
Additional local therapy before disease progression for EGFR-mutated advanced lung cancer: a systematic review and meta-analysis
Highlight box
Key findings
• Administering additional local therapy (LT) to primary and/or metastatic tumor before disease progression significantly improves survival in patients with epidermal growth factor receptor (EGFR)-mutated advanced non-small cell lung cancer (NSCLC). Furthermore, the administering LT 3 months after treatment with EGFR-tyrosine kinase inhibitor (TKI) may be a more effective approach.
What is known and what is new?
• Various treatment strategies have been investigated to overcome the acquired resistance to EGFR-TKIs.
• This study suggests that the strategically using additional LT before disease progression is a promising approach for the treatment of EGFR-mutated advanced NSCLC.
What is the implication, and what should change now?
• These results could help clinicians strategically using additional LT for EGFR-mutated advanced NSCLC. However, more prospective clinical trials are still needed to evaluate the efficacy, safety, and optimal timing of LT.
Introduction
Recent advances in targeted therapy and immunotherapy have led to personalized treatment approaches, which improve the outcome for patients with specific genetic mutations or biomarkers (1-3). Particularly, the introduction of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) has transformed the treatment possibilities for advanced non-small cell lung cancer (NSCLC) harboring EGFR mutation. This has remarkably increased the survival period of patients to 18–38.6 months (4-6). However, the emergence of acquired resistance, which leads to disease progression after treatment with first-line EGFR-TKIs, has limited the potential benefits to survival (7-9).
To overcome the acquired resistance to EGFR-TKIs, various treatment strategies have been investigated, including the detection of EGFR T790M mutation, use of first-line osimertinib, and combination of EGFR-TKIs with cytotoxic chemotherapy or vascular endothelial growth factor receptor inhibitors (10-12). After disease progression, a combination of EGFR-TKIs and additional local therapies (LTs) has also been applied. This combination can significantly extend progression-free survival (PFS) and overall survival (OS) compared to EGFR-TKIs alone, without significant differences in adverse events (13-19). Based on these findings, the National Comprehensive Cancer Network guidelines recommend that doctors consider the adoption of LT in advanced NSCLC patients who experience limited progression during the EGFR-TKIs treatment (1).
Recently, additional LT to primary tumor and/or metastatic sites before disease progression has been introduced as a promising strategy to overcome acquired resistance to EGFR-TKIs (20,21). However, the clinical outcomes of the administration of additional LT in conjunction with first-line EGFR-TKIs before disease progression have not been extensively analyzed. This meta-analysis aims to identify the efficacy and safety of additional LT in conjunction with first-line EGFR-TKIs before disease progression in patients with advanced NSCLC harboring EGFR mutations. We present this article in accordance with the PRISMA reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-830/rc) (22).
Methods
The proposed population, intervention, comparison, and outcome for our study were as follows: “In advanced NSCLC patients with EGFR mutations, does the administration of additional LT to primary tumor and/or metastatic sites before disease progression during EGFR-TKI treatment improve PFS and OS outcomes compared to EGFR-TKI monotherapy?” This review was registered on PROSPERO (registration number: CRD42023439913).
Eligible criteria
The following criteria were used to select eligible studies for the analysis: (I) studies that compare patients who received first-line EGFR-TKIs alone with patients who received additional LT in conjunction with first-line EGFR-TKIs treatment before disease progression; (II) studies that include more than ten patients with advanced NSCLC harboring EGFR mutations at the time of diagnosis in each treatment arm; and (III) studies that report either PFS or OS as a primary endpoint.
In this study, LT was defined as the removal or reduction of a tumor burden in primary lung cancer and/or metastatic lesions. The LT group consisted of patients who received first-line EGFR-TKIs in conjunction with various LT modalities, including surgery, radiotherapy, or ablation therapy. Ablation therapy includes radiofrequency ablation and microwave ablation. Studies exclusively reporting LT for metastatic lesions were excluded. The TKI group comprised participants treated with first-line EGFR-TKIs alone.
