
Effect of histological subtype on the efficacy of perioperative chemotherapy in pulmonary sarcomatoid carcinoma: a retrospective study based on SEER population
Highlight box
Key findings
• The histological subtype has an important effect on the efficacy of perioperative chemotherapy (PC) in pulmonary sarcomatoid carcinoma (PSC).
What is known and what is new?
• PSC consists of five histological subtypes. The efficacy of PC in PSC is controversial.
• We investigated the role of PC in 811 PSC patients of different histological subtypes, and found PC only brought survival benefit to carcinosarcoma patients (a PSC subtype), but not to patients of other subtypes.
What is the implication, and what should change now?
• Histological subtype is an important factor influencing the efficacy of PC in PSC, and this finding might explain the controversy about the role of PC in PSC patients. When making treatment strategy for PSC patients, especially when considering PC, physicians should take into account the histological subtype.
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare subtype of lung cancer characterized by aggressive malignancy. It accounts for less than 1% of lung cancer (1,2) and has worse prognosis than other types of lung cancer, with a 5-year survival ranging from 12.3% to 25.1% based on different patient populations (3-6). According to the latest definition of World Health Organization (WHO), PSC consists of five histological subtypes—pleomorphic carcinoma, giant cell carcinoma, spindle cell carcinoma, pulmonary blastoma, and carcinosarcoma—which are very different from other types of lung cancer in terms of morphology and content (7,8). The aggressiveness and heterogenicity have imposed challenges on the clinical treatment of PSC.
Surgery has been proved beneficial for survival and is now the standard treatment for non-advanced PSC patients (9,10). However, it was reported that the recurrence and metastasis rate was high even after complete resection (11,12). The addition of chemotherapy to surgery has been proposed to prevent recurrence and metastasis and to promote the prognosis, but the efficacy of additional chemotherapy is still controversial. Some studies reported the resistance for chemotherapy (11,13,14), while others favored chemotherapy for improved survival (15-17). But what is behind such different conclusions is largely unknown. Given that different histological subtypes of PSC might have distinct characteristics, we hypothesized that histological subtype might be a reason influencing the efficacy of additional chemotherapy. Herein, we conducted this study to investigate the effect of different subtypes of PSC on the efficacy of perioperative chemotherapy (PC) using the data from the Surveillance, Epidemiology, and End Results (SEER) database [2000–2020]. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-41/rc).
Methods
Data source
The SEER database is a U.S. population-based cancer database providing abundant clinicopathological information for most types of cancers. We retrospectively downloaded the data of interest using the software SEER Stat 8.4.2 (18). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patient enrollment
Eligible PSC patients diagnosed between 2000 to 2020 were enrolled. The site recode ICD-O-3/WHO 2008 was set as “Lung and Bronchus”; the ICD-O-3 code 8022 (pleomorphic carcinoma), 8031 (giant cell carcinoma), 8032 (spindle cell carcinoma), 8033 (pseudosarcomatous carcinoma), 8972 (pulmonary blastoma), and 8980 (carcinosarcoma) were used. Code 8033 (pseudosarcomatous carcinoma) meant that the tumor was diagnosed as PSC but the detailed histological subtype was not clear (19). The inclusion criteria of patients were: (I) aged ≥18 years; (II) histologically diagnosed with PSC; (III) underwent sublobectomy, lobectomy, or bilobectomy; (IV) tumor located in clear unilateral lobe; (V) clear survival time and cancer-specific survival (CSS) status. The exclusion criteria were: (I) unclear tumor size; (II) extended excision of the adjacent organ; (III) tumor of “distant” SEER summary stage; (IV) underwent pneumonectomy, which strongly indicated metastatic disease and severely impaired lung function; (V) the reporting source of “Autopsy only” or “Death certificate only”; (VI) underwent perioperative radiotherapy; (VII) incomplete clinical information.
