
A survival nomogram model for patients with resectable non-small cell lung cancer and lymph node metastasis (N1 or N2) based on the Surveillance, Epidemiology, and End Results Database and single-center data
Highlight box
Key findings
• A novel survival nomogram model was developed for predicting the long-term survival of patients with resectable non-small cell lung cancer (NSCLC) and positive lymph node (LN) metastasis (N1 or N2).
What is known and what is new?
• Chemotherapy is the main postoperative comprehensive treatment strategy for patients with LN metastasis of NSCLC. The necessity of radiotherapy in this particular group of patients is subject to debate, and no accurate model exists for predicting the prognosis of patients with LN metastasis.
• Our study compared the LN stage (N), number of positive lymph nodes (NPLN), lymph node ratio (LNR), and log odds of positive lymph nodes (LODDS). The nomogram containing the NPLN and LODDS could accurately predict the survival of patients with N1 or N2 NSCLC.
What is the implication, and what should change now?
• The nomogram containing NPLN and LODDS is an accurate survival prediction tool for patients with N1 or N2 NSCLC. Patients with LN metastasis can benefit from chemotherapy, but there is no evidence supporting the necessity of radiotherapy for patients with resectable NSCLC.
Introduction
Lung cancer is the leading cause of cancer-specific death in men and women. The prevalence of cancer screening contributes to improving the proportion of patients who receive localized diagnoses (1). Most patients with non-small cell lung cancer (NSCLC) are at an advanced stage when they are diagnosed, only a few patients with stage III NSCLC undergo surgery, and most patients are treated with chemotherapy and/or radiation. Some patients who receive targeted and immunotherapy drugs for advanced NSCLC can receive benefit, but the 5-year overall survival (OS) of patients with NSCLC remains at approximately 20% (2). We hypothesize that the high mortality in NSCLC is due to the high recurrence rate, the absence of specific genetic mutations, and a lack of high programmed death-ligand 1 (PD-L1) expression among patients. Despite numerous studies showing that postoperative cisplatin-based chemotherapy significantly improves survival in patients with NSCLC (3,4), there is insufficient evidence to predict prognosis and guide postoperative chemotherapy after surgery in those with NSCLC and lymph node (LN) metastasis (N1 and N2). The role of radiation therapy in patients with resectable LN-positive NSCLC is also controversial. Some researchers believe chemoradiotherapy has advantages for patients with stage III NSCLC (5). However, many patients cannot be treated with radiation due to the risks associated with higher age or those posed by multiple comorbidities, and the necessity of radiation therapy in patients with operable stage III NSCLC has not been adequately discussed. Another viewpoint is that radiotherapy with sequential adjuvant chemotherapy does not improve the survival of patients with N2 lung cancer (6).
Due to the above-described uncertainties, we established a nomogram for predicting the long-term survival outcomes of patients with NSCLC and LN metastasis (N1 or N2) based on data from the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/). This novel survival model was verified through external single-center data. Log odds of positive lymph nodes (LODDS), a novel and promising ratio-based LN staging system, was also applied in the nomogram model and compared with the N stage and number of positive lymph nodes (NPLN). We present this article in accordance with the TRIPOD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-119/rc).
Methods
Training cohort data collection and selection criteria
The data of patients with resectable NSCLC and LN metastasis (N1 or N2) were extracted from the SEER database using the SEER*Stat program (v. 8.3.9.2). The database name was Incidence-SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying), and the SEER Program permission number was 19642-Nov2020. The patients with NSCLC were diagnosed between 2010 and 2015. The selection formula was as follows: {Site and Morphology. TNM 7/CS v0204+ Schema} = “Lung” AND {Stage - 7th edition. Derived AJCC N, 7th ed (2010-2015)} = ‘N1’, ‘N1a’, ‘N1b’, ‘N1c’, ‘N1mi’, ‘N1NOS’, ‘N2’, ‘N2a’, ‘N2b’, ‘N2c’, ‘N2NOS’ AND {Race, Sex, Year Dx, Registry, County. Year of diagnosis} = ‘2010’, ‘2011’, ‘2012’, ‘2013’, ‘2014’, ‘2015’. Variables extracted from the SEER database included patient ID, age at diagnosis, sex, race recode, marital status at diagnosis, year of diagnosis, primary site, grade, laterality, International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) histology and behavior, derived American Joint Committee on Cancer (AJCC) T stage, derived AJCC N stage, derived AJCC M stage, Rx summary (surgery at primary site), radiation sequence with surgery, chemotherapy recode, regional nodes examined, regional nodes positive, survival months, SEER cause-specific death classification, SEER other cause of death classification, and vital status recode. The T stage and N stage mentioned above were pathological tumor, node, metastasis (TNM). Patients who met the following inclusion criteria were included: (I) age ≥18 years; (II) histological pathology: confirmed NSCLC including codes 8010, 8012, 8013, 8014,8015, 8020, 8021, 8022, 8031, 8032, 8046, 8050–8052, 8070–8078, 8140–8147, 8250–8255, 8260, 8310, 8323, 8430, 8480, 8481, 8482, 8490, 8560, and 8570–8575 based on the ICD-O-3 histology and behavior malignant code (7); (III) radical surgery (R0 resection) and systematic LN dissection of verified N1 or N2 stage disease; (IV) information on chemotherapy use available; and (V) complete record of sequence radiotherapy information (patients who received postoperative adjuvant radiotherapy or did not receive radiotherapy). The exclusion criteria were as follows: (I) age <18 years or >80 years; (II) pathological diagnosis of N0 or N3; (III) other malignant tumors; (IV) confirmed distant metastasis (M1); (V) preoperative radiotherapy; (VI) no radical surgery or systematic lymphadenectomy; and (VII) missing TNM staging system or survival time data. Tumor staging was performed according to the seventh edition of the AJCC.
