
Efficacy and safety of first-line PD-L1/PD-1 inhibitors in limited-stage small cell lung cancer: a multicenter propensity score matched retrospective study
Highlight box
Key findings
• We found the promising clinical efficacy and tolerable safety of first-line programmed cell death protein 1 (PD-1) inhibitors or programmed cell death ligand 1 (PD-L1) inhibitors for limited-stage small cell lung cancer (LS-SCLC) patients.
What is known and what is new?
• The prognosis of small cell lung cancer (SCLC) patients is poor, and first-line immune checkpoint inhibitors (ICIs) have been proven to significantly improve survival time in ES-SCLC patients. However, the standard first-line treatment for LS-SCLC is still chemotherapy and thoracic radiotherapy.
• LS-SCLC patients treated with first-line PD-L1/PD-1 inhibitors and chemotherapy have significant survival benefits. Additionally, lung immune prognostic index may serve as a valuable prognostic factor.
What is the implication, and what should change now?
• ICIs, which are effective in extensive-stage SCLC and have been shown to have synergy with chemoradiotherapy in the PACIFIC study, will make another breakthrough in LS-SCLC. Randomized clinical trials are needed to confirm the effectiveness of first-line ICIs in LS-SCLC.
Introduction
Approximately 15% of newly diagnosed lung cancer cases are small cell lung cancer (SCLC) patients. And it is characterized by its aggressive nature, early metastasis, and generally unfavorable prognosis (1,2). Unlike non-small cell lung cancer (NSCLC), development in the therapy strategy of SCLC has been relatively slower. Among SCLC cases, around 30% are classified as limited-stage SCLC (LS-SCLC), with a median survival period of around 20 months (3). The standard treatment strategy for LS-SCLC, recommended by the guidelines, involves chemotherapy, thoracic radiotherapy, and prophylactic cranial irradiation (PCI) (4). However, the 5-year OS rate remains low, standing at only 30–35% (5).
There have been no major breakthroughs in the treatment of SCLC for the past two decades, but the use of ICIs has ushered in significant breakthroughs in the therapy strategy of extensive-stage SCLC (ES-SCLC) (6-9). Due to the lack of adequate data, it is not recommended to offer immunotherapy to patients with LS-SCLC. Despite this, numerous preclinical studies have demonstrated the potential synergy between chemoradiotherapy (CRT) and immune checkpoint inhibitors (ICIs), serving as the basis for combining these therapeutic approaches (10-12). Currently, the utilization of concurrent or consolidation ICIs alongside with CRT remains relatively restricted, with only three phase II clinical trials reporting outcomes for LS-SCLC (13-15). Two of them presented that the use of concurrent ICIs with CRT is promising, paving the way for establishing a clinical precedent for first-line ICIs in LS-SCLC cases (14,15).
The importance of immune and inflammatory reactions in cancer progression is firmly established (16,17). Inflammatory indicators like the lung immune prognostic index (LIPI), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) have been strongly linked to poor survival outcomes among patients with NSCLC or ES-SCLC (18-20). However, few attempts have been made to explore whether inflammatory and nutritional markers could serve as indicators for the choice of immunotherapy among LS-SCLC cases.
In this real-world multicenter study, we assessed the survival outcomes and safety of programmed cell death protein 1 (PD-1) inhibitors or programmed cell death ligand 1 (PD-L1) inhibitors as an integral component of the initial treatment approach among LS-SCLC cases in practical clinical settings. Furthermore, we utilized propensity score matching (PSM) to mitigate bias stemming from variances in patients’ baseline attributes. In addition, we performed multivariate regression analyses considering the baseline information of cases involved, aiming to pinpoint the prognostic indicators in LS-SCLC patients. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-24/rc).
Methods
Patient selection
We enrolled patients with SCLC who received therapy in three medical centers (Jinling Hospital, The First Affiliated Hospital of Guangzhou Medical University, and Jiangsu Cancer Hospital) between June 2019 and March 2021. For participation in this study, it was necessary to have a confirmed diagnosis of SCLC through histological or cytological examination, received first-line standard chemotherapy comprising of etoposide and platinum (EP) with or without ICIs, and had been systematically categorized as limited disease based on the Veterans Administration Lung Study Group criteria (21). The specific chemotherapy regimen is as follows: cisplatin/carboplatin/nedaplatin/lobaplatin day 1 and etoposide 100 mg/m2 days 1, 2 and 3. The population receiving thoracic radiotherapy included both concurrent CRT (CCRT) and sequential CRT. Target areas and irradiation methods were evaluated by specialized radiation oncologists, with radiation doses typically ranging from 45 to 60 Gy. Additionally, the patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) ≥2, those who had only undergone 1–2 cycles of treatment, or those with a history of another malignancy or surgery were excluded from the study. The follow-up period of our study was up to Dec 31, 2022. Our study was carried out at multiple centers and in compliance with the Declaration of Helsinki (as revised in 2013). It received approval from the local ethics committee at Jinling Hospital (Ethics ID: 202103275) and the other two centers were informed and agreed with the study. Every patient who chose immunotherapy did so voluntarily and no patient participated in prospective clinical trials. Given the retrospective nature of our study, obtaining informed consent from enrolled patients was not deemed necessary, and patient information was kept confidential.
