
Outcomes of extracranial stereotactic body radiation therapy for induced oligometastatic non-small cell lung cancer on novel systemic therapy
Highlight box
Key findings
• The study demonstrates that stereotactic body radiation therapy (SBRT) for patients in the induced oligometastatic state with non-small cell lung cancer (NSCLC) exposed to novel therapies yields high local control, has an excellent safety profile and may lengthen the time to change of systemic treatment (TTCST).
• Patients presenting with induced oligopersistent and oligoprogressive disease who received SBRT achieved a progression-free survival (PFS) of 18.3 and 6.1 months and TTCST of 23.6 and 13.5 months respectively, which compares favorably to recent prospective studies of novel systemic therapies and to second or third line therapies.
What is known and what is new?
• Previous prospective studies have shown the benefits of radiation in the oligometastatic disease (OMD) state. However, these studies did not specifically focus on patients in the induced metastatic NSCLC getting exposed to novel therapies such as osimertinib and immune checkpoint inhibitors (pembrolizumab or nivolumab and ipilimumab). The findings suggests that the integration of SBRT should not be limited to patients in the genuine OMD state but may be widened to the induced OMD state for patients on novel systemic therapies, which challenges previous treatment paradigms.
What is the implication, and what should change now?
• SBRT should be considered as a viable treatment option for patients in the induced oligometastatic state, particularly those exposed to novel systemic therapies. This challenges the traditional approach of switching to subsequent lines of therapy and may improve PFS and TTCST.
Introduction
Lung cancer remains the leading cause of cancer-related mortality. In 2023, it is estimated that 127,070 will die from the disease in the United States alone, accounting for roughly 20% of all cancer deaths in the country (1). About 75% of non-small cell lung cancer (NSCLC) cases are diagnosed at an advanced stage and are usually treated with systemic therapy such as chemotherapy, immunotherapy and/or targeted therapy (2). Over the last few years, new systemic agents treating lung cancer have been introduced contributing to substantially improved survival. Molecular targeted therapeutics and immune checkpoint inhibitors (ICIs) have largely driven the progress. Osimertinib, a third-generation tyrosine kinase inhibitor (TKI) has demonstrated improved survival outcomes compared to first generation TKI’s in patients with epidermal growth factor receptor (EGFR)-mutated NSCLC (3). In patients with no targetable genetic alterations, ICIs such as pembrolizumab or nivolumab and ipilimumab, have become in many cases the standard of care either as monotherapy or in combination with chemotherapy (4,5).
Oligometastatic disease (OMD) describes a spectrum of limited metastatic spread (typically defined as up to 3–5 lesions) in which local therapy can potentially improve oncologic outcomes (6). The use of new imaging modalities like positron emission tomography/computed tomography (PET/CT) which enables a more sensitive identification of OMD as well as new systemic agents having better systemic response, has led to an increasing occurrence of OMD disease states, specifically up to 55% of cases in lung cancer (7). OMD was shown to be a common pattern of acquired resistance to programmed cell death-ligand 1 (PD-L1) blockers (8,9) and to TKIs with 15% to 47% of patients on first-generation TKIs and up to 73% of patients on osimertinib experiencing this pattern (10-12).
European Organisation for Research and Treatment of Cancer (EORTC) has proposed an OMD classification system of nine distinct OMD states by five classification factors to better characterize the different states of OMD (13). Therein, the authors describe the induced OMD state, a scenario in which patients with initially polymetastatic disease are exposed to an effective systemic agent and subsequently develop a limited metastatic burden. Induced OMD can be further classified as oligopersistant disease (OpersisD) which occurs when limited lesions persist after exposure to a systemic agent or oligoprogressive disease (OprogD) when a limited number of lesions progress while under the influence of a systemic agent (13-15).
Stereotactic body radiation therapy (SBRT) has emerged as a non-invasive, high-precision radiation therapy technique that results in a high local control (LC) rate (16-18). Prospective data has demonstrated that SBRT can improve oncologic outcomes of the OMD state with minimal side effects (19-23).