Variables and outcomes
We collected variables including age at which the patient was diagnosed with PSC, sex, race, year of diagnosis, primary tumor site, tumor size, surgery manner, differentiation grade, histological subtype, N stage, SEER summary stage (confirmed by the algorithm created by the SEER database: https://seer.cancer.gov/seerstat/variables/seer/lrd-stage/), PC status, survival, survival status, and whether other in situ/malignant tumor developed before and after the diagnosis of PSC. The overall survival (OS) was defined as the duration of the diagnosis of PSC and the last follow-up or the death of any reasons. The CSS was defined as the duration of the diagnosis of PSC and the last follow-up or the death because of the PSC.
Survival analysis
To investigate the efficacy of PC and the effect of histological subtype on it, we not only compared the survival of all enrolled PSC patients with and without PC, but also conducted histological subtype-specific analyses. To reduce the potential confounding bias, we used propensity score matching (PSM) method to match the independent risk factors of survival between groups. Furthermore, subgroup analyses were conducted in the pleomorphic carcinoma, giant cell carcinoma, spindle cell carcinoma, and carcinosarcoma patients with certain clinicopathological characteristics.
Statistical analysis
Continuous variables were converted into categorical variables using the cutoff values determined by X-tile tool. Categorical variables were described as absolute number and percentage and were analyzed using Pearson Chi-squared test. The Cox proportional hazards regression was used in the univariable and multivariable analyses to identify the independent risk factors of survival. Variables of P<0.1 in the univariable analysis were included into the multivariable analysis. Kaplan-Meier method was used in the survival analyses and the differences were tested by log-rank test. In the PSM, “MatchIt” R package was used and patients were matched at 1:1 ratio using “nearest” method. Hazard ratio (HR) and 95% confidence interval (CI) were used in the subgroup analyses to describe the effect of PC on the survival. The software used in this study was R (R-4.0.3). P<0.05 was considered significant and all P values were two tailed in our study.
Results
Patients and demographic characteristics
A total of 811 PSC patients were enrolled and 210 (25.89%) patients received PC. The median follow-up was 27 (interquartile range: 9–76.5) months. With respect to the treatment sequence of PC in those 210 patients, 166 (79.05%) were after surgery, 34 (16.19%) were unknown, and 10 (4.76%) were of other sequences. Compared to patients who only underwent surgery, patients who received PC were younger (proportion of patients ≥75 years old: 35.3% vs. 16.2%, P<0.001), had larger tumors (proportion of tumors >4 cm: 37.3% vs. 61.9%, P<0.001), were less likely to undergo sublobectomy (proportion: 17.0% vs. 8.1%, P=0.003), had higher N stage (proportion of N1/N2: 13.0% vs. 31.9%, P<0.001), and were at a more advanced stage (proportion of summary stage “regional”: 46.9% vs. 64.3%, P<0.001). Table 1 shows the demographic characteristics of patients. Among all enrolled PSC patients, 264 were pleomorphic carcinoma, 75 were giant cell carcinoma, 113 were spindle cell carcinoma, 21 were pulmonary blastoma, 115 were carcinosarcoma, and 223 were pseudosarcomatous carcinoma which meant detailed histological subtype was not clear other than PSC diagnosis. To better understand the effect of histological subtype on the efficacy of PC, the demographic characteristics of patients of different histological subtypes are shown in Table 2.
