
Efficacy of targeted therapy in patients with non-small cell lung cancer harboring very rare mutations in EGFR exon 18
Highlight box
Key findings
• Patients with non-small cell lung cancer (NSCLC) harboring very rare mutations in epidermal growth factor receptor (EGFR) exon 18 have a potential to exhibit a clinically meaningful outcome with EGFR targeting therapy.
What is known and what is new?
• There is no consistent treatment strategy for NSCLC patients harboring very rare mutations in EGFR exon 18 due to the rarity of such mutations.
• In this study, we observed similar progression-free survival (PFS) and overall survival (OS) between NSCLC patients with very rare mutations in EGFR exon 18 and G719X and E709X group.
What is the implication, and what should change now?
• EGFR-tyrosine kinase inhibitors (TKIs) should be one of potential treatment strategies for NSCLC patients harboring very rare mutations in EGFR exon 18, and larger studies for the selection of treatment strategy for patient harboring very rare mutations in EGFR exon 18 are needed.
Introduction
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths (1). Mutations in the epidermal growth factor receptor (EGFR) gene occur in more than 50% of Asian non-small cell lung cancer (NSCLC) patients (2). There are many EGFR mutation subtypes including common mutations, exon 19 deletions and L858R point mutation, which together account for approximately 85% of all EGFR mutations (3,4), and the remaining 15% are called uncommon or rare EGFR mutations, as represented by exon 18 mutations. Specifically, EGFR exon 18 mutations can be identified in 4.6% of east Asian patients with EGFR mutations (3,5).
The better clinical outcome of EGFR-TKIs in NSCLC patients harboring sensitive EGFR mutations has been demonstrated, including uncommon EGFR mutations such as G719X, S768I, and L861Q (6). Afatinib has been further approved for these patients (6-8), and was even a potential therapeutic option for patients harboring E709X in EGFR exon 18 (9). However, with the development of gene detection methods over the past decades, several very rare mutations have been found in exon 18 of the EGFR, including G721R, L718V/Q, and G724S (10-12). Due to the scarcity of these rare mutations, adequate treatment strategies for subgroups of patients are still debated. Recently, a few studies reported that targeted therapy was effective in very rare EGFR 18 exon mutations (12,13). Here, we report clinical outcomes of patients with very rare EGFR 18 exon mutations who had received EGFR-TKIs. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-113/rc).
Methods
Patients and methods
Patients who underwent next-generation sequencing (NGS) and had detectable mutations in EGFR exon 18 from 2015 to 2020 at West China Hospital were retrospectively identified. A total of 105 patients were included in this study, all of whom had been pathologically confirmed as having NSCLC. Out of the initial cohort, a subset of 45 patients who received EGFR-TKIs for their advanced or metastatic NSCLC were included for further analysis. The inclusion criteria for this subsequent cohort were as follows: (I) advanced or metastatic NSCLC; (II) detected mutations in EGFR exon 18; (III) treatment with EGFR-TKIs; and (IV) availability of complete prognostic data. Clinical outcome to treatment, including disease control rate (DCR), progression-free survival (PFS) and overall survival (OS). Tumor response was evaluated by Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1. The DCR was determined as the proportion of patients with complete response (CR), partial response (PR), or stable disease (SD). PFS was measured from the date of initiation of EGFR-TKI treatment until disease progression or death. OS was calculated from the date of initiation of EGFR-TKI to death due to any cause or at the follow up time. Patients were excluded from the subsequent analysis if they met the following criteria: (I) secondary mutations in EGFR exon 18; (II) had undergone surgery and/or perioperative maintenance treatment; (III) had received radiotherapy or chemotherapy only; and (IV) prognostic information for targeted therapy was missing (Figure S1). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of West China Hospital of Sichuan University (No. 2022-0606), and the requirement for individual consent for this retrospective analysis was waived.
The following information was collected from the medical records of patients retrospectively: age, sex, performance status, smoking history, EGFR mutational status, tumor-node-metastasis (TNM) stage of the primary tumor, pathology, treatment regimen, distant metastasis, and survival status. The pathological sections were reviewed independently by an experienced pathologist.
Definition of very rare mutations in exon 18
In our retrospective study, we defined very rare mutations in EGFR exon 18 as follows: mutations other than G719X or E709X. Very rare mutations in EGFR exon 18 include point mutations, deletions, and others, which were observed in approximately 15% of NSCLC patients with EGFR exon 18 mutations.