Search strategy and data collection
We conducted a systematic literature search in PubMed, Embase, and the Cochrane Library for manuscripts published until May 31, 2023. The search strategy and specific terms used are listed in Table S1. Three review authors (H.S., S.H.K., and J.S.E.) independently screened titles and abstracts. Subsequently, two review authors (H.S. and S.H.K.) reviewed the full text of all potentially relevant articles. Discrepancies were resolved through consensus and consultation with a third review author (J.S.E.). Data collection was performed using pre-standardized sheets, which included general information such as author names, affiliations, publication year, study design, number of patients, LT modalities, LT sites, and number of metastatic foci. The clinical outcome data collected included the time to LT administration after EGFR-TKI treatment, median PFS, median OS, hazard ratios (HRs) of median PFS and OS, and adverse events. PFS was defined as the time from the initiation of the first-line EGFR-TKI treatment to the confirmation of disease progression or death from any cause. OS was defined as the duration from the initiation of the first-line EGFR-TKI treatment to death from any cause. Adverse events, which refer to unfavorable or harmful events experienced by the participants during EGFR-TKI treatment or LT, were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) criteria (23).
Assessment of risk of bias in investigated studies
To assess the selection bias in randomized controlled trials (RCTs), we used the Cochrane Risk of Bias 2 tool. This tool examines domains such as random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias (24,25). For non-randomized studies, we utilized the Risk of Bias in Non-randomized Studies of Interventions tool to assess the risk of selection bias. This tool evaluates domains such as bias in confounding, selection of participants, classification of intervention, deviation from intended intervention, missing data, measurement of outcomes, and selection of reported results (26). The risk of bias was categorized as low, moderate, high, or unclear, and individual bias items were evaluated according to the methods in the Cochrane Handbook for Systematic Reviews of Interventions. Two authors (H.S. and S.H.K.) independently evaluated the risk of bias in each study. Any discrepancies were resolved through consensus or consultation with a third review author (J.S.E.).
Statistical analysis
The primary outcomes of this study were the PFS and OS values of the LT and TKI groups. Log HR and standard error were calculated using the HR and their corresponding 95% confidence intervals (CIs) of each study (27,28). To quantitatively aggregate the survival results, a meta-analysis was conducted using a random-effects model considering the confounding variables unavoidable in non-randomized studies (29). Additionally, the pooled risk ratio was estimated and used to represent the combined effect of adverse events according to the CTCAE criteria, which were applied using different versions in each study (version 3.0–5.0).
Subgroup analyses were conducted based on the factors ‘time to LT’ and ‘number of metastatic foci’. ‘Time to LT’ refers to the duration between the beginning of EGFR-TKI treatment and administration of LT. Based on previous studies that reported the maximal response to EGFR-TKIs occurring within 2–2.7 months, studies were classified into early LT (‘time to LT’ <3 months) and late LT (‘time to LT’ ≥3 months) (30-32). Each study classified patients into oligometastases (OM) or polymetastases (PM) groups based on the number of metastatic foci, but the criteria varied by study. We maintained the criteria used in each study because we were unable to obtain individual patient data.
Statistical heterogeneity was defined at P<0.01 in Cochran’s Q test and Higgins’ I2 statistics >50% (33,34). A reporting bias assessment was conducted using a visual funnel plot together with Egger’s test for the pooled analysis (35). Statistical analyses were conducted using the R statistical language (version 4.3.1; R Core Team, 2023) and additional packages (meta).
Results
Study selection and characteristics
A total of 7,506 studies were identified in the initial search. Title filtering was used to exclude studies with irrelevant topics and formats, as well as duplicate records. Subsequently, 196 selected studies were reviewed by abstract screening. Full-text screening was performed in the remaining 36 studies. Finally, 11 studies that fulfilled all eligibility criteria were included in the systematic review (Figure 1) (36-46).
Among the 11 final studies, which covered 1,313 patients, two were RCTs (n=194) (45,46), one was a phase II single-arm prospective study (n=59) (38), and eight were retrospective case-control studies (n=1,060) (36,37,39-44). There were 425 and 888 patients in the LT and TKI groups, respectively. Radiotherapy was reported as the LT modality in ten studies (36-38,40-46), whereas surgery (36,37,44) and ablation therapy (36,39,44) were performed in three studies each. All 11 studies included patients who were treated with 1st or 2nd generation EGFR-TKIs as the first-line treatment. Table 1 summarizes the general characteristics of the selected studies.