Table 1
| Characteristics | Surgery (N=601) | Surgery + chemotherapy (N=210) | P |
|---|---|---|---|
| Age (years) | <0.001 | ||
| <65 | 168 (28.0) | 87 (41.4) | |
| 65–74 | 221 (36.8) | 89 (42.4) | |
| ≥75 | 212 (35.3) | 34 (16.2) | |
| Sex | 0.72 | ||
| Female | 270 (44.9) | 98 (46.7) | |
| Male | 331 (55.1) | 112 (53.3) | |
| Race | 0.52 | ||
| White | 521 (86.7) | 180 (85.7) | |
| Black | 50 (8.3) | 22 (10.5) | |
| Others† | 30 (5.0) | 8 (3.8) | |
| Year of diagnosis | <0.001 | ||
| 2000–2006 | 174 (29.0) | 32 (15.2) | |
| 2007–2013 | 224 (37.3) | 76 (36.2) | |
| 2014–2020 | 203 (33.8) | 102 (48.6) | |
| Primary site | 0.44 | ||
| Right upper lobe | 199 (33.1) | 71 (33.8) | |
| Right middle lobe | 34 (5.7) | 12 (5.7) | |
| Right lower lobe | 110 (18.3) | 39 (18.6) | |
| Left upper lobe | 165 (27.5) | 46 (21.9) | |
| Left lower lobe | 93 (15.5) | 42 (20.0) | |
| Size (cm) | <0.001 | ||
| ≤2 | 129 (21.5) | 18 (8.6) | |
| 2–4 | 248 (41.3) | 62 (29.5) | |
| 4–6 | 119 (19.8) | 62 (29.5) | |
| >6 | 105 (17.5) | 68 (32.4) | |
| Surgery | 0.003 | ||
| Sublobectomy | 102 (17.0) | 17 (8.1) | |
| Lobectomy/bilobectomy | 499 (83.0) | 193 (91.9) | |
| Differentiation | 0.34 | ||
| Well/moderately differentiated | 26 (4.3) | 5 (2.4) | |
| Poorly differentiated | 320 (53.2) | 115 (54.8) | |
| Undifferentiated | 88 (14.6) | 38 (18.1) | |
| Unknown | 167 (27.8) | 52 (24.8) | |
| Histological subtype | 0.17 | ||
| Pseudosarcomatous carcinoma | 164 (27.3) | 59 (28.1) | |
| Pleomorphic carcinoma | 189 (31.4) | 75 (35.7) | |
| Giant cell carcinoma | 57 (9.5) | 18 (8.6) | |
| Spindle cell carcinoma | 93 (15.5) | 20 (9.5) | |
| Pulmonary blastoma | 18 (3.0) | 3 (1.4) | |
| Carcinosarcoma | 80 (13.3) | 35 (16.7) | |
| N stage | <0.001 | ||
| N0 | 482 (80.2) | 128 (61.0) | |
| N1 | 49 (8.2) | 40 (19.0) | |
| N2 | 29 (4.8) | 27 (12.9) | |
| NX‡ | 41 (6.8) | 15 (7.1) | |
| Summary stage§ | <0.001 | ||
| Localized | 319 (53.1) | 75 (35.7) | |
| Regional | 282 (46.9) | 135 (64.3) | |
| Other malignancy¶ | 0.29 | ||
| No | 322 (53.6) | 122 (58.1) | |
| Yes | 279 (46.4) | 88 (41.9) | |
Values are numbers (percentages), and percentages may not total 100 because of rounding. “2–4 cm” of tumor size indicates that the tumor size was between 2 cm (not included) and 4 cm (included). So does the expression “4–6 cm”. †, others included Asian/Pacific Islander and American Indian/Alaska Native; ‡, NX indicated that the N stage could not be assessed or the N stage was unknown; §, stage confirmed by the algorithm created by the SEER database. For more information: https://seer.cancer.gov/seerstat/variables/seer/lrd-stage/; ¶, whether developed other in situ/malignant tumor before and after the diagnosis of PSC. PSC, pulmonary sarcomatoid carcinoma; SEER, Surveillance, Epidemiology, and End Results.