Statistical analysis
The comparison of clinical characteristics between G719X or E709X group and the very rare mutation group was performed using Fisher’s exact test. Survival time was estimated using the Kaplan-Meier method, and groups were compared using log-rank tests. All statistical analyses were conducted with SPSS (version 26; IBM Corp, Armonk, NY, USA) and R software (version 4.0.3 or version 4.2.2; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Identification and distribution of different subtypes of EGFR exon 18 mutations in 105 NSCLC patients
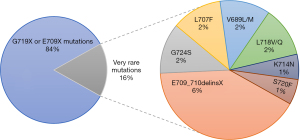
A summary of the distribution of the EGFR exon 18 mutations in 105 NSCLC patients is provided in Figure 1. Most of the patients (88/105, 84%) had G719X or E709X mutations, including G719A, G719C, G719D, G719S, E709A, E709G, E709K, E709Q, and E709V. On the other hand, 17 (16%) had very rare mutations in exon 18, among whom 7 patients had E709_710delinsX, followed by L707F, G724S, V689L/M, L718V/Q, S720F, and K714N. The baseline characteristics of NSCLC patients harboring G719X or E709X and very rare mutations were compared. We found no significant difference between these two groups (Table S1).
Clinical characteristics and the efficacy of targeted therapy in NSCLC patients harboring very rare EGFR exon 18 mutations
Patient characteristics for the subsequent analysis are shown in Table 1. Among the 45 NSCLC patients, 39 had G719X or E709X, and the remaining 6 had very rare mutations. All these patients had lung adenocarcinoma, and their pathological images were presented in Figure S2. Five of them were stage IVA NSCLC, and the remaining one was stage III. The median age was 60.5 years, male patients were predominant (5/6, 83.3%), most of them were non-smokers, and the median follow-up time was 27.6 months. In the very rare mutation group, 5 (5/6, 83.3%) patients received first-generation EGFR-TKIs, and the remaining 1 (1/6, 16.7%) patient received second-generation EGFR-TKI; 5 of them received targeted therapy as first-line therapy. The baseline characteristics were identical between patients with G719X or E709X and those with very rare mutations (Table 1).
Table 1
| Characteristics | Overall (N=45) |
G719X or E709X mutations (N=39) |
Very rare mutations (N=6) |
P value |
|---|---|---|---|---|
| Presence of common mutation, n (%) | 0.742 | |||
| Yes | 9 (20.0) | 7 (17.9) | 2 (33.3) | |
| No | 36 (80.0) | 32 (82.1) | 4 (66.7) | |
| L858R or 19del, n (%) | ||||
| L858R | 7 (15.6) | 6 (15.4) | 1 (16.7) | 1.000 |
| 19del | 2 (4.4) | 1 (2.6) | 1 (16.7) | 0.252 |
| Follow-up time (months), median (IQR) | 25.60 (16.97, 39.17) | 25.07 (15.03, 39.25) | 27.63 (23.87, 28.40) | 0.570 |
| Age (years), median (IQR) | 60.00 (55.00, 73.00) | 60.00 (55.00, 73.00) | 60.50 (52.25, 67.25) | 0.514 |
| Age (years), n (%) | 1.000 | |||
| <60 | 19 (42.2) | 16 (41.0) | 3 (50.0) | |
| ≥60 | 26 (57.8) | 23 (59.0) | 3 (50.0) | |
| Gender, n (%) | 0.488 | |||
| Female | 17 (37.8) | 16 (41.0) | 1 (16.7) | |
| Male | 28 (62.2) | 23 (59.0) | 5 (83.3) | |
| Smoking, n (%) | 0.832 | |||
| Current/former smoking | 11 (24.4) | 10 (25.6) | 1 (16.7) | |
| Never smoking | 29 (64.4) | 25 (64.1) | 4 (66.7) | |
| Unknown | 5 (11.1) | 4 (10.3) | 1 (16.7) | |
| Smoking index, n (%) | 0.631 | |||
| ≤200 | 32 (71.1) | 27 (69.2) | 5 (83.3) | |
| >200 | 5 (11.1) | 5 (12.8) | 0 (0.0) | |
| Unknown | 8 (17.8) | 7 (17.9) | 1 (16.7) | |
| ECOG PS, n (%) | 0.871 | |||
| ≥2 | 5 (11.1) | 4 (10.3) | 1 (16.7) | |
| 0–1 | 26 (57.8) | 23 (59.0) | 3 (50.0) | |
| Unknown | 14 (31.1) | 12 (30.8) | 2 (33.3) | |
| Pathology, n (%) | 1.000 | |||
| Adenocarcinoma | 43 (95.6) | 37 (94.9) | 6 (100.0) | |
| Squamous cell carcinoma | 2 (4.4) | 2 (5.1) | 0 (0.0) | |
| Stage, n (%) | 0.122 | |||
| III | 5 (11.1) | 4 (10.3) | 1 (16.7) | |