Currently, there is paucity of data on patients with induced OMD treated with SBRT while on newer systemic agents like third-generation TKI and ICIs. Therefore, we reviewed the data of all induced OMD patients treated with SBRT in our institution. We evaluated outcomes and analyzed potential prognostic factors that can predict which patients are most likely to benefit from this approach. Patients included were treated with SBRT for OpersisD as consolidation and SBRT for OprogD as an attempt to achieve improved radiographic progression-free survival (PFS) and lengthen the time to change of systemic treatment (TTCST). We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-802/rc).
Methods
Study design and patient eligibility
In this retrospective single-center study conducted at the Department of Radiation and Medical Oncology of Haddasah Medical Center, Jerusalem, we assessed the efficacy of metastatic-directed extracranial SBRT given to patients with stage IV NSCLC between January 2017 to December 2022. Inclusion criteria were: stage IV NSCLC with polymetastatic disease (>5 lesions including primary and nodes), development of induced OpersisD or OprogD during systemic treatment with either osimertinib or ICIs (pembrolizumab or nivolumab and ipilimumab) with or without chemotherapy, treatment with extracranial metastatic-directed SBRT to 1–3 lesions while continuing treatment with novel systemic therapy. Patients with initial poor response to systemic therapy who received palliative intent radiotherapy were not included in this analysis.
OpersisD was defined as 1–3 sites of persistent disease after receiving systemic therapy and OprogD was defined as 1–3 sites of disease progressing while on systemic therapy. In both OMD scenarios, PET/CT was utilized to determine whether a patient was in OpersisD or OprogD state. For the OpersisD state, if after 2–3 consecutive PET/CT scans from initiation of treatment, there was both residual CT finding and uptake on PET and the lesion was considered encompassable by treating radiation oncologist, SBRT was performed (OpersisD state). Patients were considered in OprogD state if serial PET/CT demonstrated a new lesion, an increase in size in previous existing lesion or increase in PET uptake on two serial exams of an existing lesion. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by Hadassah Medical Center institutional review board (Number: HMO-0736-21). The written informed consent was waived for this retrospective analysis.
Treatment and follow up
Patients were immobilized per the standard institutional protocol and underwent a CT simulation scan using 2- to 3-mm slice thickness. For thoracic and/or upper abdominal lesions, 4-dimensional CT data were used to create internal target volumes (ITV). For each treated site, a gross tumor volume (GTV) and/or ITV was created with an additional 3–5 mm margin for the planning target volume (PTV). Organs at risk (OARs) were delineated per RTOG consensus guidelines. The highest isodose line of the prescribed dose was required to cover >95% of PTV and 99% of the GTV/ITV. OARs were given priority over PTV coverage. The treatment plan was generated using volumetric modulated arc or intensity modulated radiation therapy and patients were treated on the Varian Truebeam. Dose fractionation schemes included 30–54 Gy in 3 fractions, 48 Gy in 4 fractions, 30–50 Gy in 5 fractions and 60 Gy in 8 fractions. The biologically effective dose (BED) was determined according to α/β=10 Gy (BED10). GTV was defined as the tumor volume visible in a PET/CT scan.
Systemic agents included osimertinib for patients with EGFR mutation and ICI (pembrolizumab/nivolumab and ipilimumab) as monotherapy or in combination with chemotherapy for patients with tumor expressing PD-L1. Radiotherapy was delivered between cycles of IV systemic therapy. Osimertinib was held 2 days prior and 2 days following the final SBRT treatment. Patients were radiographically followed up using PET/CT/CT scan and brain magnetic resonance imaging (MRI) every 3 to 6 months. Patients were invited for regular check-ups every 3 months.