Table 1
| Author, year | Study design | Stage | No. of metastases | Response of TKI (%) | LT site | LT modality (%) | Median time to LT (mo) | No. of patients | Median PFS | Median OS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LT | TKI | LT (mo) | TKI (mo) | HR (95% CI) |
LT (mo) | TKI (mo) | HR (95% CI) |
||||||||||
| Xu, 2018, (36) | R | IV | OM (≤5 foci) | PR or SD† | Lu + M | Surgery (NA); RT (NA); RFA (NA) |
E (1.5) | 51 | 39 | 20.6 | 13.9 | 0.32 (0.20–0.51) |
40.9 | 30.8 | 0.42 (0.42–0.62) |
||
| Elamin,2019, (37) | R | IV | Unlimited | PR or SD† | Lu + M | Surgery (8.3); RT (100.0) | L (4.0) | 12 | 129 | 36.0 | 14.0 | 0.29 (0.23–0.70) |
NR | 35.0 | 0.29 (0.13–0.66) |
||
| Chan, 2020, (38) | P | IIIb–IV | OM (≤4 foci) | PR (100.0) | Lu + M | RT (100.0) | L (3.0) | 16 | 43 | 18.2 | 11.0 | 0.41 (0.21–0.80) |
44.3 | NA | NA | ||
| Ni, 2020, (39) | R | IV | OM (≤5 foci) | PR (67.6); SD (32.4) | Lu + M | MWA (100.0) | E (2.0) | 34 | 52 | 16.7 | 12.9 | 0.46 (0.37–0.82) |
34.8 | 22.7 | 0.57 (0.33–0.91) |
||
| Hsu, 2021, (40) | R | IIIb–IV | Unlimited | PR (84.8); SD (15.2) | Lu | RT (100.0) | L (6.6) | 46 | 92 | 27.5 | 10.9 | 0.27 (0.17–0.44) |
NR | 38.0 | 0.11 (0.04–0.30) |
||
| Wang, 2021, (41) | R | IV | PM (>5 foci) | PR or SD† | Lu | RT (100.0) | E (2.0) | 16 | 64 | 17.8 | 10.8 | 0.54 (0.30–0.99) |
36.7 | 27.8 | NR | ||
| Deng, 2022, (42) | R | IV | PM (>3, ≤15 foci) | PR (78.6); SD (21.4) | Lu + M | RT (100.0) | L (3.3) | 46 | 77 | 13.6 | 10.6 | 0.23 (0.15–0.37) |
NA | NA | NA | ||
| Hu, 2022, (43) | R | IV | OM (≤5 foci) | SD (100.0) | Lu + M | RT (100.0) | E (1.5) | 50 | 72 | 17.0 | 12.0 | 0.32 (0.20–0.51) |
38.0 | 29.0 | 0.41 (0.27–0.63) |
||
| Kuo, 2022, (44) | R | IV | Unlimited | PR or SD† | Lu + M | Surgery (100.0); RT (23.2); RFA (1.8) |
L (5.1) | 56 | 224 | 29.6 | 13.0 | 0.37 (0.21–0.49) |
NR | 60.0 | 0.37 (0.21–0.49) |
||
| Peng, 2023, (45) | RCT | IV | OM (≤5 foci) | PR or SD† | Lu + M | RT (100.0) | L (3.0) | 30 | 31 | 17.6 | 9.0 | 0.52 (0.31–0.89) |
33.6 | 23.2 | 0.53 (0.30–0.95) |
||
| Wang, 2023, (46) | RCT | IV | OM (≤5 foci) | NA‡ | Lu + M | RT (100.0) | E (0) | 68 | 65 | 20.2 | 12.5 | 0.22 (0.17–0.46) |
25.5 | 17.6 | 0.44 (0.28–0.68) |
||
†, all patients achieved PR or SD, but this proportion was not available; ‡, LT group patients received LT and TKI, simultaneously. R, retrospective case-control study; P, phase II single-arm prospective study; RCT, randomized controlled trial; OM, oligometastases; PM, polymetastases; TKI, tyrosine kinase inhibitor; PR, partial response; SD, stable disease; NA, not available; LT, local therapy; Lu, primary lung tumor; M, metastatic sites; RT, radiotherapy; RFA, radiofrequency ablation; MWA, microwave ablation; E, early local therapy group; L, late local therapy group; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; OS, overall survival; NR, not reached.
