
Hyperprogressive disease under anti-PD-1 rechallenge after initial response to anti-PD-1 treatment for non-small cell lung cancer: a case report
Highlight box
Key findings
• Our study reported a patient who showed initial partial response to anti-programmed cell death 1 (anti-PD-1) treatment underwent hyperprogressive disease when rechallenging the same immunotherapy.
• The decreased ratio of CD8+ T cells to Foxp3+ regulatory T cells in the tumor microenvironment and the longitudinal change of neutrophil-to-lymphocyte ratios (NLR) in peripheral blood were suggested to be associated with hyperprogressive disease.
What is known and what is new?
• The rechallenge of PD-1/programmed cell death ligand 1 (PD-L1) inhibitors can be a treatment option in non-small cell lung cancer patients who once responded to them. However, the hyperprogressive phenomenon after anti-PD-1/PD-L1 rechallenge has not been reported.
• Hyperprogressive disease occurred under anti-PD-1 rechallenge despite of a favorable response to initial anti-PD-1 treatment.
• Increased regulatory T cells were suggested to be associated with hyperprogressive disease.
• NLR increased dramatically when hyperprogressive disease occurred after anti-PD-1/PD-L1 rechallenge.
What is the implication, and what should change now?
• Tumor immune microenvironment can change dynamically even after cessation of anti-PD-1/PD-L1 therapy, and may be associated with increased risk for hyperprogression during rechallenge with anti-PD-1/PD-L1 treatment.
• Patients rechallenged with anti-PD-1 after a favorable response to initial anti-PD-1 treatment should be closely monitored.
Introduction
Immune checkpoint blockade with programmed cell death 1 (PD-1) or its ligand 1 (PD-L1) inhibitors are being widely used to successfully treat non-small cell lung cancer (NSCLC) (1). Recently, hyperprogressive disease, which is characterized by an unexpected acceleration of cancer growth when treated with PD-1/PD-L1 inhibitors, has been reported in a subset of patients with NSCLC, since immune checkpoint inhibitors are increasingly used across solid cancers in clinical settings (2). Hyperprogressive disease has shown a deleterious effect on patients and is associated with worse outcomes as a serious immune-related adverse event (irAE) (3).
Our previous study demonstrated that the rechallenge of PD-1/PD-L1 inhibitors can be a treatment option in NSCLC patients who once responded to them (4). However, the hyperprogressive phenomenon during anti-PD-1/PD-L1 rechallenge has not been reported before. Herein, we report an unusual case of hyperprogressive disease when rechallenging with pembrolizumab in a patient with large cell lung cancer, who had a favorable response to the initial pembrolizumab treatment. We present this case in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-765/rc).
Case presentation
A 78-year-old male former smoker was referred to our hospital due to an abnormal shadow on chest computed tomography (CT) which was taken for the examination of cough in September 2018 (Figure 1A). The patient had no respiratory signs like hypoxia or abnormal physical examination. Brain magnetic resonance imaging (MRI), and 18F-fluorodeoxyglucose (FDG) positron-emission tomography (PET) were performed, and he was highly suspected of clinical T3N1M0 stage IIIA lung cancer. Subsequently, he underwent left upper lobe resection with mediastinal lymph node dissection in September 2018. Large cell carcinoma with 5% PD-L1 expression by tumor proportion score was reported, and the pathological stage was T3N2M0 stage IIIB [Union for International Cancer Control (UICC) TNM staging]. The tumor genetic mutation test reported no alteration in EGFR, ALK, ROS1, or BRAF.