End points
The primary endpoints were PFS and TTCST. PFS was defined as the time interval between the first course of SBRT and disease progression shown in imaging. TTCST was defined as the time interval between the first course of SBRT until an indication to change the systemic treatment either to chemotherapy or to palliative treatment. Secondary endpoints included overall survival (OS) and LC. OS was calculated as the time from first SBRT until death or final follow up. LC was calculated from the time of any SBRT course until time of local failure. Local failure was defined as progressive consolidation on CT within the treatment site confirmed with PET/CT +/− biopsy. Toxicity was determined using Common Terminology Criteria for Adverse Events (CTCAE) version 5 criteria.
Statistical analysis
For analyzing LC, Fisher’s exact test was used to test whether two categorical variables were associated. The comparison of a quantitative variable between two independent groups was performed by using the non-parametric Mann-Whitney test. Non-parametric tests were used due to the small sample size of events. The Kaplan-Meier model was applied for analyzing the effect of categorical variables on PFS or TTCST, with the log-rank test for comparing survival curves. The Cox regression model was applied for testing the effect of quantitative variables on PFS and TTCST. This model was also applied for the multivariate table used for survival analysis, yielding hazard ratios (HR) with 95% confidence intervals (CI). All statistical tests were two-tailed, and a P value of 5% or less was considered statistically significant.
Results
We identified 49 patients treated with SBRT to either OprogD NSCLC (65.3%) or OpersisD NSCLC (34.7%). Patient and tumor characteristics are summarized in Table 1 and treatment details are shown in Table 2. Patients’ median age was 64 years (range, 39–82 years), 53.1% were male. Median follow up time from first SBRT course was 18.8 months. Most patients were diagnosed with adenocarcinoma (95.9%). A total of 28 patients (57.2%) received initial treatment prior to the introduction of the novel systemic agent, including 9 patients (18.4%) treated with first generation TKI, 9 patients (18.4%) treated with stereotactic radiosurgery (SRS), 6 patients (12.2%) treated with external beam radiotherapy (EBRT), and 4 patients (8.2%) treated with standard first-line chemotherapy. ECOG for all patients was 0 or 1 prior to SBRT.
Table 1
| Characteristics | Values (n=49) |
|---|---|
| Age (years), median [range] | 64 [39–82] |
| Sex, n (%) | |
| Male | 26 (53.1) |
| Female | 23 (46.9) |
| Tumor histology, n (%) | |
| Adenocarcinoma | 47 (95.9) |
| Squamous cell carcinoma | 2 (4.1) |
| Prior treatment, n (%) | |
| First generation TKI | 9 (18.4) |
| Chemotherapy | 4 (8.2) |
| EBRT | 6 (12.2) |
| SRS | 9 (18.4) |
| Oligometastatic disease type, n (%) | |
| Oligoprogressive | 32 (65.3) |
| Oligopersistent | 17 (34.7) |
| EGFR, n (%) | |
| Ex.19 Del | 16 (32.7) |
| Ex.21L858R | 9 (18.4) |
| Ex.19 Point M | 1 (2.0) |
| Ex.21 Point M | 1 (2.0) |
| Ex.18/Ex.20 | 2 (4.1) |
| Wild type | 20 (40.8) |
| PD-L1, n (%) | |
| <1% | 4 (8.2) |
| 1–50% | 5 (10.2) |
| >50% | 13 (26.5) |
TKI, tyrosine kinase inhibitor; EBRT, external beam radiation therapy; SRS, stereotactic radiosurgery; EGFR, epidermal growth factor receptor; PD-L1, programmed cell death-ligand 1.