Bias assessment
We assessed the methodological quality and risk of selection bias in the studies using the Risk of Bias 2 and Risk of Bias in Non-randomized Studies of Interventions tools (Figure S1). None of the studies presented a high risk of bias. Therefore, all 11 studies were included in the pooled analysis.
Funnel plot analyses were conducted to evaluate the potential reporting bias. The HRs of median PFS from all 11 studies were included, and HRs of median OS from the eight studies were also included in the funnel plot analyses (Figure S2) (36,37,39,40,43-46). The funnel plots were symmetric, and Egger’s test values for them were 0.71 and 0.34, respectively, which indicated no apparent reporting bias.
Clinical outcomes
In the 11 studies, the pooled HR of median PFS between the LT and TKI groups was 0.34 (95% CI: 0.28–0.41; P<0.001), which indicated a significant benefit in favor of the LT group, with a low heterogeneity (P=0.15; I2=32%) (Figure 2A). The median PFS ranged from 13.6 to 36.0 months in the LT group, and from 9.0 to 14.0 months in the TKI group.
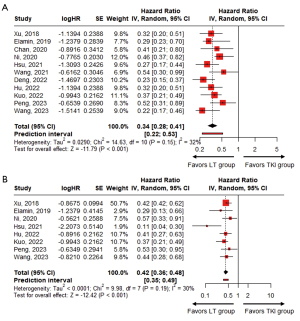
Among the 11 studies, the HRs of median OS were reported in eight studies (36,37,39,40,43-46). In the remaining three studies, Wang et al. reported median OS but did not reach an HR (41), whereas the other two studies did not report HR data of median OS (38,42). In the eight studies that provided HRs, the pooled HR of median OS between the LT and TKI groups was 0.42 (95% CI: 0.36–0.48; P<0.001), which indicated a significant benefit in favor of the LT group, with a low heterogeneity (P=0.19; I2=30%) (Figure 2B). The median OS range in the LT group was 25.5 months to not reached, whereas in the TKI group, it was 17.6 to 60.0 months (36,37,39,40,43-46).
Subgroup analyses
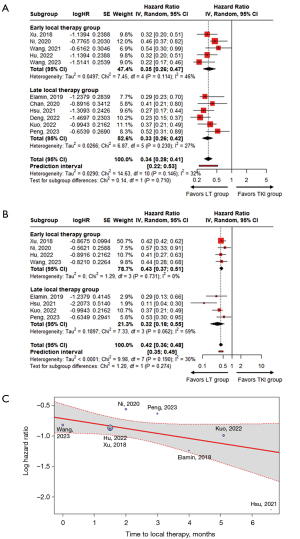
The median ‘time to LT’ ranged from 0 to 6.6 months. Five studies were classified into the early LT group (36,39,41,43,46), and the other six were classified into the late LT group (37,38,40,42,44,45). The pooled HRs of median PFS in the early and late LT groups were 0.35 (95% CI: 0.26–0.47) and 0.33 (95% CI: 0.26–0.42), respectively (P=0.710) (Figure 3A). Although not statistically significant, the pooled HR of median OS in the late LT group was lower than that in the early LT group [early LT: 0.43 (95% CI: 0.37–0.51); late LT: 0.32 (95% CI: 0.18–0.55); P=0.274] (Figure 3B). This trend was also observed as a marginally significant negative linear relationship between the log HR of median OS and the ‘time to LT’, which indicated that a delay in LT decreased the HR of median OS (P=0.086) (Figure 3C).
Based on the number of metastatic foci, six studies included patients with OM (36,38,39,43,45,46), two studies included patients with PM (41,42), and three studies included patients without any restriction on the number of metastatic foci (37,40,44). Among the OM group, five studies defined OM as five or fewer metastatic lesions (36,39,43,45,46), whereas Chan et al. defined OM as four or fewer metastatic lesions (38). In the PM group, Deng et al. defined PM as more than four metastatic lesions (42), whereas Wang et al. defined PM as more than five metastatic lesions (41). The pooled HRs of median PFS and OS in the OM group were 0.36 (95% CI: 0.28–0.46) and 0.44 (95% CI: 0.38–0.51), respectively (Figure S3). Deng et al. and Wang et al. reported median PFS HRs of 0.23 (95% CI: 0.15–0.37) and 0.54 (95% CI: 0.30–0.99) in the PM group, respectively, whereas median OS HRs were not reported (41,42).