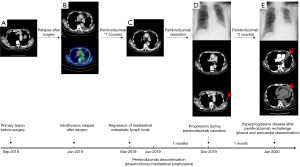
Four months after surgery, CT and FDG-PET reported recurrences at mediastinal and right supraclavicular lymph nodes, and at left ischial and right sacral bones with Eastern Cooperative Oncology Group Performance Status (ECOG PS) 1 in January 2019 (Figure 1B). The patient preferred pembrolizumab monotherapy with 200 mg q3w after treatment options including platinum-based chemotherapy or pembrolizumab monotherapy were discussed. Partial response was achieved after 2 cycles of pembrolizumab treatment according to response evaluation criteria in solid tumors (RECIST) 1.1 in March 2019 (Figure 1C). Grade 1 diarrhea occurred and was treated with probiotics and fosfomycin. After 4 cycles of pembrolizumab, grade 2 pneumothorax occurred. Pembrolizumab was suspended until the resolution of pneumothorax. After 3 more cycles of pembrolizumab, grade 2 mediastinal emphysema happened. Immunotherapy with pembrolizumab was stopped in June 2019, as a causative link between pneumothorax/mediastinal emphysema and pembrolizumab was highly suspected. The patient was stable in the state of clinical partial response for 6 months.
A left enlarged axillary lymph node was observed in CT imaging in December 2019, although no specialized respiratory symptom was reported in this patient with ECOG PS 1 (Figure 1D). Suspicious cancer cells were detected in pleural effusion thereafter. After pembrolizumab was rechallenged with 2 cycles, the patient visited the emergency room with complaints of dyspnea and peripheral edema. Chest X-ray and CT imaging showed left pleural dissemination with a new metastatic lesion near pleura and a rapid increase of pleural and pericardial effusion, compared to the images before pembrolizumab rechallenge (Figure 1E). Cytology of left pleural and pericardial effusion found malignant cells. Hyperprogressive disease after pembrolizumab rechallenge was therefore identified.
The treatment regimen was switched to tegafur/gimeracil/oteracil (S-1). The disease still progressed though S-1 was administered. Unfortunately, the patient died of lung cancer during the best supportive care.
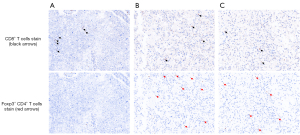
In the tumor microenvironment of surgical specimen, the infiltration of CD8+ T cells recognized as cytotoxic T cells was observed among the tumor cells (Figure 2A). Immunohistochemistry identified CD8+ cells, and more Foxp3+ T cells as regulatory T cells were distributed in the cell blocks of pleural and pericardial effusion which were taken just after hyperprogressive disease (Figure 2B,2C). The ratio of CD8+ T cells to Foxp3+ T cells in primary tumor (median: 2.6) was higher than those of subsequent hyperprogressive sites including malignant pleural effusion (median: 0.7) and pericardial effusion (median: 1.9) (P=0.029 and P=0.34).
The longitudinal neutrophil-to-lymphocyte ratio (NLR) in peripheral blood was measured (Figure 3). NLR increased at the time of intrathoracic relapse and distant metastasis, and decreased when the disease was controlled by immunotherapy with pembrolizumab. However, NLR rose dramatically when hyperprogressive disease occurred with pembrolizumab rechallenge.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for scientific publication, and it is documented in the medical record.
Discussion
This case report described a patient with recurrent large cell lung cancer undergoing hyperprogressive disease with pleural and pericardial dissemination shortly after pembrolizumab rechallenge, although he had an initial partial response to the pembrolizumab treatment.
The emerging evidence indicated that the rechallenge of immune checkpoint inhibitors is a reasonable option for selected NSCLC patients (5-7). Our recent meta-analysis study summarized the rechallenge of immune checkpoint therapy and compared the outcomes among different discontinuation reasons including immune-related adverse Events (irAEs) or a planned interruption of immunotherapy after a defined number of cycles or disease progression during immunotherapy (4). The results showed that the patients undergoing disease progression after initial discontinuation owing to irAEs or a planned interruption are more likely to benefit from the rechallenge of immunotherapy in NSCLC. Compared to the initial immunotherapy, the rechallenge was less effective but had a lower incidence of severe irAEs. Therefore, we re-treated the patient using pembrolizumab again based on that the partial response had ever been achieved in the initial treatment of pembrolizumab.