Table 2
| Characteristics | Values (n=49) |
|---|---|
| Systemic agent, n (%) | |
| Osimertinib | 27 (55.1) |
| ICI | 14 (28.6) |
| ICI + chemotherapy | 8 (16.3) |
| Initial response to systemic agent, n (%) | |
| CR | 7 (14.3) |
| PR | 35 (71.4) |
| SD | 7 (14.3) |
| Time from systemic treatment onset to SBRT (months), median (range) | |
| OpersisD | 5.8 (1.8–15.3) |
| OprogD | 15.3 (2.1–40.6) |
| Number of sites treated per patient, n (%) | |
| 1 | 29 (59.2) |
| 2 | 14 (28.6) |
| 3 | 4 (8.2) |
| 4 | 2 (4.1) |
| Lesion treated with SBRT (n=77) | |
| Site, n (%) | |
| Lung | 44 (57.1) |
| Adrenal | 10 (13.0) |
| Lymph node | 9 (11.7) |
| Bone | 7 (9.0) |
| Liver | 5 (6.5) |
| Mediastinum | 2 (2.6) |
| GTV (cc) | |
| Median | 8.2 |
| Mean | 16.7 |
| Range | 0.98–121.57 |
| BED10 (Gy) | |
| Median | 100 |
| Range | 48–151.2 |
ICI, immune checkpoint inhibitor; CR, complete response; PR, partial response; SD, stable disease; SBRT, stereotactic body radiation therapy; OprogD, oligoprogressive disease; OpersisD, oligopersistent disease; GTV, gross tumor volume; BED10, biologically effective dose (α/β=10).
All patients were treated a minimum of 8 weeks before SBRT with osimertinib (55.1%), ICI agent as monotherapy (28.6%) or ICI in combination with chemotherapy (16.3%). In response to the novel systemic agents 7 patients (14.3%) achieved complete response, 35 patients (71.4%) had a partial response and 7 patients (14.3%) had stable disease. For the entire cohort, median time from start of systemic agent to SBRT was 7.7 months (5.8 months in OpersisD group and 15.3 months in OprogD group). In 20 patients, more than one course of SBRT was administered, including 13 as salvage treatment for disease progression and 7 who received two concurrent courses to different sites. Twenty-nine patients (59.2%) received one SBRT course, 14 patients (28.6%) received two courses, 4 patients (8.2%) received three courses, and 2 patients (4.1%) received four courses. Overall, 77 lesions were treated with SBRT. Lung was the most common site (57.1%), followed by adrenal (13.0%), lymph node (11.7%), bone (9.0%), liver (6.5%) and mediastinum (2.6%). Dose fractionation schemes included 30–54 Gy in 3 fractions, 48 Gy in 4 fractions, 35–50 Gy in 5 fractions, 60 Gy in 8 fractions. Median GTV was 8.2 cc.
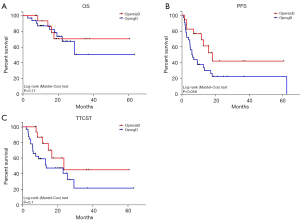
At a median of 18.8 months follow-up from first SBRT, 73.5% remained alive; 10 OprogD patients (31.25%) and 3 OpersisD patients (17.64%) passed away. The 1-year OS was 91.7% for OpersisD and 83.3% for OprogD (Figure 1A). LC was achieved in 71 lesions treated (92.2%), 6 lesions (7.8%) locally progressed at a median time of 14.0 months including 4 lung, 1 bone and 1 adrenal metastasis. In the OpersisD group, the median PFS was 18.3 months (95% CI: 10.34–26.33), while in the OprogD group, the median PFS was 6.1 months (95% CI: 3.24–8.96) (HR: 2.14; 95% CI: 0.96–4.77; P=0.06; Figure 1B). Thirteen patients received one or more salvage SBRT treatments (total: 17) after disease progression. Median TTCST was 23.6 months in the OpersisD group and 13.5 months in the OprogD group (HR: 2.11; 95% CI: 0.84–5.29; P=0.11; Figure 1C). At the last follow-up, there was no indication to change the systemic treatment for 24 patients (48.9%) of which 16 patients (32.6%) did not experience radiographic progression.
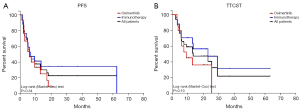
When subdivided by OMD type, OprogD patients receiving osimertinib vs. Immunotherapy had a median PFS of 6.1 vs. 6.5 months (P=0.34) respectively (Figure 2A). OprogD patients receiving osimertinib vs. immunotherapy had a median TTCST of 9.1 vs. 23.1 months (P=0.19) respectively (Figure 2B).
