
The evolving treatment landscape for BRAF-mutated non-small cell lung cancer
Oncogene-driven non-small cell lung cancer (NSCLC) represents a subgroup of lung cancers that harbors specific molecular activations, and is responsive to targeted therapies. Indeed, EGFR and ALK-inhibitors are approved in the first-line setting for NSCLC with EGFR and ALK driver alterations (1). In these subtypes of NSCLC, targeted therapies yield significant improvement in objective response rate (ORR) and progression-free survival (PFS) compared to chemotherapy (1). For other oncogenic drivers in NSCLC, such as BRAF, HER2, MET, RET, ROS1, KRAS and NTRK, targeted therapies are also approved on the basis of single-arm studies (1). Herein we will discuss the implications of the recently published phase 2 PHAROS clinical trial, that evaluated the efficacy of encorafenib and binimetinib for the treatment of BRAF mutant NSCLC (2).
BRAF is a kinase that signals in the mitogen activated protein kinase (MAPK) pathway (3). BRAF mutations confer constitutive activation of the MAPK pathway resulting in cell proliferation and tumorigenesis. BRAF mutations are found in 3–5% of NSCLC (4), and can be grouped into three classes based upon molecular characteristics. Class 1 BRAF mutations occur at the V600 residue and signal as constitutively active monomers in a RAS-independent manner (3). BRAF non-V600 mutations can be further classified as RAS-independent active dimers with intermediate to high kinase activity (Class 2), and RAS-dependent kinase-impaired dimers (Class 3) (3). Class 1 BRAF mutations make up the majority of oncogenic BRAF mutations in most cancer types. However, Class 1 mutations only comprise 33–50% of all oncogenic BRAF mutations in NSCLC (5). To date, there are only approved targeted therapies for Class 1 BRAF mutant cancers (6). In NSCLC, all classes of BRAF mutations have been reported as a negative prognostic factor compared to BRAF wild-type (WT) NSCLC (7-9).
BRAF inhibitors were first studied for Class 1 BRAF mutant melanoma, demonstrating impressive response rates but were associated with rapid resistance largely due to MAPK pathway reactivation. Indeed, single-agent BRAF inhibitors (vemurafenib or dabrafenib) have elicited ORR of 48–53% and PFS of 5.1–6.8 months in melanoma (10-12). Due to MAPK pathway reactivation and the development of rapid resistance with BRAF inhibitor monotherapy, BRAF and MEK inhibitor combinations were developed. Combinations of BRAF and MEK inhibitors successfully prolonged PFS, and ultimately overall survival (OS) compared to BRAF inhibitor monotherapy (13,14). In NSCLC, single-agent BRAF inhibitors (vemurafenib or dabrafenib) have elicited ORR of 33–45% and PFS of 5.3–12.9 months (15-17). Subsequent trials evaluating the BRAF and MEK inhibitor combination of dabrafenib and trametinib have demonstrated ORR of 40–68% and median PFS of 10.2–10.8 months (18-20) (Table 1). This data has led to the 2023 European Society for Medical Oncology (ESMO) clinical practice guidelines for oncogene-addicted metastatic NSCLC recommending this regimen in the first-line for NSCLC with BRAF V600E mutations (1). Furthermore, the dabrafenib and trametinib combination has earned tumor agnostic approval for the treatment of all metastatic non-colorectal cancers with Class 1 BRAF mutations (21).
Table 1
| Study | Therapeutic agents | No. of patients | Overall response rate | PFS, months [median (95% CI)] |
OS, months [median (95% CI)] |
Grade 3 or greater adverse events |
|---|---|---|---|---|---|---|
| Subbiah et al. JCO Precis Oncol, 2019 (15) | Vemurafenib | 62 | 81% | |||
| Previously treated | 54 | 37% | 6.1 (5.1–8.3) | 15.4 (8.2–22.6) | ||
| Naïve | 8 | 37.5% | 12.9 (4.0–NE) | NE (4.0–NE) | ||
| Mazieres et al. Ann Oncol, 2020, (16) | Vemurafenib | 101 | 45% | 5.2 (3.8–6.8) | 10.0 (6.8–15.7) | Serious adverse events—36% |
| Previously treated | 80 | |||||
| Naïve | 21 | |||||
| Planchard et al. Lancet Oncol, 2016, (17) | Dabrafenib | 84 | 45% | |||
| Previously treated | 78 | 33% | 5.5 (2.8–7.3) | 15.4 (7.3–NE) | ||
| Naïve | 6 | 67% | 8.4 | |||
| Planchard et al. Lancet Oncol, 2016, (20) | Dabrafenib + trametinib | 57 | 68% | 10.2 (6.9–16.7) | Not described | 75% |
| Previously treated | 57 | |||||
| Naïve | 0 | |||||
| Planchard et al. Lancet Oncol, 2017, (19) | Dabrafenib + trametinib | 36 | 64% | 10.9 (7.0–16.6) | 24.6 (12.3–NE) | 73% |
| Previously treated | 0 | |||||
| Naïve | 36 | |||||
| Salama et al. J Clin Oncol, 2020, (18) | Dabrafenib + trametinib | 5 | 25% | 6.6 | Not described | N/A |
| Previously treated | ||||||
| Naïve | ||||||
| Riely et al. J Clin Oncol, 2023, (2) | Encorafenib + binimetinib | 98 | NE | 42% | ||
| Previously treated | 39 | 46% | 9.3 (6.2–NE) | |||
| Naïve | 59 | 75% | NE (15.7–NE) |
NSCLC, non-small cell lung cancer; PFS, progression-free survival; CI, confidence interval; OS, overall survival; NE, not evaluable.