| IVA | 23 (51.1) | 18 (46.2) | 5 (83.3) | |
| IVB | 17 (37.8) | 17 (43.6) | 0 (0.0) | |
| Metastases, n (%) | 0.405 | |||
| Multiple organ metastasis | 17 (37.8) | 16 (41.0) | 1 (16.7) | |
| Single organ metastasis | 20 (44.4) | 17 (43.6) | 3 (50.0) | |
| No organ metastasis | 8 (17.8) | 6 (15.4) | 2 (33.3) | |
| Treatment lines, n (%) | 0.620 | |||
| First-line | 43 (95.6) | 38 (97.4) | 5 (83.3) | |
| Second-line | 2 (4.4) | 1 (2.6) | 1 (16.7) | |
| EGFR-TKIs, n (%) | 0.065 | |||
| First-generation | 18 (40.0) | 13 (33.3) | 5 (83.3) | |
| Second-generation | 24 (53.3) | 23 (59.0) | 1 (16.7) | |
| Third-generation | 3 (6.7) | 3 (7.7) | 0 (0.0) | |
| Response, n (%) | 0.622 | |||
| PD | 1 (2.2) | 1 (2.6) | 0 (0.0) | |
| PR | 14 (31.1) | 13 (33.3) | 1 (16.7) | |
| SD | 19 (42.2) | 15 (38.5) | 4 (66.7) | |
| Unknown | 11 (24.4) | 10 (25.6) | 1 (16.7) |
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; PS, performance status; common mutation, EGFR 19del and L858R; PD, progressive disease; PR, partial response; SD, stable disease.
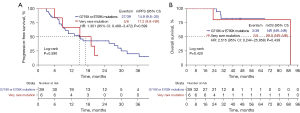
Among NSCLC patients harboring very rare EGFR exon 18 mutations, all 4 patients who received EGFR-TKIs as the first-line therapy experienced SD, and 1 patient experienced PR. The objective response was not available in the remaining one patient. A waterfall plot of the tumor retraction rate (TRR) is shown in Figure 2A. The DCR was 100% in NSCLC patients harboring very rare mutations in EGFR exon 18 and 97.4% in patients with G719X or E709X mutations, and there was no significant difference in these two groups (P>0.99) (Figure 2B). Figure 3 shows the duration of the treatment and survival time after EGFR-TKI treatment. The median PFS of NSCLC patients with very rare mutations in EGFR exon 18 was 17.2 months, which was not significantly different from that of patients with G719X or E709X group [17.2 vs. 14.9 months, P=0.59, hazard ratio (HR) =1.301, 95% confidence interval (CI): 0.488–3.472, P=0.599] (Figure 4A). For OS, as shown in Figure 4B, the very rare mutation group had a similar OS compared with G719X or E709X group (89.8 vs. not reached, P=0.42, HR =2.515, 95% CI: 0.244–25.958, P=0.439).


Discussion
In this study, we reported clinical efficacy of EGFR-TKIs in NSCLC patients harboring very rare mutations in EGFR exon 18; although no patients achieved a CR, 4 of 6 patients achieved SD. In addition, we observed no significant difference in PFS and OS between NSCLC patients harboring very rare EGFR exon 18 mutations and those with G719X or E709X mutations, suggesting that EGFR-TKI should be considered during treatment of NSCLC patients harboring any EGFR exon 18 mutations.
With the increasing use of NGS-based analysis in clinical practice of NSCLC patients, we often observe patients with very rare mutations in EGFR exon 18 (14). In this descriptive study, we investigated the distribution of mutations in EGFR exon 18 in a Chinese NSCLC cohort from a single center. We identified that the incidence of very rare mutations in exon 18 was 16% (17/105), and the majority of the very rare mutations were E709_710delinsX (N=7). In addition, there were other very rare mutations in EGFR exons 18, including G724S, L707F, K714N, L718V/Q, V689L/M, and S720F mutations. Previous work has reported a frequency of 0.44% (26/5905) for E709_710delinsX mutations in EGFR mutant lung cancer patients (15). Another study reported that 4.9% (4/82) had EGFR G724S in exon 18 (16), which was more than the incidence of EGFR G724S we identified in our study. Due to the low incidence of NSCLC patients with very rare EGFR mutations in exon 18, the information on their epidemiology is still inconsistent and incomplete, and a large cohort study of patients harboring EGFR mutations is warranted.