Hyperprogressive disease is recognized as a flair-up of tumor growth during PD-1/PD-L1 immunotherapy (8). Hyperprogressive disease was initially defined as disease progression at the first evaluation with an increase of tumor growth rate exceeding 100% by RECIST criteria (9). A recent study developed the definition of hyperprogressive disease by adding multiple new lesions into the diagnostic criteria (10). Compared to the CT image before pembrolizumab rechallenge in our case study, the new metastatic lesion near the pleura with a rapid increase of malignant pleural effusion after the 1st cycle of pembrolizumab rechallenge and the new malignant pericardial effusion was detected shortly just after the 2nd cycle, met the diagnostic criteria of hyperprogressive disease. To our knowledge, this is the first case report regarding hyperprogressive disease under anti-PD-1 rechallenge after we searched the existing literature.
Molecular mechanisms of hyperprogressive disease after immunotherapy are yet to be well elucidated (11,12). A recent study revealed that patients with hyperprogression showed marked increases of tumor-infiltrating, highly suppressive regulatory T cells in tumor tissue upon initiation of anti-PD-1/PD-L1 therapy in comparison with their decrease in patients without hyperprogression (13). Consistently, many Foxp3+ cells as regulatory T cells were observed in this patient’s malignant pleural and pericardial effusion when the hyperprogressive disease occurred. By contrast, few Foxp3+ cells as regulatory T cells were detected in the resected tumor before initial immunotherapy. This suggests tumor immune microenvironment can change dynamically even after cessation of anti-PD-1/PD-L1 therapy, and can cause hyperprogression during rechallenge of anti-PD-1/PD-L1 therapy.
The biomarker for the early detection of hyperprogressive disease during immunotherapy is not well identified. High NLR is reported to be associated with poor response to initial immune checkpoint therapy (14,15). We observed in this case that NLR was low when the disease was controlled and NLR increased when the disease progressed. Furthermore, NLR rose dramatically when hyperprogression occurred. NLR may provide useful information for evaluating the status of disease during immunotherapy including hyperprogressive disease.
Conclusions
Immunotherapy rechallenge is a field of interest in lung cancer. This report suggests that patients who showed a favorable response to initial anti-PD-1 treatment may undergo hyperprogressive disease when rechallenging the same immunotherapy. The increased Foxp3+ regulatory T cells in the tumor microenvironment and the longitudinal change of NLRs in peripheral blood were suggested to be associated with hyperprogressive disease. More clinical, biological, and histopathological data are needed to better understand the mechanisms of hyperprogression.
Acknowledgments
S.X. received Japan-China Sasakawa Medical Fellowship from the Sasakawa Memorial Health Foundation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-765/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-765/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-765/coif). T.S. received grants from AstraZeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Novartis, MSD and received payment or honoraria for lectures, presentations, speaker bureaus from AstraZeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Novartis, MSD, Taiho Pharma, Daiichi-Sankyo, Ono Pharmaceutical, Bristol-Myers Squibb, Nippon Kayaku, Pfizer, Takeda, Eli Lilly and Company, AMGEN; outside of this work. K.S. received payment or honoraria for lectures, presentations, speaker bureau from Ethicon and Intuitive. S.K. received grants from Ono Pharmaceutical, Eisai, Boehringer Ingelheim, Astellas Pharma, Daiichi Sankyo, Takara Bio, MSD Chugai, Pfizer, Astra Zeneca, Incyte, Abbvie, Takeda, GSK, LOXO/Lilly and consulting fees from Ono Pharmaceutical, Chugai, Astra Zeneca, Sumitomo Pharma, GSK, Rakuten Medical, ImmuniT