External validation cohort and selection criteria
An external validation cohort of patients treated from June 2015 to December 2016 in the Department of Thoracic Surgery of the First Affiliated Hospital, Zhejiang University School of Medicine, was used to validate the developed nomogram reliably. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. 2023-0012) and individual consent for this retrospective analysis was waived.
The validation cohort included 117 patients with postoperative N1 or N2 NSCLC. The inclusion and exclusion criteria were the same as those in the training cohort from the SEER database. The last follow-up for the cohort was in December 2022, and the primary outcome was OS.
Data processing of the number of dissected lymph nodes (NDLN), NPLN, lymph node ratio (LNR), and LODDS systems
The LNR was defined as the ratio of positive LNs divided by the total NDLN and was calculated using the following formula: NPLN/NDLN. LODDS was calculated as follows: LODDS= log(NPLN + 0.50)/(NDLN − NPLN + 0.50). 0.50 was added to the numerator and denominator (8) to avoid an infinite number.
Establishing a nomogram model for survival
The relationship between potential clinical risk factors and OS were estimated using Cox proportional hazards regression models, and the hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated. Based on the results of univariate and multivariate Cox regression conducted via R version 4.2.2 software (The R Foundation of Statistical Computing), we included the applicable independent risk factors in the nomogram to predict the 1-, 3- and 5-year OS probabilities of patients with N1 or N2 NSCLC who underwent radical surgery using.
Prediction accuracy of the nomogram model
The discrimination ability of the nomogram model was determined via the concordance index (C-index) (9). The closer the C-index is to 1, the better the prediction ability of the nomogram model. Moreover, we used the corresponding area under the curves (AUCs) generated by the 1-, 3- and 5-year OS receiver operating characteristic (ROC) curves to estimate the predictive accuracy of the nomogram (10). The calibration curve is a diagram reflecting the association between the observed outcome frequencies and the predicted probabilities. A predicted calibration curve closer to the standard curve indicates that the predictive ability of the nomogram model is more efficient.
Predictive performance of different nodal staging systems
The four different multivariable Cox regression models included model 1 (N stage), model 2 (N stage + NPLN), model 3 (N stage + LODDS), and model 4 (N stage + NPLN + LODDS). Other potential predictors in the univariate analysis were simultaneously entered into the Cox regression model. Homogeneity and discrimination ability were evaluated with the likelihood ratio (LR) test and C-index, respectively.
Statistical methods
Statistical tests were performed with R version 4.2.2 and SPSS 22.0 (IBM Corp.). HRs were determined through univariate and multivariate Cox proportional hazards models. OS was defined as the number of months from diagnosis to death or the last follow-up (December 22, 2022) for censored observations. The median risk score was used to determine the high-risk and low-risk groups and was calculated with the “survival” R package. Kaplan-Meier survival analysis of different independent risk factors included in the nomogram model was performed, and the statistical difference was analyzed using the log-rank test.