Table 2
| Characteristics | Pleomorphic carcinoma (N=264) | Giant cell carcinoma (N=75) | Spindle cell carcinoma (N=113) | Pulmonary blastoma (N=21) | Carcinosarcoma (N=115) |
|---|---|---|---|---|---|
| Age (years) | |||||
| <65 | 73 (27.7) | 38 (50.7) | 39 (34.5) | 14 (66.7) | 34 (29.6) |
| 65–74 | 112 (42.4) | 20 (26.7) | 44 (38.9) | 2 (9.5) | 45 (39.1) |
| ≥75 | 79 (29.9) | 17 (22.7) | 30 (26.5) | 5 (23.8) | 36 (31.3) |
| Sex | |||||
| Female | 114 (43.2) | 28 (37.3) | 56 (49.6) | 16 (76.2) | 58 (50.4) |
| Male | 150 (56.8) | 47 (62.7) | 57 (50.4) | 5 (23.8) | 57 (49.6) |
| Race | |||||
| White | 225 (85.2) | 61 (81.3) | 100 (88.5) | 17 (81.0) | 98 (85.2) |
| Black | 28 (10.6) | 8 (10.7) | 8 (7.1) | 3 (14.3) | 12 (10.4) |
| Others† | 11 (4.2) | 6 (8.0) | 5 (4.4) | 1 (4.8) | 5 (4.3) |
| Year of diagnosis | |||||
| 2000–2006 | 32 (12.1) | 35 (46.7) | 54 (47.8) | 12 (57.1) | 41 (35.7) |
| 2007–2013 | 88 (33.3) | 25 (33.3) | 24 (21.2) | 6 (28.6) | 36 (31.3) |
| 2014–2020 | 144 (54.5) | 15 (20.0) | 35 (31.0) | 3 (14.3) | 38 (33.0) |
| Primary site | |||||
| Right upper lobe | 95 (36.0) | 31 (41.3) | 32 (28.3) | 3 (14.3) | 37 (32.2) |
| Right middle lobe | 13 (4.9) | 5 (6.7) | 11 (9.7) | 0 (0.0) | 7 (6.1) |
| Right lower lobe | 39 (14.8) | 10 (13.3) | 27 (23.9) | 9 (42.9) | 23 (20.0) |
| Left upper lobe | 67 (25.4) | 21 (28.0) | 25 (22.1) | 3 (14.3) | 30 (26.1) |
| Left lower lobe | 50 (18.9) | 8 (10.7) | 18 (15.9) | 6 (28.6) | 18 (15.7) |
| Size (cm) | |||||
| ≤2 | 48 (18.2) | 13 (17.3) | 27 (23.9) | 1 (4.8) | 19 (16.5) |
| 2–4 | 110 (41.7) | 28 (37.3) | 46 (40.7) | 7 (33.3) | 38 (33.0) |
| 4–6 | 63 (23.9) | 19 (25.3) | 22 (19.5) | 3 (14.3) | 24 (20.9) |
| >6 | 43 (16.3) | 15 (20.0) | 18 (15.9) | 10 (47.6) | 34 (29.6) |
| Surgery | |||||
| Sublobectomy | 45 (17.0) | 8 (10.7) | 17 (15.0) | 5 (23.8) | 13 (11.3) |
| Lobectomy/bilobectomy | 219 (83.0) | 67 (89.3) | 96 (85.0) | 16 (76.2) | 102 (88.7) |
| Differentiation | |||||
| Well/moderately differentiated | 2 (0.8) | 1 (1.3) | 7 (6.2) | 9 (42.9) | 3 (2.6) |
| Poorly differentiated | 165 (62.5) | 30 (40.0) | 53 (46.9) | 2 (9.5) | 53 (46.1) |
| Undifferentiated | 39 (14.8) | 24 (32.0) | 13 (11.5) | 1 (4.8) | 15 (13.0) |
| Unknown | 58 (22.0) | 20 (26.7) | 40 (35.4) | 9 (42.9) | 44 (38.3) |
| N stage | |||||
| N0 | 195 (73.9) | 60 (80.0) | 97 (85.8) | 5 (23.8) | 66 (57.4) |
| N1 | 44 (16.7) | 7 (9.3) | 8 (7.1) | 0 (0.0) | 8 (7.0) |
| N2 | 25 (9.5) | 5 (6.7) | 7 (6.2) | 0 (0.0) | 7 (6.1) |
| NX‡ | 0 (0.0) | 3 (4.0) | 1 (0.9) | 16 (76.2) | 34 (29.6) |
| Summary stage§ | |||||
| Localized | 104 (39.4) | 38 (50.7) | 71 (62.8) | 15 (71.4) | 55 (47.8) |
| Regional | 160 (60.6) | 37 (49.3) | 42 (37.2) | 6 (28.6) | 60 (52.2) |
| Chemotherapy | |||||