Data collection and evaluation
To assess the duration from the initiation of first-line treatment to relapse or mortality, we employed the Kaplan-Meier (K-M) method to estimate both progression-free survival (PFS) and overall survival (OS). The response was evaluated in accordance with the Response Evaluation Criteria in Solid Tumors v1.1. We have collected the optimal response during the first-line treatment of each patient. The objective response rate (ORR) was computed according to the percentage of cases achieving complete response (CR) and partial response (PR), while the disease control rate (DCR) considered cases achieving CR, PR, or stable disease (SD).
In our study, we collected patient information prior to treatment, which consisted of age, sex, smoking history, ECOG PS, baseline tumor information, leukocyte, lymphocyte, neutrophil, monocyte, and platelet counts, lactate dehydrogenase (LDH), serum albumin, and tumor markers. Additionally, we utilized various indexes to evaluate inflammation and nutritional status, which included the following: NLR calculated as neutrophil/lymphocyte; lymphocyte-monocyte ratio (LMR) calculated as lymphocyte/monocyte; PLR calculated as platelet/lymphocyte; systemic immune-inflammation index (SII) calculated as platelet × NLR (22); systemic inflammation response index (SIRI) calculated as neutrophil × monocyte/lymphocyte (22); prognostic nutrition index (PNI) calculated as serum albumin (g/L) plus 5 times the total lymphocyte count (109/L) (23). LIPI was determined using the baseline LDH level and the derived neutrophil-lymphocyte ratio (dNLR), which is calculated as neutrophil count divided by the white blood cell count minus the neutrophil count. LIPI categorizes the population into three different prognostic groups: good [dNLR ≤3 and LDH ≤ upper limit of normal (ULN)], intermediate (dNLR >3 or LDH > ULN), and poor (dNLR >3 and LDH > ULN) (20). LDH and tumor markers were converted into categorical variables using the ULN. Meanwhile, the optimal cut-off value of NLR, LMR, PLR, SIRI, SII, and PNI were determined by the ’surv_cutpoint’ function.
Statistical analysis
We employed descriptive statistics, such as frequencies and percentages, to present the baseline characteristics of the enrolled cases. The Chi-square and Kruskal-Wallis tests were utilized to evaluate categorical variables.
We performed 1:1 propensity score matching with a tolerance of 0.05 between cases with first-line ICIs and those without ICIs. The covariates used for matching included age, sex, PS, smoking status, primary site, lymph node stage (N stage), tumor node metastasis (TNM) stage, and history of radiotherapy, thoracic chemoradiation, and PCI. We compared the survival outcomes of the two groups utilizing both the K-M method with log-rank test and Cox proportional hazards regression analysis.
Before the matching process, all LS-SCLC patients underwent univariate and multivariate Cox regression analyses to pinpoint prognostic factors for OS and PFS. In the univariate analysis, factors demonstrating a notable influence on OS or PFS (P<0.05) were subsequently integrated into the multivariate analysis.
Both R version 4.2.2 and SPSS 25.0 were utilized for these analyses, with statistical significance defined as a P value <0.05.
Results
Baseline characteristics
Among the 217 patients initially, 150 were eligible for inclusion, with 87 receiving first-line EP alone and 63 receiving first-line EP in combination with ICIs (Figure S1). Table 1 presents the baseline characteristics of all 150 eligible cases, among whom 82 (54.67%) were less than 65 years old, and 128 (85.33%) were male. Ninety cases (60.00%) had an ECOG PS of 1, and 140 cases (93.33%) were categorized as stage III according to the TNM stage. Additionally, 45 patients (30.00%) received PD-1 inhibitors, while 18 (12.00%) received PD-L1 inhibitors (Table S1). And 58 patients (38.67%) received CCRT, while 32 patients (21.33%) received sequential CRT. Moreover, nine patients (6.00%) received PCI (Table 1).