Results
Study cohort
Data from 5,132 patients diagnosed with NSCLC in 2010–2015 and with LN metastasis (N1 or N2) were extracted from the SEER database. A total of 117 patients diagnosed in 2015–2016 were enrolled as the external validation cohort. In the training cohort, 1,302 (25.4%) patients were aged <60 years, and 2,458 (47.9%) were female. A total of 3,546 (69.1%) patients received chemotherapy, and 1,276 (24.9%) patients received postoperative adjuvant radiotherapy. In the external validation cohort, 68 (58.1%) patients were aged <60 years, and 56 (47.9%) patients were female. Among these patients, 53 (45.3%) received adjuvant chemotherapy, and 21 (17.9%) received radiation after surgery. The baseline characteristics of the patients in the training and external validation cohorts are presented in Table 1.
Table 1
| Variables | Training cohort | Validation cohort | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (years) | |||||
| <60 | 1,302 | 25.4 | 68 | 58.1 | |
| ≥60 | 3,830 | 74.6 | 49 | 41.9 | |
| Race | |||||
| White | 4,165 | 81.2 | N/A | ||
| Black | 504 | 9.8 | N/A | ||
| Other | 463 | 9.0 | N/A | ||
| Marital status | |||||
| Single | 651 | 12.7 | N/A | ||
| Married | 3,137 | 61.1 | N/A | ||
| Other | 1,344 | 26.2 | N/A | ||
| Sex | |||||
| Female | 2,458 | 47.9 | 56 | 47.9 | |
| Male | 2,674 | 52.1 | 61 | 52.1 | |
| Primary site | |||||
| Lower lobe | 1,826 | 35.6 | 31 | 26.5 | |
| Middle lobe | 256 | 5.0 | 8 | 6.8 | |
| Upper lobe | 2,863 | 55.8 | 65 | 55.6 | |
| Overlapping lesion | 114 | 2.2 | 7 | 6.0 | |
| Main bronchus | 73 | 1.4 | 6 | 5.1 | |
| Grade | |||||
| Grade I | 286 | 5.6 | 1 | 0.9 | |
| Grade II | 2,336 | 45.5 | 27 | 23.1 | |
| Grade III | 2,415 | 47.1 | 89 | 76.1 | |
| Grade IV | 95 | 1.9 | 0 | 0 | |
| Laterality | |||||
| Left-origin of primary | 2,392 | 46.6 | 53 | 45.3 | |
| Right-origin of primary | 2,740 | 53.4 | 64 | 54.7 | |
| ICD-O-3 Histology and Behavior | |||||
| Squamous cell carcinoma | 1,462 | 28.5 | 36 | 30.8 | |
| Adenocarcinoma | 3,168 | 61.7 | 75 | 64.1 | |
| Adenosquamous carcinoma | 199 | 3.9 | 4 | 3.4 | |
| Large-cell carcinoma | 101 | 2.0 | 0 | 0 | |
| Others | 124 | 2.4 | 2 | 1.7 | |
| Pathological T stage | |||||
| T1 | 1,261 | 24.6 | 53 | 45.3 | |
| T2 | 2,583 | 50.3 | 58 | 49.6 | |
| T3 | 981 | 19.1 | 3 | 2.6 | |
| T4 | 307 | 6.0 | 3 | 2.6 | |
| Pathological N stage | |||||
| N1 | 2972 | 57.9 | 59 | 50.4 | |
| N2 | 2160 | 42.1 | 58 | 49.6 | |
| Primary surgical site | |||||
| Pneumonectomy | 602 | 11.7 | 6 | 5.1 | |
| Lobectomy or extended bilobectomy | 216 | 4.2 | 0 | 0 | |
| Lobectomy with mediastinal lymph node resection | 3,478 | 67.8 | 96 | 82.1 | |
| Resection of lobe or bilobectomy | 554 | 10.8 | 9 | 7.7 | |
| Excision of less than one lobe | 282 | 5.5 | 6 | 5.1 | |
| Radiation sequence with surgery | |||||
| No radiation | 3,856 | 75.1 | 96 | 82.1 | |
| Radiation after surgery | 1,276 | 24.9 | 21 | 17.9 | |
| Chemotherapy | |||||
| No/unknown | 1,586 | 30.9 | 64 | 54.7 | |
| Yes | 3,546 | 69.1 | 53 | 45.3 | |
| NDLN | |||||
| <10 | 3,067 | 59.8 | 11 | 9.4 | |
| ≥10 | 2,065 | 40.2 | 106 | 90.6 | |
| NPLN | |||||
| <4 | 3,824 | 74.5 | 71 | 60.7 | |
| ≥4 | 1,308 | 25.5 | 46 | 39.3 | |
| LNR | |||||
| <0.26 | 3,223 | 62.8 | 84 | 71.8 | |
| ≥0.26 | 1,909 | 37.2 | 33 | 28.2 | |
| LODDS | |||||
| <−0.25 | 3,704 | 72.2 | 99 | 84.6 | |
| ≥−0.25 | 1,428 | 27.8 | 18 | 15.4 | |
Grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated/anaplastic. ICD-O-3, International Classification of Diseases for Oncology, 3rd edition; NDLN, number of dissected lymph nodes; NPLN, number of positive lymph nodes; LNR, lymph node ratio; LODDS, log odds of positive lymph nodes; N/A, not available.