| No/unknown | 189 (71.6) | 57 (76.0) | 93 (82.3) | 18 (85.7) | 80 (69.6) |
| Yes | 75 (28.4) | 18 (24.0) | 20 (17.7) | 3 (14.3) | 35 (30.4) |
| Other malignancy¶ | |||||
| No | 152 (57.6) | 46 (61.3) | 54 (47.8) | 12 (57.1) | 69 (60.0) |
| Yes | 112 (42.4) | 29 (38.7) | 59 (52.2) | 9 (42.9) | 46 (40.0) |
Values are numbers (percentages), and percentages may not total 100 because of rounding. “2–4 cm” of tumor size indicates that the tumor size was between 2 cm (not included) and 4 cm (included). So does the expression “4–6 cm”. †, others included Asian/Pacific Islander and American Indian/Alaska Native; ‡, NX indicated that the N stage could not be assessed or the N stage was unknown; §, stage confirmed by the algorithm created by the SEER database. For more information: https://seer.cancer.gov/seerstat/variables/seer/lrd-stage/; ¶, whether developed other in situ/malignant tumor before and after the diagnosis of PSC. PSC, pulmonary sarcomatoid carcinoma; SEER, Surveillance, Epidemiology, and End Results.
Survival analysis before PSM
Of patients who only underwent surgery, the 1-, 3-, 5-year OS rate were 70.96%, 51.95%, 44.22% respectively, and the 1-, 3-, 5-year CSS rate were 76.68%, 61.64%, 55.15% respectively. Of patients who received PC, the 1-, 3-, 5-year OS rate were 83.45%, 62.15%, 49.71% respectively, and the 1-, 3-, 5-year CSS rate were 85.36%, 65.58%, 58.43% respectively. In all enrolled patients, PC was associated with better OS (Figure 1A, P=0.02), but not when it came to CSS (Figure 1B, P=0.24). In specific histological subtype, PC showed no benefit for OS or CSS in pleomorphic carcinoma patients (Figure 1C,1D), giant cell carcinoma patients (Figure 1E,1F), or spindle cell carcinoma patients (Figure 1G,1H). However, in carcinosarcoma patients, PS was associated with improved OS (Figure 1I, P=0.01) and CSS (Figure 1J, P=0.04). In pulmonary blastoma patients, although the result seemed that PC might bring survival benefit to them, the difference was not statistically significant due to the small sample size (Figure S1). In pseudosarcomatous carcinoma patients who were diagnosed with PSC but without detailed histological subtype, PC was associated with better OS (Figure 1K, P=0.02), but not as to CSS (Figure 1L, P=0.11).
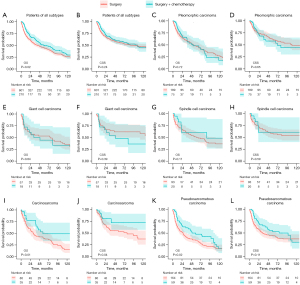
Survival analysis after PSM
The result of univariable and multivariable analyses showed that age, sex, year of diagnosis, tumor size, histological subtype, N stage, summary stage, and chemotherapy were independent risk factors of OS or CSS (Tables S1,S2). And the risk factors were matched between the surgery only and the surgery plus chemotherapy groups in the survival comparison. The clinicopathological characteristics of the matched groups were balanced (Table 3, Tables S3-S7, Figures S2-S7).