Table 1
| Characteristic | Subcategories | All patients (n=150), n (%) |
Before matching, n (%) | After matching, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| EP (n=87) | EP + ICIs (n=63) | P | EP (n=41) | EP + ICIs (n=41) | P | ||||
| Age (years) | <65 | 82 (54.67) | 52 (59.77) | 30 (47.62) | 0.14 | 21 (51.22) | 22 (53.66) | 0.82 | |
| ≥65 | 68 (45.33) | 35 (40.23) | 33 (52.38) | 20 (48.78) | 19 (46.34) | ||||
| Sex | Male | 128 (85.33) | 75 (86.21) | 53 (84.13) | 0.72 | 33 (80.49) | 34 (82.93) | 0.77 | |
| Female | 22 (14.67) | 12 (13.79) | 10 (15.87) | 8 (19.51) | 7 (17.07) | ||||
| Smoking history | Never | 36 (24.00) | 16 (18.39) | 20 (31.75) | 0.059 | 11 (26.83) | 14 (34.15) | 0.47 | |
| Former/current | 114 (76.00) | 71 (81.61) | 43 (68.25) | 30 (73.17) | 27 (65.85) | ||||
| ECOG PS | 0 | 60 (40.00) | 38 (43.68) | 22 (34.92) | 0.28 | 18 (43.90) | 16 (39.02) | 0.65 | |
| 1 | 90 (60.00) | 49 (56.32) | 41 (65.08) | 23 (56.10) | 25 (60.98) | ||||
| Primary site | Left | 66 (44.00) | 36 (41.38) | 30 (47.62) | 0.44 | 17 (41.46) | 19 (46.34) | 0.65 | |
| Right | 84 (56.00) | 51 (58.62) | 33 (52.38) | 24 (58.54) | 22 (53.66) | ||||
| N stage | N0 | 13 (8.67) | 12 (13.79) | 1 (1.59) | <0.001 | 0 (0.00) | 0 (0.00) | 0.88 | |
| N1 | 7 (4.67) | 6 (6.90) | 1 (1.59) | 1 (2.44) | 1 (2.44) | ||||
| N2 | 89 (59.33) | 56 (64.37) | 33 (52.38) | 27 (65.85) | 29 (70.73) | ||||
| N3 | 41 (27.33) | 13 (14.94) | 28 (44.44) | 13 (31.71) | 11 (26.83) | ||||
| TNM stage | I–II | 10 (6.67) | 8 (9.20) | 2 (3.17) | 0.26 | 1 (2.44) | 1 (2.44) | >0.99 | |
| III | 140 (93.33) | 79 (90.80) | 61 (96.83) | 40 (97.56) | 40 (97.56) | ||||
| Radiotherapy† | No | 58 (38.67) | 30 (34.48) | 28 (44.44) | 0.21 | 18 (43.90) | 13 (31.71) | 0.25 | |
| Yes | 92 (61.33) | 57 (65.52) | 35 (55.56) | 23 (56.10) | 28 (68.29) | ||||
| Thoracic chemoradiation | No | 60 (40.00) | 32 (36.78) | 28 (44.44) | 0.33 | 18 (43.90) | 13 (31.71) | 0.50 | |
| Concurrent | 58 (38.67) | 38 (43.68) | 20 (31.75) | 13 (31.71) | 17 (41.46) | ||||
| Sequential | 32 (21.33) | 17 (19.54) | 15 (23.81) | 10 (24.39) | 11 (26.83) | ||||
| PCI | No | 141 (94.00) | 80 (91.95) | 61 (96.83) | 0.37 | 39 (95.12) | 39 (95.12) | >0.99 | |
| Yes | 9 (6.00) | 7 (8.05) | 2 (3.17) | 2 (4.88) | 2 (4.88) | ||||
†, radiotherapy included first-line thoracic radiotherapy and PCI. EP, etoposide and platinum; ICIs, immune checkpoint inhibitors; ECOG PS, Eastern Cooperative Oncology Group performance status; N stage, node stage; TNM, tumor node metastasis; PCI, prophylactic cranial irradiation.
After propensity score-matching, 41 pairs of patients were matched, exhibiting comparable baseline characteristics (P>0.05). Notably, before matching, 20 patients (31.75%) received CCRT in the EP + ICIs group, 15 patients (23.81%) received sequential CRT, and two patients (3.17%) underwent PCI, compared to 38 (43.68%), 17 (19.54%), and seven (8.05%) patients, respectively, in the EP group (Table 1).
Survival analysis
In the EP + ICIs group, the median OS (mOS) was 29.5 months [95% confidence interval (CI): 13.9–45.1], while in the EP group, it was 20.0 months [95% CI: 14.4–25.6; hazard ratio (HR) =0.64 (95% CI: 0.41–1.02), P=0.059) (Figure 1A). Disappointingly, the difference was not statistically significant. On the other hand, the median PFS (mPFS) was significantly prolonged in the EP + ICIs group, with a duration of 14.6 months (95% CI: 8.7–20.6), compared to the EP group of 8.6 months [95% CI: 8.0–9.2; HR =0.42 (95% CI: 0.28–0.63), P<0.001] (Figure 1B). This difference in mPFS was statistically significant.
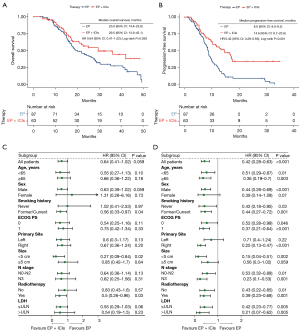
Subgroup analysis suggested that patients with combined chemotherapy and radiotherapy [HR =0.5 (95% CI: 0.26–0.96), P=0.03] could obtain significantly longer OS from immunotherapy (Figure 1C). In addition, the PFS benefit was consistent in most subgroups (Figure 1D).
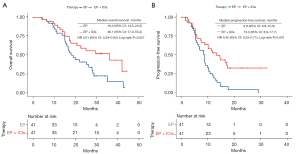
As outlined in the methods, we conducted PSM. In the group receiving both chemotherapy and immunotherapy, the mOS stood at 36.1 months (95% CI: 17.0–55.2), demonstrating a significant increase compared to the group receiving chemotherapy alone, of which the mOS was 19.0 months (95% CI: 14.5–23.5; HR =0.51 (95% CI: 0.28–0.93), P=0.02] (Figure 2A). Similarly, the mPFS was also notably prolonged in the EP + ICIs group, measuring 13.3 months (95% CI: 8.9–17.7), in contrast to the EP cohort, which had an mPFS of 8.8 months (95% CI: 6.8–10.8; HR =0.42 (95% CI: 0.25–0.71), P=0.001] (Figure 2B).