Research, United Immunity, Astellas Pharma. SK received lecture fee from Ono Pharmaceutical, Bristol-Myers Squibb, Astra Zeneca, Chugai, MSD, Merck KGaA; outside of this work. K.T. received grants from Chugai Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., NIPPON SHINYAKU Co., Ltd., TSUMURA & Co., Pfizer Inc, TAIHO, PHARMACEUTICAL Co., Ltd., KYORIN Pharmaceutical Co., Ltd., TEIJIN PHARMA LIMITED, Sanofi K. K., ONO PHARMACEUTICAL Co., Ltd., Novartis Pharma K. K, SHIONOGI & Co., Ltd., Eli Lilly Japan K. K., Bayer Yakuhin, Ltd, DAIICHI SANKYO Co., Ltd., NIPRO PHARMA CORPORATION, Asahi Kasei Pharma Corporation, Nippon Kayaku Co., Ltd., Takeda, Pharmaceutical Company Limited, Kyowa Kirin Co., Ltd., and honoraria for speaking at sponsored meetings including Chugai Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., MSD K. K, Pfizer Inc, AstraZeneca K. K, TAIHO PHARMACEUTICAL Co., Ltd., KYORIN Pharmaceutical Co., Ltd., Merck Biopharma Co., Ltd., ONO PHARMACEUTICAL Co., Ltd., Nippon Kayaku Co., Ltd., Novartis Pharma K. K, Eli Lilly Japan K. K, Sumitomo Dainippon Pharma Co., Ltd., Bristol-Myers K. K, Meiji Seika Pharma Co, Ltd., Takeda Pharmaceutical Company Limited, Viatris Inc, Janssen Pharmaceutical K.K, Abbott Japan LLC, Thermo Fisher Scientific Inc. K.T. obtains patents “METHOD FOR DETECTING CELLS” (issued), “THE METHOD FOR DETECTING CIRCULATING TUMOR CELLS USING VIRUS” (pending), and “CALML5 is a novel diagnostic marker for differentiating thymic squamous cell carcinoma from type B3 thymoma” (pending). K.T. serves as Board of Director in The Japan Lung Cancer Society and The Japanese Respiratory Society. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for scientific publication, and it is documented in the medical record.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol 2018;4:1543-52. [Crossref] [PubMed]
- Kim CG, Kim KH, Pyo KH, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol 2019;30:1104-13. [Crossref] [PubMed]
- Xu S, Shukuya T, Tamura J, et al. Heterogeneous Outcomes of Immune Checkpoint Inhibitor Rechallenge in Patients With NSCLC: A Systematic Review and Meta-Analysis. JTO Clin Res Rep 2022;3:100309. [Crossref] [PubMed]
- Fujita K, Uchida N, Kanai O, et al. Retreatment with pembrolizumab in advanced non-small cell lung cancer patients previously treated with nivolumab: emerging reports of 12 cases. Cancer Chemother Pharmacol 2018;81:1105-9. [Crossref] [PubMed]
- Bernard-Tessier A, Baldini C, Martin P, et al. Outcomes of long-term responders to anti-programmed death 1 and anti-programmed death ligand 1 when being rechallenged with the same anti-programmed death 1 and anti-programmed death ligand 1 at progression. Eur J Cancer 2018;101:160-4. [Crossref] [PubMed]
- Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol 2019;5:1310-7. [Crossref] [PubMed]
- Kas B, Talbot H, Ferrara R, et al. Clarification of Definitions of Hyperprogressive Disease During Immunotherapy for Non-Small Cell Lung Cancer. JAMA Oncol 2020;6:1039-46. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]
- Wang M, Huang H, Xu Z, et al. Proposal for multiple new lesions as complement of hyperprogressive disease in NSCLC patients treated with PD-1/PD-L1 immunotherapy. Lung Cancer 2022;173:28-34. [Crossref] [PubMed]
- Lin M, Vanneste BGL, Yu Q, et al. Hyperprogression under immunotherapy: a new form of immunotherapy response?-a narrative literature review. Transl Lung Cancer Res 2021;10:3276-91. [Crossref] [PubMed]
- Champiat S, Ferrara R, Massard C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 2018;15:748-62. [Crossref] [PubMed]
- Kamada T, Togashi Y, Tay C, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A 2019;116:9999-10008. [Crossref] [PubMed]
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Ruste V, Goldschmidt V, Laparra A, et al. The determinants of very severe immune-related adverse events associated with immune checkpoint inhibitors: A prospective study of the French REISAMIC registry. Eur J Cancer 2021;158:217-24. [Crossref] [PubMed]