Table 3
| Characteristics | Surgery (N=35) | Surgery + chemotherapy (N=35) | P |
|---|---|---|---|
| Age (years) | 0.76 | ||
| <65 | 12 (34.3) | 15 (42.9) | |
| 65–74 | 17 (48.6) | 15 (42.9) | |
| ≥75 | 6 (17.1) | 5 (14.3) | |
| Sex | > 0.99 | ||
| Female | 18 (51.4) | 19 (54.3) | |
| Male | 17 (48.6) | 16 (45.7) | |
| Race | 0.48 | ||
| White | 32 (91.4) | 29 (82.9) | |
| Black | 3 (8.6) | 6 (17.1) | |
| Year of diagnosis | 0.36 | ||
| 2000–2006 | 11 (31.4) | 6 (17.1) | |
| 2007–2013 | 9 (25.7) | 12 (34.3) | |
| 2014–2020 | 15 (42.9) | 17 (48.6) | |
| Primary site | 0.85 | ||
| Right upper lobe | 12 (34.3) | 13 (37.1) | |
| Right middle lobe | 4 (11.4) | 2 (5.7) | |
| Right lower lobe | 8 (22.9) | 6 (17.1) | |
| Left upper lobe | 7 (20.0) | 9 (25.7) | |
| Left lower lobe | 4 (11.4) | 5 (14.3) | |
| Size (cm) | 0.51 | ||
| ≤2 | 3 (8.6) | 2 (5.7) | |
| 2–4 | 14 (40.0) | 10 (28.6) | |
| 4–6 | 8 (22.9) | 7 (20.0) | |
| >6 | 10 (28.6) | 16 (45.7) | |
| Surgery | >0.99 | ||
| Sublobectomy | 4 (11.4) | 3 (8.6) | |
| Lobectomy/bilobectomy | 31 (88.6) | 32 (91.4) | |
| Differentiation | 0.69 | ||
| Well/moderately differentiated | 1 (2.9) | 1 (2.9) | |
| Poorly differentiated | 14 (40.0) | 19 (54.3) | |
| Undifferentiated | 5 (14.3) | 4 (11.4) | |
| Unknown | 15 (42.9) | 11 (31.4) | |
| N stage | 0.96 | ||
| N0 | 18 (51.4) | 18 (51.4) | |
| N1 | 3 (8.6) | 4 (11.4) | |
| N2 | 4 (11.4) | 3 (8.6) | |
| NX‡ | 10 (28.6) | 10 (28.6) | |
| Summary stage§ | 0.81 | ||
| Localized | 16 (45.7) | 14 (40.0) | |
| Regional | 19 (54.3) | 21 (60.0) | |
| Other malignancy¶ | 0.62 | ||
| No | 24 (68.6) | 21 (60.0) | |
| Yes | 11 (31.4) | 14 (40.0) | |
Values are numbers (percentages), and percentages may not total 100 because of rounding. “2–4 cm” of tumor size indicates that the tumor size was between 2 cm (not included) and 4 cm (included). So does the expression “4–6 cm”. ‡, NX indicated that the N stage could not be assessed or the N stage was unknown; §, stage confirmed by the algorithm created by the SEER database. For more information: https://seer.cancer.gov/seerstat/variables/seer/lrd-stage/; ¶, whether developed other in situ/malignant tumor before and after the diagnosis of PSC. PSC, pulmonary sarcomatoid carcinoma; SEER, Surveillance, Epidemiology, and End Results.
In patients of all subtypes of PSC, PC was associated with better OS (Figure 2A, P<0.001) and CSS (Figure 2B, P=0.005). In specific histological subtype, PC still showed no benefit for OS or CSS in pleomorphic carcinoma patients (Figure 2C,2D), giant cell carcinoma patients (Figure 2E,2F), or spindle cell carcinoma patients (Figure 2G,2H). Meanwhile, in carcinosarcoma patients, PS was still associated with improved OS (Figure 2I, P=0.003) and CSS (Figure 2J, P=0.008). In pseudosarcomatous carcinoma patients, PC showed no significant benefit for OS (Figure 2K, P=0.06) nor CSS (Figure 2L, P=0.07). As for pulmonary blastoma patients, we did not conduct survival analysis after matching because of the small sample size.

Subgroup survival analyses
To further validate our findings, we conducted histological subtype-specific subgroup analyses according to age, sex, tumor size, surgery manner, summary stage, and other malignancy status (Figure 3; Figure S8). In carcinosarcoma patients, apart from the ≤4 cm subgroup and the sublobectomy subgroup, PC appeared to be associated with improved OS across all subgroups, although the differences were not significant in some subgroups, possibly due to the small sample size in each subgroup. In pleomorphic carcinoma patients and giant cell carcinoma patients, no significant association between PC and survival was found across subgroups. In spindle cell carcinoma patients, there seemed to be a trend suggesting potential survival benefit from the PC, but the effect was not significant, which might also due to the small sample size.