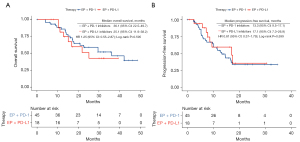
Our analysis found no statistically significant differences in survival outcomes between patients who received PD-L1 inhibitors and those who received PD-1 inhibitors [OS: HR =1.25 (95% CI: 0.55–2.87), P=0.59; PFS: HR =0.81 (95% CI: 0.37–1.79), P=0.59] (Figure 3).
Treatment response and safety
The response was evaluated in cases with complete data. All the enrolled cases had a DCR of 100% and an ORR of 84.9%. For patients receiving EP, EP + PD-1 inhibitors, and EP + PD-L1 inhibitors, the ORR were 81.0%, 90.9%, and 87.5% respectively. There was no significant distinction in ORR and DCR between the EP + ICIs group and the EP group (ORR: EP, 81.0%; EP + ICIs, 90.0%, P=0.14; DCR: EP, 100%; EP + ICIs, 100%, P>0.99) (Figure 4A).
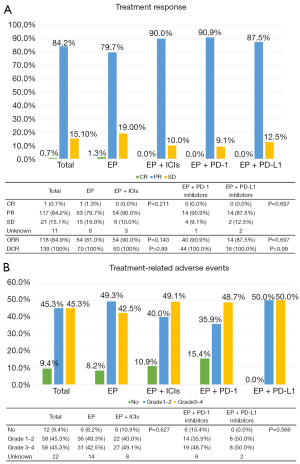
Regarding treatment-related adverse events (trAEs), Figure 4B presents that 116 of all eligible patients (90.6%) experienced at least one trAE, with 58 (45.3%) experiencing grade 1–2 trAEs and another 58 (45.3%) facing grade 3–4 trAEs. There was no fatal adverse event. It is noteworthy that the incidence rates of trAEs in the EP + ICIs group and the EP group did not exhibit statistical significance (EP: grade 1–2, 49.3%; grade 3–4, 42.5%; EP + ICIs: grade 1–2, 40.0%; grade 3–4, 49.1%; P=0.62).
Moreover, the response and the adverse events in the group treated with EP + PD-1 inhibitors were comparable to those in the group treated with EP + PD-L1 inhibitors (ORR: P=0.69; DCR: P>0.99; trAEs: P=0.56) (Figure 4).
Prognostic factors
Table 2 summarizes the results of our investigation into the correlation between baseline characters or inflammation and tumor markers with the survival outcomes of patients with LS-SCLC. This was achieved through conducting both univariate and multivariate analyses on PFS and OS.
Table 2
| Characteristics | Subcategories | Progression-free survival | Overall survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||||
| Age (years) | <65 | – | – | – | – | – | – | – | – | |||
| ≥65 | 0.85 (0.57–1.26) | 0.41 | – | – | 1.51 (0.98–2.33) | 0.06 | – | – | ||||
| Sex | Male | – | – | – | – | – | – | – | – | |||
| Female | 0.79 (0.47–1.33) | 0.37 | – | – | 0.43 (0.21–0.9) | 0.02 | 0.46 (0.14–1.53) | 0.20 | ||||
| Smoking history | Never | – | – | – | – | – | – | – | – | |||
| Former/current | 1.19 (0.75–1.89) | 0.45 | – | – | 1.17 (0.71–1.94) | 0.53 | – | – | ||||
| ECOG PS | 0 | – | – | – | – | – | – | – | – | |||
| 1 | 0.76 (0.51–1.12) | 0.16 | – | – | 0.72 (0.47–1.11) | 0.13 | – | – | ||||
| Primary site | Left | – | – | – | – | – | – | – | – | |||
| Right | 0.74 (0.51–1.09) | 0.13 | – | – | 0.82 (0.53–1.28) | 0.38 | – | – | ||||
| Size (cm) | <5 | – | – | – | – | – | – | – | – | |||
| ≥5 | 1.19 (0.73–1.95) | 0.48 | – | – | 1.06 (0.61–1.83) | 0.84 | – | – | ||||
| N stage | N0–N2 | – | – | – | – | – | – | – | – | |||
| N3 | 0.69 (0.44–1.07) | 0.09 | – | – | 0.9 (0.55–1.5) | 0.69 | – | – | ||||
| TNM stage | I–II | – | – | – | – | – | – | – | – | |||
| III | 1.27 (0.59–2.76) | 0.53 | – | – | 2.63 (0.82–8.44) | 0.10 | – | – | ||||
| Thoracic chemoradiation | No | – | – | – | – | – | – | – | – | |||
| Concurrent | 0.74 (0.48–1.13) | 0.15 | – | – | 0.54 (0.33–0.87) | 0.01 | 0.70 (0.31–1.54) | 0.36 | ||||
| Sequential | 0.66 (0.39–1.11) | 0.11 | – | – | 0.58 (0.32–1.04) | 0.06 | 077 (0.34–1.75) | 0.52 | ||||
| PCI | No | – | – | – | – | – | – | – | – | |||