Adverse events
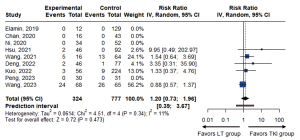
Ten studies reported grade 3 or higher serious adverse events according to the CTCAE criteria (Table 2) (37-46). Among them, five studies reported these events at rates of 1.8–40.0% (40-42,44,46), whereas four studies reported none (37-39,45). The pooled risk ratio for adverse events was 1.20 (95% CI: 0.73–1.96; P=0.473), which indicated no significant differences between the LT and TKI groups (Figure 4).
Table 2
| Author, year | Total | Pulmonological | GE | Hepatological | Cardiological | Dermatological | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LT | TKI | LT | TKI | LT | TKI | LT | TKI | LT | TKI | LT | TKI | ||||||
| Elamin, 2019, (37) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Chan, 2020, (38) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Ni, 2020, (39) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Hsu, 2021, (40) | 2 (4.3) | 0 | 2 (4.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Wang, 2021, (41) | 5 (31.3) | 13 (20.3) | 1 (6.2) | 0 | 0 | 0 | 2 (12.5) | 6 (9.4) | 0 | 0 | 2 (12.5) | 7 (10.9) | |||||
| Deng, 2022, (42) | 2 (4.3) | 1 (1.8) | 1 (2.2) | 1 (1.8) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.2) | 0 | |||||
| Hu, 2022, (43) | NA | NA | 3 (6.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 (4.9) | ||||||
| Kuo, 2022, (44) | 3 (5.4) | 9 (4.0) | 0 | 0 | 0 | 0 | 1 (1.8) | 4 (1.8) | 0 | 0 | 2 (3.6) | 5 (2.2) | |||||
| Peng, 2023, (45) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Wang, 2023, (46) | 24 (35.3) | 26 (40.0) | 5 (7.4) | 4 (6.2) | 3 (4.4) | 2 (3.1) | 1 (1.5) | 1 (1.5) | 0 | 2 (3.1) | 15 (22.1) | 17 (26.2) | |||||
Data are presented as n (%). LT, local therapy; TKI, tyrosine kinase inhibitors; GE, gastroenterological; NA, not available.
Discussion
Our analyses demonstrated that the administration of additional LT to a primary tumor and/or metastatic sites before disease progression can be an effective and safe strategy to prevent or delay the development of acquired resistance to EGFR-TKIs. The median PFS and OS were significantly better in the LT group than in the TKI group. Moreover, there was no significant difference between the LT and TKI groups regarding grade ≥3 adverse events.
Previous studies have compared EGFR-TKIs alone with LT for oligoprogression sites during EGFR-TKIs treatment. The reported HRs of median PFS and OS were 0.54 and 0.48, respectively, with median PFS of 6.7–18.3 months, and median OS of 20.0–37.3 months (47-51). In this meta-analysis, when LT was administered to primary tumors and/or metastatic sites before disease progression, the HRs of median PFS and OS were numerically lower compared to historical comparators of LT for oligoprogression sites (HR of median PFS: 0.34 and 0.54; HR of median OS: 0.42 to 0.48, respectively). The administration of LT to primary tumors and/or metastatic sites before disease progression can reduce the burden of treatment-resistant cells to EGFR-TKIs and potentiate the effect of systemic therapy (51). Ultimately, this can lead to prolonged survival compared to the results of LT performed after disease progression.
According to the ‘time to LT’ analysis, the pooled HR of median OS was numerically lower in the late LT group (0.32) than in the early LT group (0.43). This trend was further confirmed by the correlation between the log HR of median OS and the ‘time to LT’, which demonstrated that HR decreased when LT was performed later. Although an optimal LT timing has not been established, possible options to obtain the greatest tumor shrinkage are: (I) at the time of diagnosis, (II) at the initial response to EGFR-TKIs, and (III) at the maximal response to EGFR-TKIs. The overall response and disease control rates of EGFR-TKI treatment are 59.1% and 81.8%, respectively, and they lead to the shrinkage of both primary tumor and metastatic foci (52). Therefore, the use of LT after the maximal response to EGFR-TKI treatment can better reduce the required radiation field or surgical extent compared to its application at the initial diagnosis or response (53). This reduction is potentially associated with a decrease in the incidence of LT-related morbidities, such as radiation pneumonitis or exacerbation of pre-existing lung disease (obstructive lung disease or interstitial lung disease). Additionally, EGFR-TKI treatment has the potential to transform PM into an OM state, which can impact prognosis (54). Furthermore, LT for residual resistance clones of high heterogeneity can induce a fundamental change in biological behavior, potentially delaying progression (55). Ultimately, these outcomes can synergistically contribute to improving the overall treatment performance and thereby extend the survival period.