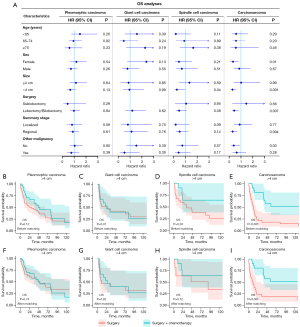
Because PC was more often recommended for tumors >4 cm, we further conducted survival comparison in patients with tumors >4 cm. Consistently, the survival of carcinosarcoma patients who received PC was better as compared to patients who did not (before PSM: P<0.001, Figure 3E; after PSM: P=0.001, Figure 3I). In pleomorphic carcinoma patients and giant cell carcinoma patients, no significant benefit from PC was found neither before nor after match (Figure 3B,3C,3F,3G). In spindle cell carcinoma patients, PC was associated with better OS before PSM (Figure 3D, P=0.03) but not after PSM (Figure 3H, P=0.12).
Discussion
PSC is characterized by its aggressive malignancy and poor prognosis and whether PC could improve the survival of PSC patients has been a controversial topic. We investigated the effect of different histological subtypes of PSC on the efficacy of PC and found that survival benefit of PC was only observed in carcinosarcoma patients but not in pleomorphic carcinoma, giant cell carcinoma, or spindle cell carcinoma neither before nor after PSM. For pulmonary blastoma patients, PC appeared to be beneficial but the difference was not significant because of the small sample size.
Due to the rarity of PSC, no prospective randomized controlled trial has investigated the potential of PC to improve the prognosis of PSC patients. In the recent decades, several retrospective studies have explored this problem but reported opposite results. On one hand, some studies found that PSC patients could not benefit from PC (9,11,13,14,20). In the study by Karim et al., the median OS of patients who received PC was even shorter than that of patients who received surgery only (457.6 vs. 713.5 days), though the difference was not significant (14). In the study by Huang et al., PC was associated with better survival in the univariable analysis but not in the multivariable analysis (21). On the other hand, some studies reported that PC could improve the survival of PSC patients (2,3,15-17,22). Among these studies, some found that the survival benefit of PC was observed in stage II/III patients, but not in stage I patients (2,3,15,16). This difference might be a result of the influence of confounding factors, as some studies included patients with metastasis, some included patient who received radiotherapy, some included patients who underwent pneumonectomy, and some utilized PSM method or multivariable analysis while others did not.
In terms of histological subtype, in studies that did not support the use of PC, only two studies described the proportion of each subtype of PSC and the proportion of carcinosarcoma in their enrolled patients was less than 1% (9,11). In studies favoring PC, Cen et al. reported a 12.4% proportion of carcinosarcoma in their study. However, in the subgroup analysis they found that PC was not associated survival benefit in all five WHO-defined specific subtypes of PSC, including carcinosarcoma (16). Their finding in carcinosarcoma patients was different from ours. In their study, they enrolled patients of stage IV and patients who underwent pneumonectomy, meanwhile, whether the characteristics of carcinosarcoma patients in the subgroup analysis of the PC group and the surgery only group were balanced was not reported. In our study, patients with advanced stage and patients who received pneumonectomy or extended lobectomy were excluded, and the characteristics of carcinosarcoma patients between groups were balanced after PSM. These heterogenicity between the two studies might account for the difference.