Independent risk factors in the training cohort
Age, sex, T stage, N stage, chemotherapy, radiation sequence with surgery, regional nodes examined, NPLN, LNR, and LODDS were significantly associated with OS in the univariate analysis (P value <0.01). According to univariate analysis, 14 independent risk factors were selected for inclusion in the multivariate analysis using Cox regression. The seven following factors were found to be significant: age (age ≥60 years: HR =1.341, 95% CI: 1.288–1.394; P<0.001), sex (male: HR =1.367, 95% CI: 1.323–1.411; P<0.001), T stage (T2: HR =1.191, 95% CI: 1.134–1.248, P=0.002; T3: HR =1.821, 95% CI: 1.755–1.887, P<0.001; T4: HR =1.754, 95% CI: 1.712–1.848, P<0.001), N stage (N2: HR =1.293, 95% CI: 1.244–1.342; P<0.001), chemotherapy (yes: HR =0.604, 95% CI: 0.557–0.651; P<0.001), NPLN (≥4: HR =1.212, 95% CI: 1.151–1.273; P=0.002), and LODDS (≥−0.25: HR =1.378, 95% CI: 1.298–1.458; P<0.001). In contrast, primary site, grade, ICD-O-3 histology and behavior, Radiation sequence with surgery, regional nodes examined, surgery primary site, and LNR were not statistically significant. The results of the univariate and multivariate analyses are presented in Table 2.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| <60 | Reference | Reference | |||
| ≥60 | 1.398 (1.264–1.546) | <0.001 | 1.341 (1.288–1.394) | <0.001 | |
| Race | |||||
| White | Reference | N/A | |||
| Black | 1.183 (1.017–1.375) | 0.029 | N/A | ||
| Other | 0.956 (0.781–1.169) | 0.659 | N/A | ||
| Marital status | |||||
| Single | Reference | N/A | |||
| Married | 1.011 (0.879–1.163) | 0.878 | N/A | ||
| Other | 0.923 (0.839–1.016) | 0.104 | N/A | ||
| Sex | |||||
| Female | Reference | Reference | |||
| Male | 0.732 (0.673–0.796) | <0.001 | 1.367 (1.323–1.411) | <0.001 | |
| Primary site | |||||
| Lower lobe | Reference | Reference | |||
| Middle lobe | 0.817 (0.576–1.157) | 0.254 | 0.810 (0.702–0.918) | 0.050 | |
| Upper lobe | 0.623 (0.420–0.924) | 0.019 | 0.948 (0.902–0.994) | 0.248 | |
| Overlapping lesion | 0.762 (0.539–1.076) | 0.123 | 1.291 (1.157–1.425) | 0.056 | |
| Main bronchus | 1.142 (0.750–1.738) | 0.535 | 1.107 (0.920–1.294) | 0.587 | |
| Grade | |||||
| Grade I | Reference | Reference | |||
| Grade II | 0.744 (0.526–1.052) | 0.095 | 1.022 (0.923–1.121) | 0.828 | |
| Grade III | 0.748 (0.553–1.011) | 0.059 | 1.240 (1.140–1.340) | 0.030 | |
| Grade IV | 0.945 (0.699–1.277) | 0.711 | 1.120 (0.932–1.308) | 0.548 | |
| Laterality | |||||
| Left origin of primary site | Reference | N/A | |||
| Right origin of primary site | 0.967 (0.890–1.051) | 0.435 | N/A | ||
| ICD-O-3 histology and behavior | |||||
| Squamous cell carcinoma | Reference | Reference | |||
| Adenocarcinoma | 1.028 (0.783–1.350) | 0.844 | 0.934 (0.882–0.986) | 0.186 | |
| Adenosquamous carcinoma | 0.890 (0.681–1.163) | 0.392 | 1.108 (0.999–1.217) | 0.347 | |
| Large-cell carcinoma | 0.914 (0.609–1.372) | 0.665 | 1.439 (1.289–1.589) | 0.015 | |
| Others | 1.409 (0.973–2.041) | 0.069 | 0.956 (0.815–1.097) | 0.752 | |
| Pathological T stage | |||||
| T1 | Reference | Reference | |||
| T2 | 0.538 (0.450–0.643) | <0.001 | 1.191 (1.134–1.248) | 0.002 | |
| T3 | 0.665 (0.565–0.784) | <0.001 | 1.821 (1.755–1.887) | <0.001 | |
| T4 | 1.017 (0.854–1.211) | 0.848 | 1.754 (1.712–1.848) | <0.001 | |
| Pathological N stage | |||||
| N1 | Reference | Reference | |||
| N2 | 0.750 (0.691–0.815) | <0.001 | 1.293 (1.244–1.342) | <0.001 | |