LT showed a favorable efficacy not only in the OM group but also in the PM group. Although LT is typically recommended for cases of OM NSCLC (56), these results suggest that LT can also be considered for cases of PM NSCLC. However, the different definitions of OM and PM used in each study hindered a comparison of the effectiveness of LT between OM and PM groups. Further research is needed to compare the difference between OM and PM groups under additional LT.
This study has some limitations. First, the number of studies and patients included in the analysis was relatively small. The limited number of trials and participants restricted our ability to detect differences between subgroups. Second, although RCTs are considered the gold standard for evaluating intervention efficacy, this study had access to only two RCTs. Between these, the study by Peng et al. did not reach the planned number of patients (45). Third, given that meta-analysis is based on the results of published articles and entails integrating various clinical details, such as LT modalities and types of EGFR-TKIs, a certain degree of heterogeneity is inevitable. Fourth, evaluating treatment outcomes in patients with advanced NSCLC largely depends on factors such as the overall response rate, the prevalence of T790M mutation, the presence of unfavorable prognostic factors, and the type of EGFR-TKI. However, this information was not available. Finally, 3rd-generation EGFR-TKIs are preferentially used as the initial treatment for advanced NSCLC with EGFR mutation. However, previous studies have not specifically investigated the combination of additional LT with 3rd-generation TKIs. This lack of data may lead to a deviation from the current treatment trends. Therefore, future trials such as NCT03410043 and NCT05167851 will provide valuable information on the efficacy and safety of 3rd-generation EGFR-TKIs in combination with LT. Despite these limitations, this meta-analysis provides valuable insights into the potential benefits of combining LT and EGFR-TKIs before disease progression in patients with advanced NSCLC with EGFR mutations.
Conclusions
In conclusion, the use of additional LT to primary tumor and/or metastatic sites before disease progression in patients with advanced NSCLC during first-line EGFR-TKI treatment leads to more favorable outcomes compared to EGFR-TKI monotherapy. Moreover, the use of LT 3 months after EGFR-TKI treatment might be a more effective approach.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for editing and reviewing this manuscript for English language.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-830/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-830/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-830/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Li S, de Camargo Correia GS, Wang J, et al. Emerging Targeted Therapies in Advanced Non-Small-Cell Lung Cancer. Cancers (Basel) 2023;15:2899. [Crossref] [PubMed]
- Majeed U, Manochakian R, Zhao Y, et al. Targeted therapy in advanced non-small cell lung cancer: current advances and future trends. J Hematol Oncol 2021;14:108. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref] [PubMed]
- Hsu WH, Yang JC, Mok TS, et al. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol 2018;29:i3-9. [Crossref] [PubMed]
- Lin JJ, Cardarella S, Lydon CA, et al. Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J Thorac Oncol 2016;11:556-65. [Crossref] [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46.