Clinical efficacy and safety of encorafenib + binimetinib for BRAF V600E mutant metastatic NSCLC
The PHAROS trial was a single-arm, open label study that enrolled 98 patients with BRAF V600E mutant metastatic NSCLC. Amongst these, 59 patients were treatment-naïve, and 39 patients had previously received systemic therapy for metastatic NSCLC. The ORR and median PFS was 75% and not reached (>15.7 months) in treatment-naïve patients, and 46% and 9.3 months in previously treated patients. Grade 3 and 4 treatment-related adverse events (TRAEs) occurred in 38% and 3% of all patients, respectively. The most frequently reported TRAEs of any grade were nausea, diarrhea, fatigue, and vomiting.
The PHAROS trial establishes that encorafenib and binimetinib has clinical activity in BRAF V600E mutant metastatic NSCLC. The efficacy data compare favorably with dabrafenib and trametinib for BRAF V600E NSCLC (Table 1). However, due to differences in the patient populations between these trials, encorafenib and binimetinib is not clearly superior to dabrafenib and trametinib in Class 1 BRAF mutant NSCLC.
The adverse event profile of encorafenib and binimetinib is distinct from dabrafenib and trametinib, and these differences should be considered when choosing the optimal targeted therapy regimen for patients with BRAF mutant NSCLC. While the most common types of TRAEs observed in both were nausea, vomiting, diarrhea and fatigue, there was a notable difference in the rate of pyrexia. The rate of pyrexia of any grade with encorafenib and binimetinib was 22%, and with dabrafenib and trametinib in previously untreated patients, the rate of grade 1 or 2 and grade 3 were 53% and 11%, respectively (19). Additionally, there were numerically fewer TRAEs that led to treatment discontinuation, dose interruption or delay, and dose reduction in those treated with encorafenib and binimetinib (15%, 44%, and 24% of patients, respectively) compared to dabrafenib and trametinib in both previously treated (12%, 61%, and 35% of patients, respectively) or untreated patients (22%, 75%, and 39%) (19,20).
Optimal sequencing of BRAF targeted therapy in NSCLC
While Class 1 BRAF mutations unequivocally predict response to BRAF/MEK inhibitors, the role of these mutations as predictive biomarkers for other therapeutic modalities such as immune checkpoint inhibitors (ICIs) is less clear. NSCLC with Class 1 BRAF mutations experience inferior responses to ICI compared to non-oncogene driven NSCLC, but Class 1 BRAF mutated and KRAS-mutated NSCLC are likely to derive more benefit from ICI than EGFR-mutated or ALK-rearranged NSCLC (22-25). This may be due to the fact that BRAF and KRAS mutations in lung cancer occur more frequently in smokers than EGFR and ALK mutations (26), whereby the carcinogen may establish a more mutagenic cancer that forms an immunoreactive microenvironment primed to respond to immune checkpoint inhibition (27).
The comparison between the therapeutic landscape in BRAF V600E mutated melanoma and NSCLC is also relevant. In melanoma, BRAF and MEK inhibition has fallen out of favor in the first-line compared to immune checkpoint inhibition based on the DREAMseq and SEACOMBIT randomized controlled trials (28,29). These trials randomized the sequence of BRAF and MEK inhibitor combinations with immunotherapy, both demonstrating the superiority of using immunotherapy first. While no such study has been performed in NSCLC, the biological underpinnings of MAPK inhibition establishing an immunosuppressed microenvironment may similarly exist in NSCLC (30,31). While difficult to compare trial populations, data for BRAF and MEK inhibitors in the first line setting compare favourably to frontline immunotherapy and chemotherapy in NSCLC. In the small number of 59 previously untreated patients in the PHAROS trial, median PFS was >15.7 months with encorafenib and binimetinib (2), while immunotherapy plus chemotherapy combinations demonstrated PFS of 8.8 months in metastatic NSCLC patients without EGFR or ALK driver mutations (32). The existing data highlights the need for randomized treatment sequencing data for patients with metastatic NSCLC with BRAF V600E mutations. But based on the data that are currently available, first-line or later line treatment with BRAF and MEK inhibitors are appropriate treatment options for Class 1 BRAF mutant NSCLC.