| Primary surgical site | |||||
| Pneumonectomy | Reference | Reference | |||
| Lobectomy or extended bilobectomy | 1.012 (0.824–1.243) | 0.906 | 1.152 (1.039–1.265) | 0.210 | |
| Lobectomy with mediastinal lymph node resection | 1.164 (0.906–1.495) | 0.236 | 0.929 (0.857–1.001) | 0.306 | |
| Resection of lobe or bilobectomy | 0.799 (0.668–0.955) | 0.014 | 1.032 (0.939–1.125) | 0.734 | |
| Excision of less than one lobe | 0.897 (0.726–1.107) | 0.309 | 0.963 (0.848–1.078) | 0.743 | |
| Radiation sequence with surgery | |||||
| No radiation | Reference | Reference | |||
| Radiation after surgery | 0.877 (0.799–0.963) | 0.006 | 1.085 (1.031–1.139) | 0.130 | |
| Chemotherapy | |||||
| No/unknown | Reference | Reference | |||
| Yes | 1.528 (1.402–1.665) | <0.001 | 0.604 (0.557–0.651) | <0.001 | |
| NDLN | |||||
| <10 | 1.131 (1.041–1.230) | 0.004 | Reference | ||
| ≥10 | Reference | 1.101 (1.049-1.153) | 0.065 | ||
| NPLN | |||||
| <4 | Reference | Reference | |||
| ≥4 | 1.447 (1.323–1.583) | <0.001 | 1.212 (1.151–1.273) | 0.002 | |
| LNR | |||||
| <0.26 | Reference | Reference | |||
| ≥0.26 | 0.662 (0.609–0.719) | <0.001 | 1.057 (0.978–1.136) | 0.477 | |
| LODDS | |||||
| <−0.25 | Reference | Reference | |||
| ≥−0.25 | 0.629 (0.577–0.686) | <0.001 | 1.378 (1.298–1.458) | <0.001 | |
Grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated/anaplastic. OS, overall survival; SEER, Surveillance, Epidemiology, and End Results; HR, hazard ratio; CI, confidence interval; N/A, not available; ICD-O-3, International Classification of Diseases for Oncology, 3rd edition; NDLN, number of dissected lymph nodes; NPLN, number of positive lymph nodes; LNR, lymph node ratio; LODDS, log odds of positive lymph nodes.
The nomogram model for predicting OS
The nomogram (Figure 1) for predicting OS was constructed based on the following seven independent risk factors: age (<60 or ≥60 years), sex (male or female), T stage (T1, T2, T3, or T4), N stage (N1 or N2), chemotherapy (yes or no), LODDS (<−0.25 or ≥−0.25), and NPLN (<4 or ≥4). Each independent risk factor corresponded to a distinct score, and the total score compared to 1-, 3-, and 5-year OS.
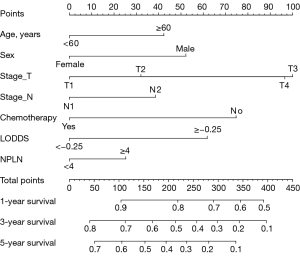
Discrimination accuracy of the nomogram model
In the training and external validation cohorts, we found favorable consistency between the nomogram predictions and the actual observed outcomes of the 1-, 3-, and 5-year OS according to the calibration curves (Figure 2). We evaluated the discrimination ability of the nomogram using C-index values, which were 0.648 (95% CI: 0.636–0.659) in the training cohort and 0.807 (95% CI: 0.751–0.863) in the external validation cohort. The AUCs generated by the 1-, 3-, and 5-year OS ROC curves were 0.7, 0.663, and 0.6476 in the training cohort, respectively, while the AUCs in the external validation cohort were 0.8, 0.834, and 0.84571, respectively (Figure 3).


Comparison of different nodal staging systems
The four different multivariable Cox regression models included model 1 (N stage), model 2 (N stage + NPLN), model 3 (N stage + LODDS), and model 4 (N stage + NPLN + LODDS). The predictive performance values were as follows: model 1, LR test 380.23 and C-index 0.635; model 2, LR test 422.43 and C-index 0.641; model 3, LR test 477.65 and C-index 0.647; and model 4, LR test 487.69 and C-index 0.648. Based on these results (Table 3), we selected model 4 to assess LNs.