- Lee CK, Davies L, Wu YL, et al. Gefitinib or Erlotinib vs Chemotherapy for EGFR Mutation-Positive Lung Cancer: Individual Patient Data Meta-Analysis of Overall Survival. J Natl Cancer Inst 2017; [Crossref]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- La Monica S, Madeddu D, Tiseo M, et al. Combination of Gefitinib and Pemetrexed Prevents the Acquisition of TKI Resistance in NSCLC Cell Lines Carrying EGFR-Activating Mutation. J Thorac Oncol 2016;11:1051-63. [Crossref] [PubMed]
- Hata A, Katakami N, Kaji R, et al. Afatinib plus bevacizumab combination after acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: Multicenter, single-arm, phase 2 trial (ABC Study). Cancer 2018;124:3830-8. [Crossref] [PubMed]
- Lo PC, Dahlberg SE, Nishino M, et al. Delay of treatment change after objective progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer 2015;121:2570-7. [Crossref] [PubMed]
- Santarpia M, Altavilla G, Borsellino N, et al. High-dose Radiotherapy for Oligo-progressive NSCLC Receiving EGFR Tyrosine Kinase Inhibitors: Real World Data. In Vivo 2020;34:2009-14. [Crossref] [PubMed]
- Wang C, Lu X, Zhou Z, et al. The Efficacy of Upfront Intracranial Radiation with TKI Compared to TKI Alone in the NSCLC Patients Harboring EGFR Mutation and Brain Metastases. J Cancer 2019;10:1985-90. [Crossref] [PubMed]
- Goto Y, Tanai C, Yoh K, et al. Continuing EGFR-TKI beyond radiological progression in patients with advanced or recurrent, EGFR mutation-positive non-small-cell lung cancer: an observational study. ESMO Open 2017;2:e000214. [Crossref] [PubMed]
- Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:346-51. [Crossref] [PubMed]
- Ni Y, Bi J, Ye X, et al. Local microwave ablation with continued EGFR tyrosine kinase inhibitor as a treatment strategy in advanced non-small cell lung cancers that developed extra-central nervous system oligoprogressive disease during EGFR tyrosine kinase inhibitor treatment: A pilot study. Medicine (Baltimore) 2016;95:e3998. [Crossref] [PubMed]
- Kroeze SGC, Fritz C, Schaule J, et al. Continued versus Interrupted Targeted Therapy during Metastasis-Directed Stereotactic Radiotherapy: A Retrospective Multi-Center Safety and Efficacy Analysis. Cancers (Basel) 2021;13:4780.
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Suh YG, Cho J. Local ablative radiotherapy for oligometastatic non-small cell lung cancer. Radiat Oncol J 2019;37:149-55. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [Crossref] [PubMed]
- National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
- Cochrane Handbook for Systematic Reviews of Interventions. 1st ed. John Wiley & Sons, Ltd; 2008.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337-51. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16.
- Reeves BC, Deeks JJ, Higgins JP, et al. Including non-randomized studies on intervention effects. In: Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd.; 2019:595-620.
- Wu TH, Hsiue EH, Lee JH, et al. Best Response According to RECIST During First-line EGFR-TKI Treatment Predicts Survival in EGFR Mutation-positive Non-Small-cell Lung Cancer Patients. Clin Lung Cancer 2018;19:e361-72. [Crossref] [PubMed]
- Tang Y, Xia B, Xie R, et al. Timing in combination with radiotherapy and patterns of disease progression in non-small cell lung cancer treated with EGFR-TKI. Lung Cancer 2020;140:65-70. [Crossref] [PubMed]
- Al-Halabi H, Sayegh K, Digamurthy SR, et al. Pattern of Failure Analysis in Metastatic EGFR-Mutant Lung Cancer Treated with Tyrosine Kinase Inhibitors to Identify Candidates for Consolidation Stereotactic Body Radiation Therapy. J Thorac Oncol 2015;10:1601-7. [Crossref] [PubMed]
- Cochran WG. The Combination of Estimates from Different Experiments. Biometrics 1954;10:101-29.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [Crossref] [PubMed]
- Xu Q, Zhou F, Liu H, et al. Consolidative Local Ablative Therapy Improves the Survival of Patients With Synchronous Oligometastatic NSCLC Harboring EGFR Activating Mutation Treated With First-Line EGFR-TKIs. J Thorac Oncol 2018;13:1383-92. [Crossref] [PubMed]
- Elamin YY, Gomez DR, Antonoff MB, et al. Local Consolidation Therapy (LCT) After First Line Tyrosine Kinase Inhibitor (TKI) for Patients With EGFR Mutant Metastatic Non-small-cell Lung Cancer (NSCLC). Clin Lung Cancer 2019;20:43-7. [Crossref] [PubMed]