A multivariable model was used to better assess the effect of the different OMD groups and to investigate potential prognostic factors (Table 3). Patients who had OprogD type of disease had shorter PFS (HR: 2.73; 95% CI: 1.17–6.39; P=0.02) and shorter TTCST (HR: 3.07; 95% CI: 1.12–8.45; P=0.03). Patients treated with osimertinib had shorter PFS (HR: 2.20; 95% CI: 1.01–4.82; P=0.048) and shorter TTCST (HR: 2.83; 95% CI: 1.09–7.33; P=0.032). GTV, age and gender had no effect on PFS and TTCST. Male gender demonstrated a trend toward shorter PFS (HR: 2.10; 95% CI: 0.99–4.35; P=0.052) and a similar trend toward TTCST (HR: 2.34; 95% CI: 0.99–5.53; P=0.053).
Table 3
| Variable | PFS from SBRT | TTCST from SBRT | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| GTV | 1.01 | 0.10–1.03 | 0.103 | 1.02 | 0.10–1.03 | 0.088 | |
| Age | 1.02 | 0.98–1.06 | 0.365 | 1.01 | 0.96–1.06 | 0.746 | |
| Male vs. female | 2.10 | 0.99–4.35 | 0.052 | 2.34 | 0.99–5.53 | 0.053 | |
| Osimertinib vs. ICI | 2.20 | 1.01–4.82 | 0.048 | 2.83 | 1.09–7.33 | 0.032 | |
| OprogD vs. OpersisD | 2.73 | 1.17–6.39 | 0.020 | 3.07 | 1.12–8.45 | 0.030 | |
PFS, progression-free survival; TTCST, time to change of systemic treatment; OMD, oligometastatic disease; NSCLC, non-small cell lung cancer; SBRT, stereotactic body radiation therapy; GTV, gross tumor volume; ICI, immune checkpoint inhibitor; OprogD, oligoprogressive disease; OpersisD, oligopersistent disease.
In regards to toxicity, 9 patients (18.4%) had grade 1 pneumonitis, 1 patient (2%) had grade 2 pneumonitis and grade 2 bronchoconstriction, 1 patient (2%) had grade 2 pneumonitis and grade 2 pain and 1 patient (2%) had grade 3 pneumonitis.
Discussion
EORTC has classified nine distinct subtypes of OMD and created a distinction between those patients with development of genuine OMD and induced OMD (13). The evolution of treatment paradigms in NSCLC, including the use of mutation-specific biological agents and immunotherapy, has led to an increasing number of patients transitioning from polymetastatic disease to an induced OMD state due to persistent clones (OpersisD) or resistant clones (OprogD) (7-12). The utilization of ablative radiotherapy in the induced OMD state, however, remains a subject of debate, given potential for high-risk toxicity with concurrent systemic therapy. It remains unclear whether the benefit of local therapy applies to the induced OMD state and there are uncertainties surrounding the timing of delivery in the era of novel systemic therapies. Our study highlights the potential of SBRT for patients with induced OpersisD or OprogD in NSCLC who are concurrently receiving novel therapies, demonstrating a robust capacity for local disease control. Furthermore, our approach suggests that SBRT may extend the duration of effective treatment with novel systemic agents with an excellent safety profile. This underlines the significance of SBRT as a feasible and safe strategy for patients in the induced OMD state, contributing not only to LC but also to the preservation of meaningful additional time on novel systemic therapies.