Table 3
| Measures | LR test | C-index |
|---|---|---|
| Model 1 (N) | 380.23 | 0.635 |
| Model 2 (N + NPLN) | 422.43 | 0.641 |
| Model 3 (N + LODDS) | 477.65 | 0.647 |
| Model4 (N + NPLN + LODDS) | 487.69 | 0.648 |
LR, likelihood ratio; C-index, concordance index; N, N stage; NPLN, number of positive lymph nodes; LODDS, log odds of positive lymph nodes.
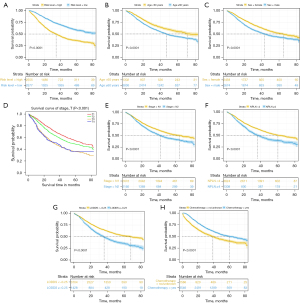
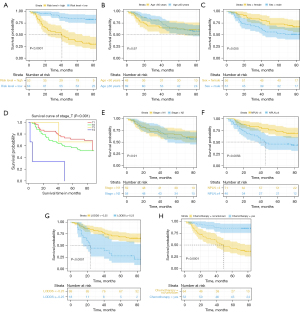
OS of patients with different risk factors
As shown in Figure 4, we observed that those in the low-risk group, aged <60 years, female, with T stage 1, with N stage 1, with NPLN <4, with LODDS <−0.25, and treated with chemotherapy had a significantly longer OS. In our external validation cohort, T stage, NPLN, LODDS, and chemotherapy also exhibited excellent discriminatory power in stratifying prognoses (Figure 5).
Discussion
According to the National Comprehensive Cancer Network (NCCN) guidelines, adjuvant chemotherapy is recommended for patients with stage II and IIIA NSCLC following radical surgery (9,10). For patients with stage IIIB resectable NSCLC, the recommended adjuvant therapy is chemotherapy or radiotherapy. Therefore, the necessity of radiotherapy remains controversial, and with accessibility to targeted therapy and immunotherapy, selecting a therapeutic strategy is also a challenge. Metastasis to ipsilateral peribronchial nodes, hilar nodes, or intrapulmonary nodes is considered to indicate pathological N stage 1; meanwhile, metastasis to ipsilateral mediastinal and/or subcarinal nodes is supposed to indicate pathological N stage 2 (11,12). The TNM system is a simple way to distinguish the LN situation of patients but is not a good predictor of prognosis. Hence, it is urgent to develop a new LN evaluation system. Complete LN dissection is not only associated with the quality of the surgery but also means achieving a sufficient number of resected positive LNs. The ratio-based LN evaluation systems of LODDS and LNR include NDLN and NPLN. To some extent, the LODDS can overcome the limitation of LNR because LNR = NPLN/NDLN could be used to obtain the same result with different NPLN and NDLN values. Different NPLN values are consistently associated with different prognoses, and the resulting clinical decisions may also vary considerably.
In our study, the data of patients with N1 or N2 resectable NSCLC were obtained from the SEER database, and the external validation cohort data were collected from the Department of Thoracic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine. The enrolled validation cohort had good universality and representativeness for Chinese patients with N1 or N2 resectable NSCLC. By performing univariate and multivariate analyses, we identified seven independent risk factors, including age, sex, T stage, N stage, NPLN, LODDS, and chemotherapy. The cutoff values for classification were obtained via ROC curves. A recent study reported that skip N2 might be a favorable factor for patients with N2 lung cancer (13). Therefore, these patients cannot simply be classified as stage N2. In lung adenocarcinoma, Wang et al. found that LNR showed a slightly better prediction performance than did LODDS (14). At the same time, Yu et al. demonstrated that LODDS had a better predictive performance among patients with node-positive lung squamous cell carcinoma after surgery than N, NPLN, and LNR (15). In other tumors, Cao et al. reported that among patients undergoing resection of esophageal cancer, LODDS predicted survival more accurately than did either LNM or LNR (16). Another study reported that the NPLN was superior to LNR and LODDS for predicting the prognosis of pancreatic neuroendocrine neoplasms (17). LODDS has also been applied to predict the survival of patients with NSCLC receiving neoadjuvant therapy (18).