- Chan OSH, Lam KC, Li JYC, et al. ATOM: A phase II study to assess efficacy of preemptive local ablative therapy to residual oligometastases of NSCLC after EGFR TKI. Lung Cancer 2020;142:41-6. [Crossref] [PubMed]
- Ni Y, Ye X, Yang X, et al. Microwave ablation as local consolidative therapy for patients with extracranial oligometastatic EGFR-mutant non-small cell lung cancer without progression after first-line EGFR-TKIs treatment. J Cancer Res Clin Oncol 2020;146:197-203. [Crossref] [PubMed]
- Hsu KH, Huang JW, Tseng JS, et al. Primary Tumor Radiotherapy During EGFR-TKI Disease Control Improves Survival of Treatment Naïve Advanced EGFR-Mutant Lung Adenocarcinoma Patients. Onco Targets Ther 2021;14:2139-48. [Crossref] [PubMed]
- Wang X, Lu Z, Zeng Z, et al. Thoracic stereotactic body radiation therapy plus first-line tyrosine kinase inhibitors for patients with epidermal growth factor receptor-mutant polymetastatic non-small-cell lung cancer: A propensity-matched retrospective study. Medicine (Baltimore) 2021;100:e27279. [Crossref] [PubMed]
- Deng R, Liu J, Song T, et al. Primary lesion radiotherapy during first-line icotinib treatment in EGFR-mutated NSCLC patients with multiple metastases and no brain metastases: a single-center retrospective study. Strahlenther Onkol 2022;198:1082-93. [Crossref] [PubMed]
- Hu X, Li H, Kang X, et al. First-Line Tyrosine Kinase Inhibitors Combined With Local Consolidative Radiation Therapy for Elderly Patients With Oligometastatic Non-Small Cell Lung Cancer Harboring EGFR Activating Mutations. Front Oncol 2022;12:766066. [Crossref] [PubMed]
- Kuo SW, Chen PH, Lu TP, et al. Primary Tumor Resection for Stage IV Non-small-cell Lung Cancer Without Progression After First-Line Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor Treatment: A Retrospective Case-Control Study. Ann Surg Oncol 2022;29:4873-84. [Crossref] [PubMed]
- Peng P, Gong J, Zhang Y, et al. EGFR-TKIs plus stereotactic body radiation therapy (SBRT) for stage IV Non-small cell lung cancer (NSCLC): A prospective, multicenter, randomized, controlled phase II study. Radiother Oncol 2023;184:109681. [Crossref] [PubMed]
- Wang XS, Bai YF, Verma V, et al. Randomized Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated Non-Small Cell Lung Cancer. J Natl Cancer Inst 2023;115:742-8. [Crossref] [PubMed]
- Rossi S, Finocchiaro G, Noia VD, et al. Survival outcome of tyrosine kinase inhibitors beyond progression in association to radiotherapy in oligoprogressive EGFR-mutant non-small-cell lung cancer. Future Oncol 2019;15:3775-82. [Crossref] [PubMed]
- Kim H, Venkatesulu BP, McMillan MT, et al. Local Therapy for Oligoprogressive Disease: A Systematic Review of Prospective Trials. Int J Radiat Oncol Biol Phys 2022;114:676-83. [Crossref] [PubMed]
- Weiss J, Kavanagh B, Deal A, et al. Phase II study of stereotactic radiosurgery for the treatment of patients with oligoprogression on erlotinib. Cancer Treat Res Commun 2019;19:100126. [Crossref] [PubMed]
- Mok FST, Tong M, Loong HH, et al. Local ablative radiotherapy on oligo-progression while continued on epidermal growth factor receptor tyrosine kinase inhibitors in advanced non-small cell lung cancer patients: A longer cohort. Asia Pac J Clin Oncol 2022;18:614-24. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Kanazu M, Mori M, Kimura M, et al. Effectiveness of EGFR tyrosine kinase inhibitors in advanced non-small cell lung cancer patients with uncommon EGFR mutations: A multicenter observational study. Thorac Cancer 2021;12:90-6. [Crossref] [PubMed]
- Tjong MC, Louie AV, Iyengar P, et al. Local ablative therapies in oligometastatic NSCLC-upfront or outback?-a narrative review. Transl Lung Cancer Res 2021;10:3446-56. [Crossref] [PubMed]
- Petrelli F, Ghidini A, Ghidini M, et al. Better survival of patients with oligo- compared with polymetastatic cancers: a systematic review and meta-analysis of 173 studies. F1000Res 2021;10:423. [Crossref] [PubMed]
- Hu X, Li H, Liu H, et al. Assessing efficacy and safety of stereotactic body radiation therapy for oligometastatic non-small cell lung cancer with epidermal growth factor receptor (EGFR) wild type. Transl Cancer Res 2021;10:184-94. [Crossref] [PubMed]
- Wu YL, Planchard D, Lu S, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol 2019;30:171-210. [Crossref] [PubMed]