Biomarkers for BRAF mutant NSCLC
Novel biomarkers may optimize personalized treatment decisions for patients with BRAF V600E NSCLC. Existing biomarkers used in lung cancer such as programmed death-ligand 1 (PD-L1) or tumor mutation burden may predict which patients are likely to derive optimal benefit from immunotherapy versus targeted therapy. Circulating tumor DNA (ctDNA) may similarly offer a modality by which patients with NSCLC harboring BRAF V600E can be stratified for first-line therapy. In the PHAROS trial, an attempt was made to identify molecular alterations to predict response. While CDKN2A and FLT1 alterations showed potential correlation with response, statistical significance was lost upon false discovery correction (2).
Implications for non-V600 BRAF mutant NSCLC
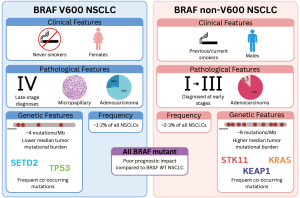
Given that the majority of oncogenic BRAF mutations in NSCLC are non-V600 BRAF mutations, it is imperative to establish novel and improved treatment strategies for these patients. Patients with non-V600 BRAF mutations were not included in the PHAROS trial and prospective data are lacking for the clinical efficacy of targeted therapies in non-V600 BRAF mutant NSCLC. However, a recent systematic review of mostly retrospective data reported response rates of 41% and 12% for BRAF and MEK inhibitor combinations in Class 2 and 3 BRAF mutant NSCLC, respectively (6). It is important to note that these data represent a small cohort of NSCLC patients (n=60) and may over-represent the true response rate, given the retrospective nature of the majority of included patients. Furthermore, the genomic and transcriptomic landscape of non-V600 BRAF mutant NSCLC is distinct from V600 BRAF mutant NSCLC (5), and these differences may influence therapeutic responses (Figure 1). Although both BRAF V600 and non-V600 mutant NSCLC are diagnosed at all stages, BRAF V600 are more likely to be diagnosed at stage IV, whereas non-V600 are more likely to be diagnosed at stages I–III (33). While it is clear that some patients with non-V600 BRAF mutant metastatic NSCLC derive benefit from BRAF and MEK inhibitors, prospective clinical trials are needed to better evaluate the effectiveness of this approach.
In summary, the phase 2 PHAROS trial represents an important step forward in the treatment of patients with NSCLC with BRAF V600E mutations. Encorafenib and binimetinib demonstrated safety and efficacy in this setting, with the outcomes of these patients suggesting signs of improvement compared to previous generations of BRAF and MEK inhibitor combinations and other standard first-line therapies for NSCLC. Despite these promising results, the optimal sequence of therapeutic regimens has not been definitively established; randomized clinical trials exploring this question are warranted.