Gomez and Iyenger both demonstrated that the addition of consolidative radiation to the OpersisD state after 3 months of receiving systemic therapy improves PFS (14.7 vs. 4.4 months and 9.7 vs. 3.5 months) with the former also demonstrating an OS benefit. In both trials, patients with both genuine and induced OMD were randomized after 3 months of systemic therapy and none were treated with current third-generation TKIs or ICI (19,22). Our study specifically studied patients in the induced OMD state exposed to novel therapies and compared favorably to these studies. After a median of 7.7 months of systemic therapy before SBRT, patients treated with SBRT for local consolidation achieved a radiographic PFS of 18.3 months, and TTCST of 23.6 months and a median OS not yet reached. TTCST endpoint was collected in addition to PFS, as salvage SBRT can often be delivered in an attempt to prolong time on first-line agents as second- and third-line therapies are often less effective (24). Moreover, this endpoint is being used in current ongoing prospective studies on the role of local therapy in OMD as it can represent the timing of polymetastatic progression in which, to date, there is no role for ablative radiotherapy (25).
The only phase 3 data available in OMD, is the SINDAS trial, which randomized patients with genuine OMD EGFR mutated NSCLC to first generation TKI with or without SBRT to all sites of disease. This study demonstrated both a median PFS and OS benefit (23). Given the inability to treat all sites of disease upfront in polymetastatic cancer, our study differs in that we first expose the patient to the novel systemic therapy and then utilize PET/CT follow up to determine which patients may benefit from local therapy. Currently, we are awaiting the results from phase 2 Northstar trial randomizing patients with EGFR mutation after three months of osimertinib to local consolidative therapy (26). Recently the NRG LU002 trial randomizing patients after 3 months of systemic therapy including immunotherapy was closed to accrual as it did not meet the phase II PFS endpoint (27). We await for final analysis from this trial however it is important to consider that an ablative dose was not required for radiotherapy treatment (BED more than 100 Gy) which may have impacted the results. Possibly future studies may consider endpoints such as TTCST in order to capture patients who might benefit from salvage SBRT without the need to switch therapies. Indeed, while patients who undergo repeat SBRT have shorter PFS, there is retrospective data to suggest that they may have an improved OS compared to single course SBRT (28).
Our investigation also extends to patients with oligoprogressive NSCLC, offering insights into the benefits of SBRT as an alternative to switching to subsequent lines of therapy. Aligning with recent findings from the CURB trial (20), our study underscores the potential of SBRT to prolong radiographic PFS of 6.1 months and TTCST of 13.5 months. Interestingly we did note that those patients with EGFR mutation receiving osimertinib had similar PFS but numerically shorter TTCST than the immunotherapy group, suggesting faster progression to a polymetastatic state in the osimertinib group. This may support that the role of additional biopsy at the time of oligoprogression in order to look for new targetable mutations (29). Of note, the CURB trial only enrolled 14% of 59 patients with EGFR mutation on TKI, therefore, demonstrating the need for prospective data on each specific subtype and suggesting maybe upfront or consolidation radiation in EGFR may be a better approach than waiting for progression, a hypothesis also supported by an analysis of Zeng and colleagues (30). Imperative to note that our study included a total of 27 patients on osimertinib in both groups and despite retrospective data highlighting higher risks of complications with osimertinib (31) our study demonstrated a favorable safety profile with concurrent treatment. In the entire cohort, only one grade 3 and no grade 4–5 toxicities were seen.
Our study also highlights the novel use of serial PET/CT imaging to determine which patients may benefit from local treatment. Most of the prospective data on the treatment of OMD primarily used CT imaging without PET adjunct (19,22). Recently, Christ and colleagues demonstrated that OMD state can be frequently found during their disease course when utilizing serial PET/CT and MRI imaging, including 55% of patients with lung cancer (7). In the Oriole trial, treatment guidance with PET PSMA in prostate cancer demonstrated improved PFS and distant metastasis-free survival compared to those patients in which all PET avid lesions were not treated (32). Further study into whether PET/CT should be performed routinely for follow up in stage IV NSCLC and how it affects management and treatment outcomes should be studied.