In our study, although LNR showed statistical significance in the univariate analysis, it was not significant in the multivariate analysis, with a P value of 0.274. Therefore, LNR was not included in our nomogram model. As shown in Table 2, model 4, including N stage, NPLN, and LODDS, exhibited the most powerful prognostic predictive ability. According to this model, patients with a higher age, a higher T or N stage, no chemotherapy, more NPLNs, and a larger LODDS had worse OS. Thus, according to this model, we suggest more aggressive therapeutic strategies and close follow-up for patients with lower scores. The novel survival nomogram model we built combining N stage, NPLN, and LODDS could better predict postoperative long-term OS for patients with N1 or N2 NSCLC. Previous articles have compared the predictive power and accuracy of N stage, LNR, and LODDS (19,20). We incorporated N stage, NPLN, LODDS, and chemotherapy into a prognostic model. In a sense, we altered the traditional evaluation of TNM classification, and by taking full advantage of the pathological information retrieved from surgery, we could develop better adjuvant treatment strategies. The role of postoperative radiotherapy (PORT) in patients with completely resected NSCLC remains debatable. From the univariate Cox analysis results, we did not discover survival benefits consistent with those reported in previous studies (21,22). Chien et al. reported that PORT prolongs disease-free survival (DFS) and OS in selected patients, such as those with epidermal growth factor receptor (EGFR) mutation. Still, the sample size was only 82 patients (23). Hui et al. demonstrated that in patients with pIIIA-N2 NSCLC after complete resection and adjuvant chemotherapy, PORT did not improve DFS (24). According to a meta-analysis, PORT should not be considered routine treatment of patients with completely resected NSCLC (25). The Lung ART trial found that PORT was not associated with a better DFS than no PORT (26).
There are some limitations to our study. First, selection bias was unavoidable due to the retrospective nature of the study design. Second, some essential detailed data were missing, such as visceral pleural invasion, lymphovascular invasion, nerve invasion, bronchogenic spread, cancer thrombus occurrence, types of gene mutations, targeted therapy, and the usage of immune checkpoint inhibitors. These missing data might be important for predicting the prognosis of patients with NSCLC. The SEER database has some limits that it lacks any details about chemotherapeutic regimens and details about recurrence [DFS and progression-free survival (PFS)]. DFS and PFS were other significant parameters related to patients’ adjuvant therapy and quality of life.
There were some strengths to our study as well. Our study included the LODDS system and further compared it with NPLN and LNR. We confirmed the superiority of LODDS in evaluating the survival prognosis of patients with N1 or N2 NSCLC. LODDS was also applied in the construction of the nomogram model. This study was based on large-scale population data from the SEER database that were used to establish a nomogram model, and the reliability of our nomogram model was validated through internal and external samples. Moreover, our model could accurately predict the OS due to the adequate follow-up time and comparatively complete survival data, which provided sufficient power to assess the nomogram model.
Conclusions
Our novel survival nomogram model confirmed the prognostic value of LODDS for predicting long-term OS in patients with N1 or N2 NSCLC after undergoing surgical intervention. In addition, the LODDS system might have better prognostic effectiveness than the AJCC N classification or the NPLN and LNR systems. Adjuvant chemotherapy confers a significant survival benefit for patients with N1 or N2 NSCLC. In terms of radiation therapy, we did not find that it provided any survival benefits. Thus, we believe that radiotherapy might not be necessary for those with N1 or N2 resectable NSCLC. Radiotherapy might be advantageous in patients with unresectable NSCLC lesions. The treatment strategy of targeted drugs and immune checkpoint inhibitors as neoadjuvant therapy is becoming more mainstream. Therefore, matching strategy in patients considering surgery is emerging as an important issue. Prospective studies are needed in the future to identify the optimal therapeutic regimen.