| Yes | 1.95 (0.94–4.02) | 0.07 | – | – | 1.73 (0.79–3.76) | 0.16 | – | – | ||||
| Chemo-immunotherapy | EP | – | – | – | – | – | – | – | – | |||
| EP + PD-1 inhibitors | 0.43 (0.27–0.68) | <0.001 | 0.33 (0.17–0.62) | 0.001 | 0.61 (0.36–1.01) | 0.055 | – | – | ||||
| EP + PD-L1 inhibitors | 0.38 (0.18–0.80) | 0.01 | 0.18 (0.06–0.60) | 0.005 | 0.78 (0.37–1.64) | 0.50 | – | – | ||||
| CEA (ng/mL) | ≤5 | – | – | – | – | – | – | – | – | |||
| >5 | 0.76 (0.45–1.27) | 0.29 | – | – | 0.9 (0.51–1.57) | 0.70 | – | – | ||||
| CA125 (U/mL) | ≤35 | – | – | – | – | – | – | – | – | |||
| >35 | 0.65 (0.37–1.14) | 0.13 | – | – | 1.03 (0.6–1.79) | 0.91 | – | – | ||||
| CA153 (U/mL) | ≤25 | – | – | – | – | – | – | – | – | |||
| >25 | 1.49 (0.46–4.81) | 0.50 | – | – | 1.11 (0.34–3.59) | 0.86 | – | – | ||||
| SCC antigen (ng/mL) | ≤1.5 | – | – | – | – | – | – | – | – | |||
| >1.5 | 1.03 (0.37–2.9) | 0.95 | – | – | 2.53 (1.05–6.12) | 0.03 | 2.13 (0.87–5.21) | 0.09 | ||||
| NSE (ng/mL) | ≤16.3 | – | – | – | – | – | – | – | – | |||
| >16.3 | 0.84 (0.43–1.62) | 0.60 | – | – | 1.35 (0.58–3.17) | 0.48 | – | – | ||||
| Cyfra21-1 (ng/mL) | ≤3.3 | – | – | – | – | – | – | – | – | |||
| >3.3 | 1.12 (0.64–1.97) | 0.69 | – | – | 1.57 (0.82–2.99) | 0.17 | – | – | ||||
| LDH (U/L) | ≤245 | – | – | – | – | – | – | – | – | |||
| >245 | 1.47 (0.84–2.56) | 0.17 | – | – | 1.52 (0.84–2.75) | 0.16 | – | – | ||||
| LIPI | Good | – | – | – | – | – | – | – | – | |||
| Intermediate | 1.35 (0.79–2.32) | 0.27 | 2.22 (1.20–4.13) | 0.01 | 1.16 (0.63–2.13) | 0.64 | – | – | ||||
| Poor | 2.92 (1.03–8.27) | 0.04 | 2.03 (0.71–5.77) | 0.18 | 1.36 (0.47–3.96) | 0.57 | – | – | ||||
| NLR | ≤1.70 | – | – | – | – | – | – | – | – | |||
| >1.70 | 1.27 (0.72–2.22) | 0.41 | – | – | 1.66 (0.85–3.23) | 0.13 | – | – | ||||
| PLR | ≤152.12 | – | – | – | – | – | – | – | – | |||
| >152.12 | 1.42 (0.87–2.32) | 0.15 | – | – | 1.59 (0.92–2.74) | 0.09 | – | – | ||||
| LMR | ≤3.90 | – | – | – | – | – | – | – | – | |||
| >3.90 | 0.71 (0.43–1.17) | 0.17 | – | – | 0.67 (0.39–1.17) | 0.16 | – | – | ||||
| SII | ≤663.67 | – | – | – | – | – | – | – | – | |||
| >663.67 | 1.11 (0.69–1.8) | 0.66 | – | – | 0.71 (0.41–1.22) | 0.21 | – | – | ||||
| SIRI | ≤0.94 | – | – | – | – | – | – | – | – | |||
| >0.94 | 1.16 (0.72–1.89) | 0.54 | – | – | 1.51 (0.87–2.63) | 0.14 | – | – | ||||
| PNI | ≤54.15 | – | – | – | – | – | – | – | – | |||
| >54.15 | 0.66 (0.3–1.46) | 0.30 | – | – | 0.47 (0.17–1.3) | 0.14 | – | – | ||||
PFS, progression-free survival; OS, overall survival; LS-SCLC, limited-stage small cell lung cancer; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; N stage, node stage; TNM, tumor node metastasis; PCI, prophylactic cranial irradiation; EP, etoposide and platinum; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; CEA, carcinoembryonic antigen; CA125, carbohydrate antigen 125; CA153, carbohydrate antigen 153; SCC, squamous cell carcinoma; NSE, neuron-specific enolase; Cyfra21-1, cytokeratin 19 fragment; LDH, lactate dehydrogenase; LIPI, lung immune prognostic index [good (dNLR ≤3 and LDH ≤ ULN), intermediate (dNLR >3 or LDH > ULN), and poor (dNLR >3 and LDH > ULN)]; dNLR, derived neutrophil-lymphocyte ratio; ULN, upper limit of normal; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; LMR, lymphocyte-monocyte ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; PNI, prognostic nutrition index.