While our results highlight the favorable outcomes in OpersisD compared to OprogD, the optimal timing for SBRT intervention remains inconclusive. Given the favorable outcomes in both groups compared to recently published prospective data of osimertinib (33) and chemotherapy-ICI (34-36), this study suggests that the integration of SBRT should not be limited to patients in the genuine OMD state or oligorecurrent state and may be widened to the induced OMD state for patients on novel systemic therapies. Similarly, a validation study of the EORTC classification schema, the SABR-5 trial including patients of all histologies demonstrated favorable outcomes; however, it only included 5 patients in the induced OpersisD state and 27 in the induced OprogD state (37). Given the low numbers of patients in OpersisD, the prognostic power of the cohort was largely attributable to chronicity and oligoprogression. While our study included more patients and was limited purely to NSCLC, it is subject to limitations inherent to its retrospective design and patient selection. Moreover, selection bias and confounding factors, such as varied initial reactions to immunotherapy, may influence the observed outcomes.
Conclusions
Our study contributes to the ongoing discourse surrounding the role of SBRT in induced OMD lung cancer states exposed to novel systemic therapies. At a median time of 18.8 months follow-up SBRT yielded a high LC rate of 92.2% with an excellent safety profile for patients with induced oligometastatic NSCLC while on novel therapies. Patients presenting with induced oligopersistent and OprogD who received SBRT achieved a PFS of 18.3 and 6.1 months and TTCST of 23.6 and 13.5 months respectively. Additionally, routine follow-up PET/CT imaging was used in our study to better diagnose OMD thereby capturing patients who might benefit from local treatment. While acknowledging the limitations, the findings underscore the potential benefits of SBRT in terms of LC and extended TTCST in a cohort of induced OMD with NSCLC on novel therapies. As the field continues to evolve, prospective trials and further investigation are warranted to unravel the optimal integration of SBRT into the treatment landscape for such patients.
Acknowledgments
This article was written as part of the requirements of the Faculty of Medicine, The Hebrew University-Hadassah Medical School, Jerusalem 91120, Israel (Department of Military Medicine “Tzameret”) for an MD degree.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-802/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-802/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-802/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-802/coif). The authors have no conflicts of interest to declare.
Ethical Statement: the authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the Hadassah Medical Center institutional review board (Number: HMO-0736-21), and the written informed consent was waived for this retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Blandin Knight S, Crosbie PA, Balata H, et al. Progress and prospects of early detection in lung cancer. Open Biol 2017;7:170070. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:198-211. [Crossref] [PubMed]
- Beckham TH, Yang TJ, Gomez D, et al. Metastasis-directed therapy for oligometastasis and beyond. Br J Cancer 2021;124:136-41. [Crossref] [PubMed]
- Christ SM, Pohl K, Muehlematter UJ, et al. Imaging-Based Prevalence of Oligometastatic Disease: A Single-Center Cross-Sectional Study. Int J Radiat Oncol Biol Phys 2022;114:596-602. [Crossref] [PubMed]
- Schoenfeld AJ, Rizvi HA, Memon D, et al. Systemic and Oligo-Acquired Resistance to PD-(L)1 Blockade in Lung Cancer. Clin Cancer Res 2022;28:3797-803. [Crossref] [PubMed]
- Heo JY, Yoo SH, Suh KJ, et al. Clinical pattern of failure after a durable response to immune check inhibitors in non-small cell lung cancer patients. Sci Rep 2021;11:2514. [Crossref] [PubMed]