Furthermore, due to the low incidence of EGFR exon 18 mutations observed in approximately 5% of EGFR-mutant NSCLC patients and because EGFR mutations cause inconsistent responses to EGFR-TKIs, many clinical trials have been conducted to explore the efficacy of diverse treatments for EGFR exon 18-mutant NSCLC patients (17). Xu et al. discovered that there was no significant difference in PFS between second-generation EGFR-TKIs and first-generation EGFR-TKIs (16). Passaro et al. found that EGFR exon 18 and combination mutations could be considered potentially sensitive rare mutations because of a similar survival compared with common EGFR mutations (18). Currently, afatinib, a second-generation irreversible ErbB family blocker, has been sufficiently validated to have clinical activity in NSCLC patients harboring major rare mutations and compound EGFR mutations (7,19). The efficacy of osimertinib for NSCLC patients with these rare EGFR mutations has also been studied, and in a single arm phase II study, Cho et al. demonstrated favorable activity in patients with NSCLC harboring uncommon EGFR mutations (20), and other studies also found similarly favorable clinical activities (21-24).
However, the efficacy of EGFR-TKIs for NSCLC patients harboring very rare EGFR mutations is still unclear due to the exclusion of patients harboring very rare EGFR mutations from many previous studies and the low incidence of these mutations (23,25-27). For patients with very rare mutations in EGFR exon 18, there was no consensus concerning the treatment outcome and treatment strategy of these patients, and only some case reports and small sample studies had reported diverse therapeutic strategies and clinical outcomes (10,12,15,28). Lee et al. reported a patient harboring the EGFR G724S mutation who was susceptible to gefitinib and observed a long-term response (12). Jelli et al. reported a NSCLC patient harboring the EGFR E709_710delinsD mutation who achieved a complete response to afatinib (13). However, in addition to the favorable response reported in the above cases, some case reports have described that patients with very rare mutations in EGFR exon 18 did not experience clinical benefit from EGFR-TKIs. Furthermore, some case studies have demonstrated that EGFR L718V/Q is a mechanism of resistance to osimertinib but potentially retains sensitivity to afatinib (10,29-31). To identify the efficacy of targeted therapy in patients harboring very rare mutations in EGFR exon 18, we initiated the first descriptive study to investigate the therapeutic responses in these patients. In the present study, we found that patients with very rare EGFR exon 18 mutations achieved a favorable DCR (100%) and a median PFS of 17.2 months, which was identical to that of patients harboring G719X or E709X mutations. Furthermore, in a previous study, some patients with uncommon EGFR alterations were reported to derive clinical benefit from immune checkpoint inhibitors and patients with uncommon EGFR mutations had high rates of programmed cell death ligand 1 (PD-L1) expression and CD8+ tumor infiltrating lymphocytes (TILs) (32). Thus, to date, the predominant treatment strategy for patients with very rare EGFR exon 18 has remained uncertain; our study found that EGFR-TKIs could serve as a potential treatment strategy for patients harboring very rare mutations in EGFR exon 18 despite the quite limited number of patients.
This retrospective study is subject to several inherent limitations that warrant consideration. First, the present study included only 6 patients who harbored very rare mutations in EGFR exon 18 and received EGFR-TKIs. Since the very rare EGFR exon 18 mutations were heterogeneous mutations, the therapeutic strategy selected for patients with these mutations was diverse, resulting in a limited number of patients receiving EGFR-TKIs. However, appropriate selections of treatment are important, and retrospective studies are the first step in establishing effective treatment strategies. Another limitation could have been the use of first- and second-line EGFR-TKIs since nowadays osimertinib could be considered the standard in first-line treatment, and in the present study, there were no patients harboring very rare mutations received osimertinib. Additionally, a significant limitation is that we did not analyze the influence on the treatment outcome of compound mutations, defined as harboring sensitive EGFR 19 del or L858R missense mutations in exon 21. Attili et al. reported that NSCLC patients with combined common plus uncommon EGFR mutations achieved response rates of 40–80% and 100% with first-generation EGFR-TKIs and afatinib, respectively (33), and another study identified that complex mutations are similarly sensitive to EGFR-TKIs treatment as are classical mutations (8). This lack of compound mutation data creates a challenge to draw definitive conclusions about the response rate impact of very rare mutations in EGFR exon 18 in this population. We acknowledge that these limitations may affect the robustness of our results and the strength of our conclusions. Therefore, the outcomes of this study should be interpreted cautiously and further validated in a large cohort study with exclusion of the impact of compound mutations.