Acknowledgments
We appreciate the efforts of the SEER tumor registry team in establishing the database.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-119/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-119/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-119/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-119/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. 2023-0012) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363-85. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Price A. Emerging developments of chemoradiotherapy in stage III NSCLC. Nat Rev Clin Oncol 2012;9:591-8. [Crossref] [PubMed]
- Drake JA, Portnoy DC, Tauer K, et al. Adding Radiotherapy to Adjuvant Chemotherapy Does Not Improve Survival of Patients With N2 Lung Cancer. Ann Thorac Surg 2018;106:959-65. [Crossref] [PubMed]
- Jia B, Zheng Q, Wang J, et al. A nomogram model to predict death rate among non-small cell lung cancer (NSCLC) patients with surgery in surveillance, epidemiology, and end results (SEER) database. BMC Cancer 2020;20:666. [Crossref] [PubMed]
- Deng W, Xu T, Wang Y, et al. Log odds of positive lymph nodes may predict survival benefit in patients with node-positive non-small cell lung cancer. Lung Cancer 2018;122:60-6. [Crossref] [PubMed]
- Cao X, Zheng YZ, Liao HY, et al. A clinical nomogram and heat map for assessing survival in patients with stage I non-small cell lung cancer after complete resection. Ther Adv Med Oncol 2020;12:1758835920970063. [Crossref] [PubMed]
- Parodi S, Verda D, Bagnasco F, et al. The clinical meaning of the area under a receiver operating characteristic curve for the evaluation of the performance of disease markers. Epidemiol Health 2022;44:e2022088. [Crossref] [PubMed]
- De Giglio A, Di Federico A, Gelsomino F, et al. Prognostic relevance of pleural invasion for resected NSCLC patients undergoing adjuvant treatments: A propensity score-matched analysis of SEER database. Lung Cancer 2021;161:18-25. [Crossref] [PubMed]
- Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015;2015:CD011430. [Crossref] [PubMed]
- Taylor M, Soliman N, Paoletti E, et al. Impact of skip mediastinal lymph node metastasis on outcomes after resection for primary lung cancer. Lung Cancer 2023;184:107341. [Crossref] [PubMed]
- Wang S, Yu Y, Xu W, et al. Dynamic nomograms combining N classification with ratio-based nodal classifications to predict long-term survival for patients with lung adenocarcinoma after surgery: a SEER population-based study. BMC Cancer 2021;21:653. [Crossref] [PubMed]
- Yu Y, Zhang P, Yao R, et al. Prognostic value of log odds of positive lymph nodes in node-positive lung squamous cell carcinoma patients after surgery: a SEER population-based study. Transl Lung Cancer Res 2020;9:1285-301. [Crossref] [PubMed]
- Cao J, Yuan P, Ma H, et al. Log Odds of Positive Lymph Nodes Predicts Survival in Patients After Resection for Esophageal Cancer. Ann Thorac Surg 2016;102:424-32. [Crossref] [PubMed]
- Gao B, Zhou D, Qian X, et al. Number of Positive Lymph Nodes Is Superior to LNR and LODDS for Predicting the Prognosis of Pancreatic Neuroendocrine Neoplasms. Front Endocrinol (Lausanne) 2021;12:613755. [Crossref] [PubMed]
- Wang Q, Wang S, Sun Z, et al. Evaluation of log odds of positive lymph nodes in predicting the survival of patients with non-small cell lung cancer treated with neoadjuvant therapy and surgery: a SEER cohort-based study. BMC Cancer 2022;22:801. [Crossref] [PubMed]
- Prassas D, Safi SA, Stylianidi MC, et al. N, LNR or LODDS: Which Is the Most Appropriate Lymph Node Classification Scheme for Patients with Radically Resected Pancreatic Cancer? Cancers (Basel) 2022;14:1834. [Crossref] [PubMed]
- Prassas D, Verde PE, Pavljak C, et al. Prognostic Discrimination of Alternative Lymph Node Classification Systems for Patients with Radically Resected Non-Metastatic Colorectal Cancer: A Cohort Study from a Single Tertiary Referral Center. Cancers (Basel) 2021;13:3898. [Crossref] [PubMed]
- Wang L, Chen W, Xu X, et al. Effect of postoperative radiotherapy on survival in patients with completely resected and pathologically confirmed stage N2 non-small-cell lung cancer: a systematic review and meta-analysis. Ther Adv Chronic Dis 2023;14:20406223231195622. [Crossref] [PubMed]
- Ku HY, Lin SM, Wang CL, et al. Impact of pathological nodal staging and tumour differentiation on survival and postoperative radiotherapy in completely resected stage IIIA Non-small-cell lung cancer. Lung Cancer 2023;184:107357. [Crossref] [PubMed]
- Chien JC, Hu YC, Tsai YJ, et al. Predictive Value of Clinicopathological Factors to Guide Post-Operative Radiotherapy in Completely Resected pN2-Stage III Non-Small Cell Lung Cancer. Diagnostics (Basel) 2023;13:3095. [Crossref] [PubMed]
- Hui Z, Men Y, Hu C, et al. Effect of Postoperative Radiotherapy for Patients With pIIIA-N2 Non-Small Cell Lung Cancer After Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial. JAMA Oncol 2021;7:1178-85. [Crossref] [PubMed]
- Burdett S, Rydzewska L, Tierney J, et al. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2016;10:CD002142. [Crossref] [PubMed]
- Le Pechoux C, Pourel N, Barlesi F, et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:104-14. [Crossref] [PubMed]