In the univariate analysis, our findings indicated that the history of chemo-immunotherapy (EP + PD-1: P<0.001; EP + PD-L1: P=0.01) and baseline better LIPI (intermediate: P=0.27; poor: P=0.04) were linked to improved PFS in patients with LS-SCLC. After conducting the multivariate analysis, the results showed that the history of chemo-immunotherapy [EP + PD-1: HR =0.33 (95% CI: 0.17–0.62), P=0.001; EP + PD-L1: HR =0.18 (95% CI: 0.06–0.60), P=0.005] and baseline LIPI [intermediate: HR =2.22 (95% CI: 1.20–4.13), P=0.01; poor: HR =2.03 (95% CI: 0.71–5.77), P=0.18] emerged as independent prognostic factors for PFS (Table 2). Furthermore, our study presented that female (P=0.02), the history of thoracic chemoradiation (concurrent: P=0.01; sequential: P=0.06), and baseline squamous cell carcinoma antigen (SCC) antigen ≤1.5 ng/mL (P=0.03) were significantly linked to prolonged OS in the univariate analysis. However, in the multivariate analysis, we were unable to identify independent prognostic factors for OS (Table 2).
Discussion
The recent standard therapy strategy for LS-SCLC involves a combination of chemotherapy and thoracic radiotherapy (4). Despite several attempts to enhance the survival outcomes of LS-SCLC patients through modifications to thoracic radiotherapy, the results have been unsatisfactory (3,24). Recent clinical evidence has shown significant survival benefits when first-line ICIs are combined with chemotherapy in cases with ES-SCLC (6-9). And the success of ICIs combined with CCRT in PACIFIC trial has generated interest in incorporating ICIs into the treatment of LS-SCLC (25). Exploring the real-world clinical benefits of ICIs therapy in LS-SCLC patients is a valuable endeavor. In this study, we investigated the effectiveness and safety of first-line PD-1 inhibitors or PD-L1 inhibitors when used in CCRT or chemotherapy alone for patients with LS-SCLC.
We carried out a retrospective study in three medical centers in China, utilizing propensity score-matched analysis. Through our study, PFS was significantly longer in the patients who were treated with chemotherapy and ICIs both before and after matching, and after the propensity score-matched analysis the OS of patients treated with chemo-immunotherapy was also longer. But there was no significant difference in OS before matching. Although PSM could minimize the differences resulting from baseline characteristics, the matched sample may not accurately present the entire population due to the limitations of the statistical technique, which may account for the differences before and after matching (26). Despite the absence of a significant difference, OS was numerically improved in the immunotherapy combination cohort than in the chemotherapy alone cohort, which suggests the promise of ICIs in the first-line treatment of LS-SCLC. Three prospective clinical trials have previously assessed the importance of ICIs in LS-SCLC cases (13-15). One study showed that pembrolizumab as concurrent or consolidation therapy with CRT was linked to encouraging effectiveness (mPFS: 19.7 months; mOS: 39.5 months) (14). Another study presented the superior survival outcomes of durvalumab with CCRT (2-year PFS rate: 42%; mPFS: 14.4 months) compared to the historical control cohort (2-year PFS rate: 24%) (14). The results of our study were similar to these two studies and both demonstrated that ICIs have great potential for improved survival in patients with LS-SCLC. Moreover, both of these studies and ours effectively managed the frequency and severity of trAEs (14,15). Disappointingly, the trial STIMULI found no significant difference between the consolidation nivolumab + ipilimumab arm and the control arm in patients treated with CCRT (13). Notably, because of trAEs, this trail had a high drop-out rate of 55.1%. Some scholars speculate that ipilimumab, a kind of CTLA-4 inhibitor, may be suspected as the contributing factor (13). Overall, these findings emphasize the importance of conducting randomized clinical trials to confirm the effectiveness of first-line ICIs in LS-SCLC cases.
We collected patient data from three provincial medical centers over nearly 2 years, making it a relatively large-scale retrospective study. Unfortunately, due to a lack of indication support, the sample size included in our study is relatively small. According to previous studies, LS-SCLC patients indeed constitute only a small fraction of lung cancer patients (3). The two mentioned phase II clinical trials investigating the efficacy of first-line CRT + ICIs in LS-SCLC patients included only 40 and 50 patients, respectively (14,15). In our study, we included 63 patients who received ICIs, which is a relatively decent sample size. With a limited sample size, there may be challenges in achieving well-balanced treatment groups, especially when matching multiple covariates. And the wide 95% CI observed and the significant overlap between treatment groups may raise concerns about the precision of our estimates. These findings indeed reflected the limitations imposed by our study’s small sample size. However, phase III clinical trials for LS-SCLC have only just begun, requiring a longer research period to obtain survival outcomes. Retrospective studies can be used to explore potential associations or propose new research hypotheses, as well as to provide supplementary evidence for clinical trials. We believe that despite these limitations, our study contributes valuable insights and complements the existing literature on this topic.