Conclusions
Our study is the first descriptive study examining the response of patients with very rare EGFR mutations in exon 18 to EGFR-TKIs. EGFR-TKIs showed promising results in the treatment of very rare mutations in EGFR exon 18. Further studies, especially on the selection of treatment strategies, are needed.
Acknowledgments
We thank Dr. Ping Zhou of Department of Pathology in West China Hospital of Sichuan University for reviewing pathological sections.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-113/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-113/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-113/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-113/coif). K.S. has received research grant from AstraZeneca, and has received honoraria from AstraZeneca, Chugai, Boehringer Ingelheim, and Taiho, outside the submitted work. M.S. reported speakers bureaus from ROCHE, BMS, ASTRA ZENECA and NOVARTIS. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of West China Hospital of Sichuan University (No. 2022-0606), and the requirement for individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Cheng C, Wang R, Li Y, et al. EGFR Exon 18 Mutations in East Asian Patients with Lung Adenocarcinomas: A Comprehensive Investigation of Prevalence, Clinicopathologic Characteristics and Prognosis. Sci Rep 2015;5:13959. [Crossref] [PubMed]
- Yuan M, Huang LL, Chen JH, et al. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther 2019;4:61. [Crossref] [PubMed]
- Zhou S, Hu X, Wang Y, et al. Clinicopathologic characteristics and outcome of patients with different EGFR mutations. Asia Pac J Clin Oncol 2019;15:166-71. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Yang JC, Schuler M, Popat S, et al. Afatinib for the Treatment of Non-Small Cell Lung Cancer Harboring Uncommon EGFR Mutations: An Updated Database of 1023 Cases Brief Report. Front Oncol 2022;12:834704. [Crossref] [PubMed]
- Gursoy P, Tatli AM, Erdem D, et al. Real-life comparison of afatinib and erlotinib in non-small cell lung cancer with rare EGFR exon 18 and exon 20 mutations: a Turkish Oncology Group (TOG) study. J Cancer Res Clin Oncol 2023;149:865-75. [Crossref] [PubMed]
- Hao Y, Xu M, Zhou H, et al. Efficacy of EGFR-Tyrosine Kinase Inhibitors for advanced non-small cell lung cancer patients harboring rare EGFR mutations of exon 18 E709X. Med Oncol 2022;40:34. [Crossref] [PubMed]
- Li M, Qin J, Xie F, et al. L718Q/V mutation in exon 18 of EGFR mediates resistance to osimertinib: clinical features and treatment. Discov Oncol 2022;13:72. [Crossref] [PubMed]
- Velcheti V, Khunger M, Abazeed ME. Novel EGFR Exon 18 (G721R) Mutation in a Patient with Non-Small Cell Lung Carcinoma with Lack of Response to Afatinib. J Thorac Oncol 2017;12:e16-8. [Crossref] [PubMed]
- Lee TH, Yang CJ. De novo exon 18 G724S point mutation may be sensitive to Gefitinib. Kaohsiung J Med Sci 2021;37:918-9. [Crossref] [PubMed]
- Jelli B, Taton O, D'Haene N, et al. Complete Response to Afatinib of an EGFR Exon 18 delE709_T710insD-Mutated Stage IV Lung Adenocarcinoma. Eur J Case Rep Intern Med 2021;8:002749. [Crossref] [PubMed]
- Prabhash K, Advani SH, Batra U, et al. Biomarkers in Non-Small Cell Lung Cancers: Indian Consensus Guidelines for Molecular Testing. Adv Ther 2019;36:766-85. [Crossref] [PubMed]
- Huang Y, Xu C, Sun Y, et al. Rare EGFR E709-T710delinsX: Molecular characteristics and superior response to afatinib treatment in NSCLC patients. Lung Cancer 2022;172:117-23. [Crossref] [PubMed]
- Xu H, Yang G, Li W, et al. EGFR Exon 18 Mutations in Advanced Non-Small Cell Lung Cancer: A Real-World Study on Diverse Treatment Patterns and Clinical Outcomes. Front Oncol 2021;11:713483. [Crossref] [PubMed]
