [Crossref] [PubMed]
- Chen H, Zhang K, Bai M, et al. Recurrent transmural tracheal schwannoma resected by video-assisted thoracoscopic window resection: A case report. Medicine (Baltimore) 2019;98:e18180. [Crossref] [PubMed]
- Ishibashi H, Wakejima R, Takasaki C, et al. Successful Excision of Endobronchial Cellular Schwannoma With Right Lower Sleeve Lobectomy. Ann Thorac Surg 2019;107:e203-5. [Crossref] [PubMed]
- Chávez-Fernández DA, Zúñiga-Garza E, López-Saucedo RA. Primary tracheal schwannoma resected in a Turner syndrome patient: a case report. J Surg Case Rep 2020;2020:rjaa430. [Crossref] [PubMed]
- Zhang L, Tang W, Hong QS, et al. Case report: A tracheobronchial schwannoma in a child. Respir Med Case Rep 2020;30:101047. [Crossref] [PubMed]
- Zhou D, Xing X, Fan J, et al. PD-1/PD-L1 negative schwannoma mimicking obstructive bronchial malignancy: A case report. Thorac Cancer 2020;11:2335-8. [Crossref] [PubMed]
- Imen T, Sadok BM, Raoudha A, et al. Endobronchial schwannoma in adult: A case report. Respir Med Case Rep 2021;33:101396. [Crossref] [PubMed]
- Esch M, Teschner M. Primary tracheal microcystic reticular schwannoma - Case report of a rare neurogenic tumor treated by segmental tracheal resection. Int J Surg Case Rep 2021;79:251-4. [Crossref] [PubMed]
- Jin B, Wang T, Wang J, et al. Interventional bronchoscopic therapy in adult patients with tracheobronchial schwannoma. Ann Palliat Med 2021;10:6279-86. [Crossref] [PubMed]
- Jahromi MG, Yakhdani AS, Saeedi-Moghadam M, et al. Incidental diagnosis of a rare endobronchial schwannoma in a 7-year-old girl: A case report. Radiol Case Rep 2022;17:4043-5. [Crossref] [PubMed]
- Nishi Y, Handa H, Tsuruoka H, et al. Successful Treatment with Radiation Therapy in an Older Patient with Endobronchial Schwannoma. Case Rep Oncol 2022;15:212-7. [Crossref] [PubMed]
- Shen YS, Tian XD, Pan Y, et al. Treatment of primary tracheal schwannoma with endoscopic resection: A case report. World J Clin Cases 2022;10:10279-85. [Crossref] [PubMed]
- Shimada T, Okuzumi S, Kakimoto T, et al. Pneumothorax as a rare presentation of bronchial schwannoma. Respir Med Case Rep 2022;36:101590. [Crossref] [PubMed]
- Afsin E, Yaksi O, Önal A, et al. Tracheal schwannoma mimicking asthma. Int J Neurosci 2022; Epub ahead of print. [Crossref]
- Xia C, Liu M, Niu X, et al. Molecular Features of a Primary Transmural Tracheal Schwannoma: Clinical Experience and Review of the Literature. Cancer Manag Res 2022;14:1125-9. [Crossref] [PubMed]
- Lina G, Pengguo H, Zhihua X, et al. Tracheobronchial schwannoma: a case report and literature review. J Int Med Res 2023;51:3000605221149891. [Crossref] [PubMed]
- Alkhars HF, Al Muhaimid T, Al Abdulwahid F, et al. Endoscopic Excision of Primary Tracheal Schwannoma: A Case Report. Am J Case Rep 2023;24:e939823. [Crossref] [PubMed]
- Burton KA, Karulf M, Pahn B. Bronchial Schwannoma Incidentally Discovered via Bronchoscopy: A Case Report. Cureus 2023;15:e38011. [Crossref] [PubMed]
- Botero JD, Pérez Restrepo M, Murillo MA, et al. An Unusual Finding of a Neurogenic Tumor of the Trachea: A Tracheal Schwannoma. Cureus 2023;15:e48172. [Crossref] [PubMed]
- Karam C, Abou Nafeh N, Aouad MT, et al. Harlequin syndrome during peripheral cardiopulmonary bypass in a patient with an obstructing tracheal schwannoma: A case report. Clin Case Rep 2023;11:e7509. [Crossref] [PubMed]
- Jin D, Dai Q, Han S, et al. Effect of da Vinci robot-assisted versus traditional thoracoscopic bronchial sleeve lobectomy. Asian J Surg 2023;46:4191-5. [Crossref] [PubMed]
- de'Angelis N, Khan J, Marchegiani F, et al. Robotic surgery in emergency setting: 2021 WSES position paper. World J Emerg Surg 2022;17:4. [Crossref] [PubMed]