One aspect that requires attention in our study. Treatment decision-making in real-world clinical practice exists the complexity. Due to the comprehensive consideration of the patient’s physical condition, preferences, and economic status, some LS-SCLC patients were not treated with standard first-line concurrent or sequential chemoradiation therapy, but with chemotherapy alone. Some patients refuse radiotherapy due to old age, emphysema, or pulmonary fibrosis. Some patients are concerned about the potential severe side effects of combined radiotherapy, chemotherapy, and immunotherapy, leading them to refuse radiotherapy. In addition, radiotherapy requires patients to visit the radiotherapy department for further treatment after discharge, and the course of treatment is relatively long. Some patients did not undergo radiotherapy after discharge due to inconvenience. For these reasons, we adjusted the design strategy, primarily comparing the differences between the immune and non-immune groups. Analyzing the full population provides valuable insights into the broader patient population and allows for a comprehensive understanding. For patients receiving first-line radiotherapy, only subgroup analysis was performed. Interestingly, our subgroup analysis found that patients with combined radiotherapy could obtain significantly longer OS and PFS from chemo-immunotherapy. This result suggested that the incorporation of ICIs with CRT is feasible. A lot of studies have found a synergistic relationship between radiation therapy and immunotherapy, paving the way for innovative combinations that harness both treatment approaches (12,27,28). In NSCLC, the PACIFIC study has reported that consolidation therapy with durvalumab after CCRT significantly improved PFS and OS (25). We believe that ICIs, which are effective in ES-SCLC and have been shown to have optimal synergy with radiotherapy in the PACIFIC study, will make another breakthrough in LS-SCLC.
Based on our discovery, there was no notable distinction between the groups that were administered PD-1 inhibitors versus those given PD-L1 inhibitors. Results of clinical studies in ES-SCLC suggested controversy over the efficacy of PD-1 inhibitors. While the KEYNOTE-604 study did not yield consistent results with PD-L1 inhibitors, the ASTRUM-005 study demonstrated the most substantial OS benefit among the published trials so far (8,29). The inquiry into whether PD-1 and PD-L1 inhibitors differing clinical outcomes remains an unsettled matter, demanding additional scrutiny and research (30).
Previous studies found that SCLC patients do not exhibit significant predictive value with regard to PD-L1 expression or tumor mutation burden (TMB) (1,31). To date, there are no widely accepted biomarkers available for predicting the prognosis of LS-SCLC patients. Our study, through multivariate analysis, has revealed that the history of chemo-immunotherapy and baseline LIPI independently influence PFS in LS-SCLC patients. Notably, recent studies have identified LIPI as a prognostic indicator among NSCLC cases undergoing combined chemotherapy and ICIs (32,33). A study reported that baseline LIPI was linked to the survival outcomes of ES-SCLC cases undergoing chemotherapy alone (34). Furthermore, Li et al. conducted an analysis involving 100 ES-SCLC cases, corroborating the utility of LIPI in predicting both OS and PFS among cases undergoing chemoimmunotherapy (20). Sun et al. also found the significant role of LIPI stratification within the LS-SCLC patient population, which was consistent with our outcome (35). However, we have not found the value of LIPI for OS in multivariate analysis. Regression models derived from a limited sample size must be interpreted with caution. Overall, more substantial evidence is required to establish the importance of inflammatory biomarkers in the treatment of LS-SCLC cases.
Our research does have several limitations that should be acknowledged. Firstly, we acknowledge that our patient population is heterogeneous. Because the study’s design is retrospective, it was not possible to entirely eliminate confounding variables and selective bias, even with the use of PSM and subgroup analysis. Secondly, although the primary chemotherapy regimens were similar between the two cohorts, we cannot ascertain whether variations in the timing of immunotherapy administration and differences in radiotherapy techniques might have influenced treatment efficacy. Lastly, our study included only patients with a good PS score, which may not fully represent the entire LS-SCLC population. It would be valuable to investigate whether patients with a PS =2 would also derive benefits from chemotherapy combined with ICIs, and we eagerly await future research in this regard.