- Leduc C, Merlio JP, Besse B, et al. Clinical and molecular characteristics of non-small-cell lung cancer (NSCLC) harboring EGFR mutation: results of the nationwide French Cooperative Thoracic Intergroup (IFCT) program. Ann Oncol 2017;28:2715-24. [Crossref] [PubMed]
- Passaro A, Prelaj A, Bonanno L, et al. Activity of EGFR TKIs in Caucasian Patients With NSCLC Harboring Potentially Sensitive Uncommon EGFR Mutations. Clin Lung Cancer 2019;20:e186-94. [Crossref] [PubMed]
- Heigener DF, Schumann C, Sebastian M, et al. Afatinib in Non-Small Cell Lung Cancer Harboring Uncommon EGFR Mutations Pretreated With Reversible EGFR Inhibitors. Oncologist 2015;20:1167-74. [Crossref] [PubMed]
- Cho JH, Lim SH, An HJ, et al. Osimertinib for Patients With Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J Clin Oncol 2020;38:488-95. [Crossref] [PubMed]
- Wang C, Zhao K, Hu S, et al. Clinical Outcomes of Afatinib Versus Osimertinib in Patients With Non-Small Cell Lung Cancer With Uncommon EGFR Mutations: A Pooled Analysis. Oncologist 2023;28:e397-405. [Crossref] [PubMed]
- Bar J, Peled N, Schokrpur S, et al. UNcommon EGFR Mutations: International Case Series on Efficacy of Osimertinib in Real-Life Practice in First-LiNe Setting (UNICORN). J Thorac Oncol 2023;18:169-80. [Crossref] [PubMed]
- Villaruz LC, Wang X, Bertino EM, et al. A single-arm, multicenter, phase II trial of osimertinib in patients with epidermal growth factor receptor exon 18 G719X, exon 20 S768I, or exon 21 L861Q mutations. ESMO Open 2023;8:101183. [Crossref] [PubMed]
- Ji J, Aredo JV, Piper-Vallillo A, et al. Osimertinib in NSCLC With Atypical EGFR-Activating Mutations: A Retrospective Multicenter Study. JTO Clin Res Rep 2023;4:100459. [Crossref] [PubMed]
- Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 2014;25:126-31. [Crossref] [PubMed]
- Pretelli G, Spagnolo CC, Ciappina G, et al. Overview on Therapeutic Options in Uncommon EGFR Mutant Non-Small Cell Lung Cancer (NSCLC): New Lights for an Unmet Medical Need. Int J Mol Sci 2023;24:8878. [Crossref] [PubMed]
- Dong W, Wang C, Wang C, et al. Inconsistent clinical outcomes following afatinib treatment in NSCLC patients harboring uncommon epidermal growth factor receptor mutation. Front Oncol 2022;12:999606. [Crossref] [PubMed]
- Wei Y, Jiang B, Liu S, et al. Afatinib as a Potential Therapeutic Option for Patients With NSCLC With EGFR G724S. JTO Clin Res Rep 2021;2:100193. [Crossref] [PubMed]
- Raez LE, Carracedo C, Drusbosky LM, et al. EGFR L718V (+)/T790M (-) as a Mechanism of Resistance in Patients with Metastatic Non-small-cell Lung Cancer with EGFR L858R Mutations. Clin Lung Cancer 2021;22:e817-9. [Crossref] [PubMed]
- Fang W, Gan J, Huang Y, et al. Acquired EGFR L718V Mutation and Loss of T790M-Mediated Resistance to Osimertinib in a Patient With NSCLC Who Responded to Afatinib. J Thorac Oncol 2019;14:e274-5. [Crossref] [PubMed]
- Liu Y, Li Y, Ou Q, et al. Acquired EGFR L718V mutation mediates resistance to osimertinib in non-small cell lung cancer but retains sensitivity to afatinib. Lung Cancer 2018;118:1-5. [Crossref] [PubMed]
- Chen K, Cheng G, Zhang F, et al. PD-L1 expression and T cells infiltration in patients with uncommon EGFR-mutant non-small cell lung cancer and the response to immunotherapy. Lung Cancer 2020;142:98-105. [Crossref] [PubMed]
- Attili I, Passaro A, Pisapia P, et al. Uncommon EGFR Compound Mutations in Non-Small Cell Lung Cancer (NSCLC): A Systematic Review of Available Evidence. Curr Oncol 2022;29:255-66. [Crossref] [PubMed]