Acknowledgments
Funding: This work was supported by
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-117/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-117/coif). A.A.N.R. reports a Canadian Cancer Society Challenge Grant (#707457), honoraria from Advanced Accelerator Applications for lectures, and honoraria from EMD Serono for participating in an advisory board, which is unrelated to the topics discussed in this manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:339-57. [Crossref] [PubMed]
- Riely GJ, Smit EF, Ahn MJ, et al. Phase II, Open-Label Study of Encorafenib Plus Binimetinib in Patients With BRAFV600-Mutant Metastatic Non-Small-Cell Lung Cancer. J Clin Oncol 2023;41:3700-11. Erratum in: J Clin Oncol 2024;42:245. [Crossref] [PubMed]
- Dankner M, Rose AAN, Rajkumar S, et al. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene 2018;37:3183-99. [Crossref] [PubMed]
- Guaitoli G, Zullo L, Tiseo M, et al. Non-small-cell lung cancer: how to manage BRAF-mutated disease. Drugs Context 2023;12:2022-11-3. [Crossref] [PubMed]
- Kazandjian S, Rousselle E, Dankner M, et al. The Clinical, Genomic, and Transcriptomic Landscape of BRAF Mutant Cancers. Cancers (Basel) 2024;16:445. [Crossref] [PubMed]
- Dankner M, Wang Y, Fazelzad R, et al. Clinical Activity of Mitogen-Activated Protein Kinase-Targeted Therapies in Patients With Non-V600 BRAF-Mutant Tumors. JCO Precis Oncol 2022;6:e2200107. [Crossref] [PubMed]
- Dagogo-Jack I, Martinez P, Yeap BY, et al. Impact of BRAF Mutation Class on Disease Characteristics and Clinical Outcomes in BRAF-mutant Lung Cancer. Clin Cancer Res 2019;25:158-65. [Crossref] [PubMed]
- Wiesweg M, Preuß C, Roeper J, et al. BRAF mutations and BRAF mutation functional class have no negative impact on the clinical outcome of advanced NSCLC and associate with susceptibility to immunotherapy. Eur J Cancer 2021;149:211-21. [Crossref] [PubMed]
- Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol 2011;29:3574-9. [Crossref] [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [Crossref] [PubMed]
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707-14. [Crossref] [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [Crossref] [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [Crossref] [PubMed]
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867-76. [Crossref] [PubMed]
- Subbiah V, Gervais R, Riely G, et al. Efficacy of Vemurafenib in Patients With Non-Small-Cell Lung Cancer With BRAF V600 Mutation: An Open-Label, Single-Arm Cohort of the Histology-Independent VE-BASKET Study. JCO Precis Oncol 2019;3:PO.18.00266.
- Mazieres J, Cropet C, Montané L, et al. Vemurafenib in non-small-cell lung cancer patients with BRAF(V600) and BRAF(nonV600) mutations. Ann Oncol 2020;31:289-94. [Crossref] [PubMed]
- Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:642-50. [Crossref] [PubMed]
- Salama AKS, Li S, Macrae ER, et al. Dabrafenib and Trametinib in Patients With Tumors With BRAF(V600E) Mutations: Results of the NCI-MATCH Trial Subprotocol H. J Clin Oncol 2020;38:3895-904. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. [Crossref] [PubMed]
- Gouda MA, Subbiah V. Expanding the Benefit: Dabrafenib/Trametinib as Tissue-Agnostic Therapy for BRAF V600E-Positive Adult and Pediatric Solid Tumors. Am Soc Clin Oncol Educ Book 2023;43:e404770. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Rihawi K, Giannarelli D, Galetta D, et al. BRAF Mutant NSCLC and Immune Checkpoint Inhibitors: Results From a Real-World Experience. J Thorac Oncol 2019;14:e57-9. [Crossref] [PubMed]
- Murciano-Goroff YR, Pak T, Mondaca S, et al. Immune biomarkers and response to checkpoint inhibition of BRAF(V600) and BRAF non-V600 altered lung cancers. Br J Cancer 2022;126:889-98. [Crossref] [PubMed]
- Guisier F, Dubos-Arvis C, Viñas F, et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients With Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J Thorac Oncol 2020;15:628-36. [Crossref] [PubMed]
- Sasaki H, Shitara M, Yokota K, et al. Braf and erbB2 mutations correlate with smoking status in lung cancer patients. Exp Ther Med 2012;3:771-5. [Crossref] [PubMed]
- Sorin M, Rezanejad M, Karimi E, et al. Single-cell spatial landscapes of the lung tumour immune microenvironment. Nature 2023;614:548-54. [Crossref] [PubMed]
- Atkins MB, Lee SJ, Chmielowski B, et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients With Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J Clin Oncol 2023;41:186-97. [Crossref] [PubMed]
- Ascierto PA, Mandalà M, Ferrucci PF, et al. Sequencing of Ipilimumab Plus Nivolumab and Encorafenib Plus Binimetinib for Untreated BRAF-Mutated Metastatic Melanoma (SECOMBIT): A Randomized, Three-Arm, Open-Label Phase II Trial. J Clin Oncol 2023;41:212-21. [Crossref] [PubMed]
- Haas L, Elewaut A, Gerard CL, et al. Acquired resistance to anti-MAPK targeted therapy confers an immune-evasive tumor microenvironment and cross-resistance to immunotherapy in melanoma. Nat Cancer 2021;2:693-708. [Crossref] [PubMed]
- Yang Z, Wang Y, Liu S, et al. Enhancing PD-L1 Degradation by ITCH during MAPK Inhibitor Therapy Suppresses Acquired Resistance. Cancer Discov 2022;12:1942-59. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Litvak AM, Paik PK, Woo KM, et al. Clinical characteristics and course of 63 patients with BRAF mutant lung cancers. J Thorac Oncol 2014;9:1669-74. [Crossref] [PubMed]