- Basler L, Kroeze SG, Guckenberger M. SBRT for oligoprogressive oncogene addicted NSCLC. Lung Cancer 2017;106:50-7. [Crossref] [PubMed]
- Zhou Y, Yu F, Zhao Y, et al. A narrative review of evolving roles of radiotherapy in advanced non-small cell lung cancer: from palliative care to active player. Transl Lung Cancer Res 2020;9:2479-93. [Crossref] [PubMed]
- Schmid S, Klingbiel D, Aeppli S, et al. Patterns of progression on osimertinib in EGFR T790M positive NSCLC: A Swiss cohort study. Lung Cancer 2019;130:149-55. [Crossref] [PubMed]
- Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020;21:e18-28. [Crossref] [PubMed]
- Nguyen KT, Sakthivel G, Milano MT, et al. Oligoprogression in non-small cell lung cancer: a narrative review. J Thorac Dis 2022;14:4998-5011. [Crossref] [PubMed]
- Tjong MC, Louie AV, Iyengar P, et al. Local ablative therapies in oligometastatic NSCLC-upfront or outback?-a narrative review. Transl Lung Cancer Res 2021;10:3446-56. [Crossref] [PubMed]
- Tsao MN, Ven L. Stereotactic Body Radiation Therapy for Extracranial Oligometastatic Non-small-cell Lung Cancer: A Systematic Review. Clin Lung Cancer 2020;21:95-105.e1. [Crossref] [PubMed]
- Mavrikios A, Remon J, Quevrin C, et al. Local control strategies for management of NSCLC with oligoprogressive disease. Cancer Treat Rev 2023;120:102621. [Crossref] [PubMed]
- Ouyang W, Yu J, Nuerjiang S, et al. Stereotactic body radiotherapy improves the survival of patients with oligometastatic non-small cell lung cancer. Cancer Med 2019;8:4605-14. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Tsai CJ, Yang JT, Shaverdian N, et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study. Lancet 2024;403:171-82.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. [Crossref] [PubMed]
- Wang XS, Bai YF, Verma V, et al. Randomized Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated Non-Small Cell Lung Cancer. J Natl Cancer Inst 2023;115:742-8. [Crossref] [PubMed]
- Auclin E, Benitez-Montanez J, Tagliamento M, et al. Second-line treatment outcomes after progression from first-line chemotherapy plus immunotherapy in patients with advanced non-small cell lung cancer. Lung Cancer 2023;178:116-22. [Crossref] [PubMed]
- Alomran R, White M, Bruce M, et al. Stereotactic radiotherapy for oligoprogressive ER-positive breast cancer (AVATAR). BMC Cancer 2021;21:303. [Crossref] [PubMed]
- Gandhi S. Osimertinib, Surgery, and Radiation Therapy in Treating Patients With Stage IIIB or IV Non-small Cell Lung Cancer With EGFR Mutations, NORTHSTAR Study. NCT03410043, Updated: September 28, 2023, Accessed November 23.
- Iyengar P. Maintenance Chemotherapy With or Without Local Consolidative Therapy in Treating Patients With Stage IV Non-small Cell Lung Cancer. NCT03137771, Updated: December 08, 2023, Accessed January 2024. Available online: https://www.nrgoncology.org/Clinical-Trials/Protocol/nrg-lu002?filter=nrg-lu002
- Willmann J, Adilovic S, Vlaskou Badra E, et al. Repeat stereotactic body radiotherapy for oligometastatic disease. Radiother Oncol 2023;184:109671. [Crossref] [PubMed]
- Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:339-57. [Crossref] [PubMed]
- Zeng Y, Ni J, Yu F, et al. The value of local consolidative therapy in Osimertinib-treated non-small cell lung cancer with oligo-residual disease. Radiat Oncol 2020;15:207. [Crossref] [PubMed]
- Smith CP, Xiang M, Yoon SM, et al. Brief Report: Severe Pneumonitis After Combined Thoracic Radiotherapy and Osimertinib. JTO Clin Res Rep 2023;4:100468. [Crossref] [PubMed]
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020;6:650-9. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Novello S, Kowalski DM, Luft A, et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J Clin Oncol 2023;41:1999-2006. [Crossref] [PubMed]
- Baker S, Mou B, Jiang W, et al. Validation of the Prognostic Utility of ESTRO/EORTC Oligometastatic Disease Classification: A Secondary Analysis From the Population-Based Phase II SABR-5 Trial. Int J Radiat Oncol Biol Phys 2022;114:849-55. [Crossref] [PubMed]