Conclusions
To summarize, our study has demonstrated encouraging clinical effectiveness and acceptable safety when utilizing first-line PD-1 inhibitors or PD-L1 inhibitors in combination with CRT or chemotherapy alone for LS-SCLC patients. Furthermore, our findings highlight the potential significance of LIPI as a valuable prognostic indicator among LS-SCLC patients. The insights from our study will prove beneficial for guiding the development of treatment protocols and data analysis in future research endeavors.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-24/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-24/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-24/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-24/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the local ethics committee of Jinling Hospital (Ethics ID: 202103275) and the other two centers were informed and agreed with the study. Informed consent from individuals was waived based on the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol 2020;17:300-12. [Crossref] [PubMed]
- Xiong J, Barayan R, Louie AV, et al. Novel therapeutic combinations with PARP inhibitors for small cell lung cancer: A bench-to-bedside review. Semin Cancer Biol 2022;86:521-42. [Crossref] [PubMed]
- Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116-25. [Crossref] [PubMed]
- Ganti AKP, Loo BW, Bassetti M, et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:1441-64. [Crossref] [PubMed]
- Higgins KA, Gorgens S, Sudmeier LJ, et al. Recent developments in limited stage small cell lung cancer. Transl Lung Cancer Res 2019;8:S147-52. [Crossref] [PubMed]
- Liu SV, Reck M, Mansfield AS, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol 2021;39:619-30. [Crossref] [PubMed]
- Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51-65. [Crossref] [PubMed]
- Cheng Y, Han L, Wu L, et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022;328:1223-32. [Crossref] [PubMed]
- Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:739-47. [Crossref] [PubMed]
- Lhuillier C, Rudqvist NP, Elemento O, et al. Radiation therapy and anti-tumor immunity: exposing immunogenic mutations to the immune system. Genome Med 2019;11:40. [Crossref] [PubMed]
- Esposito A, Criscitiello C, Curigliano G. Immune checkpoint inhibitors with radiotherapy and locoregional treatment: synergism and potential clinical implications. Curr Opin Oncol 2015;27:445-51. [Crossref] [PubMed]
- De Martino M, Daviaud C, Vanpouille-Box C. Radiotherapy: An immune response modifier for immuno-oncology. Semin Immunol 2021;52:101474. [Crossref] [PubMed]
- Peters S, Pujol JL, Dafni U, et al. Consolidation nivolumab and ipilimumab versus observation in limited-disease small-cell lung cancer after chemo-radiotherapy - results from the randomised phase II ETOP/IFCT 4-12 STIMULI trial. Ann Oncol 2022;33:67-79. [Crossref] [PubMed]
- Welsh JW, Heymach JV, Guo C, et al. Phase 1/2 Trial of Pembrolizumab and Concurrent Chemoradiation Therapy for Limited-Stage SCLC. J Thorac Oncol 2020;15:1919-27. [Crossref] [PubMed]
- Park S, Noh JM, Choi YL, et al. Durvalumab with chemoradiotherapy for limited-stage small-cell lung cancer. Eur J Cancer 2022;169:42-53. [Crossref] [PubMed]
- Wang S, Sun J, Chen K, et al. Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med 2021;19:140. [Crossref] [PubMed]
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Mandaliya H, Jones M, Oldmeadow C, et al. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res 2019;8:886-94. [Crossref] [PubMed]
- Xiong Q, Huang Z, Xin L, et al. Post-treatment neutrophil-to-lymphocyte ratio (NLR) predicts response to anti-PD-1/PD-L1 antibody in SCLC patients at early phase. Cancer Immunol Immunother 2021;70:713-20. [Crossref] [PubMed]
- Li L, Pi C, Yan X, et al. Prognostic Value of the Pretreatment Lung Immune Prognostic Index in Advanced Small Cell Lung Cancer Patients Treated With First-Line PD-1/PD-L1 Inhibitors Plus Chemotherapy. Front Oncol 2021;11:697865. [Crossref] [PubMed]
- Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease? Lung Cancer 2002;37:271-6. [Crossref] [PubMed]
- Dziedzic EA, Gąsior JS, Tuzimek A, et al. The Association between Serum Vitamin D Concentration and New Inflammatory Biomarkers-Systemic Inflammatory Index (SII) and Systemic Inflammatory Response (SIRI)-In Patients with Ischemic Heart Disease. Nutrients 2022;14:4212. [Crossref] [PubMed]
- Chen L, Bai P, Kong X, et al. Prognostic Nutritional Index (PNI) in Patients With Breast Cancer Treated With Neoadjuvant Chemotherapy as a Useful Prognostic Indicator. Front Cell Dev Biol 2021;9:656741. [Crossref] [PubMed]
- Kazemi M, Ladbury C, Liu J, et al. Thoracic Radiation in Limited Stage Small Cell Lung Cancer: Trends in Radiation Fractionation. Clin Lung Cancer 2023;24:322-8. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399-424.
- Weichselbaum RR, Liang H, Deng L, et al. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol 2017;14:365-79. [Crossref] [PubMed]
- Charpentier M, Spada S, Van Nest SJ, et al. Radiation therapy-induced remodeling of the tumor immune microenvironment. Semin Cancer Biol 2022;86:737-47. [Crossref] [PubMed]
- Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol 2020;38:2369-79. [Crossref] [PubMed]
- Duan J, Cui L, Zhao X, et al. Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2020;6:375-84. [Crossref] [PubMed]
- Keogh A, Finn S, Radonic T. Emerging Biomarkers and the Changing Landscape of Small Cell Lung Cancer. Cancers (Basel) 2022;14:3772. [Crossref] [PubMed]
- Mezquita L, Auclin E, Ferrara R, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:351-7. [Crossref] [PubMed]
- Sorich MJ, Rowland A, Karapetis CS, et al. Evaluation of the Lung Immune Prognostic Index for Prediction of Survival and Response in Patients Treated With Atezolizumab for NSCLC: Pooled Analysis of Clinical Trials. J Thorac Oncol 2019;14:1440-6. [Crossref] [PubMed]
- Qi W, Zhao S, Chen J. Prognostic role of pretreatment lung immune prognostic index in extensive-stage small-cell lung cancer treated with platinum plus etoposide chemotherapy. Cancer Biomark 2021;31:177-85. [Crossref] [PubMed]
- Sun B, Hou Q, Liang Y, et al. Prognostic ability of lung immune prognostic index in limited-stage small cell lung cancer. BMC Cancer 2022;22:1233. [Crossref] [PubMed]