According to the definition of WHO, pleomorphic carcinoma is a poorly differentiated non-small cell lung cancer (NSCLC, including squamous cell carcinoma, adenocarcinoma), or undifferentiated NSCLC that contains at least 10% spindle and/or giant cells or a carcinoma consisting only of spindle and giant cells (8). Spindle cell carcinoma and giant cell carcinoma are almost entirely composed of epithelial spindle cells and malignant giant cells, respectively, without differentiated carcinomatous elements (8). In our study, although in the three subtypes, PC was not associated with better survival, it seemed that spindle cell carcinoma patients were more likely to benefit from PC as compared to giant cell carcinoma patients, especially spindle cell carcinoma patients whose tumor size >4 cm. In the study by Hendriksen et al., they reported that PC was beneficial in stage II/III/IV but bot in stage I pleomorphic carcinoma patients (3). In our subgroup analysis about tumor stage, PC was neither associated with better survival in stage “localized” pleomorphic carcinoma patients nor in stage “regional” pleomorphic carcinoma patients. Due to the significant variation in the proportion of spindle cells, giant cells, and other tumor cells in pleomorphic carcinoma, the internal heterogenicity between pleomorphic carcinomas was evident (23). This heterogenicity might result in the difference between our study and their study. And we agree that further studies taking into account of the cellular composition of pleomorphic carcinoma are needed.
The diagnosis of PSC was mainly based on morphology and immunohistochemistry as needed. Pleomorphic carcinoma, spindle cell carcinoma, and giant cell carcinoma did not contain true sarcomatous element and rarely express markers of sarcomatous differentiation in the immunohistochemistry, while pulmonary blastoma and carcinosarcoma did (8,24). In the study by Weissferdt et al., they proposed that the conflicting results of clinicopathologic features and prognostic data might result from the heterogenous composition of PSC, and in their study, carcinosarcoma and pulmonary blastoma were considered as different clinicopathologic entities from the other three subtypes (23). Our result supported this opinion and emphasized the importance of histological subtype when considering PC.
In addition to the difference in morphology and immunohistochemistry, different molecular profiles of each subtype of PSC have been identified. Studies found that pleomorphic carcinoma was prone to have KRAS mutation or EGFR mutation, while these mutations rarely present in carcinosarcoma (25-30). And it has been reported that KRAS mutation was associated with resistance to chemotherapy (31). Nowadays, in the era of target therapy and immunotherapy, studies have tried to uncover the characteristic of targetable driven mutations and immune check points (32-34). MET exon alteration has been found to be more enriched in PSC than in other NSCLC and has been suggested as a promising targetable mutation (32). But the MET mutation in carcinosarcoma also seems to be rare (25). Studies take into account the molecular profiles and histological subtypes of PSC to investigate the efficacy of PC might help individualize treatment in the future.
There were some limitations in our study. First, our study was a retrospective study and selection bias might exist. Second, although in the subgroup analysis spindle cell carcinoma patients whose tumor >4 cm were likely to benefit from PC, we could not investigate the reason behind it as no more pathological information or molecular profile information was available. Third, due to the lack of information in the SEER database, we could not assess whether the type of drug (platinum-based vs. non-platinum-based), dosage, and duration of PC might affect our findings. Forth, it was widely reported that the smoking rate was high in PSC patients (4,9,10), but we could not evaluate the effect of smoking on the efficacy of PC because there was also no smoking information in the SEER database.
With regard to statistics, there were also some limitations to note in our study. First, because PSC is a rare disease, the sample size of each histological subgroup is limited, though our study was already based on a database of large sample size. No benefit of PC was found in pleomorphic carcinoma patients, giant cell carcinoma patients, or spindle cell carcinoma patients in our study, but it should be noted that there might be a lack of statistical power due to the small sample size. Second, in the subgroup analysis, the sample size further decreased, so the result of subgroup analyses might only be suitable for investigating the trend of effect rather than evaluating the absolute effect. Third, as we conducted numerous survival comparisons among PSC patients of different histological subtypes, there might be a multiplicity issue in our study, which suggested that the small P value observed in carcinosarcoma patients was merely a chance finding. But as discussed above, pleomorphic carcinoma, spindle cell carcinoma, and giant cell carcinoma did not contain true sarcomatous element but carcinosarcoma did. Based on the histological characteristic, the possibility that our finding was just a chance finding resulting from multiplicity issue was slim.
Conclusions
We investigated the effect of different histological subtypes of PSC on the efficacy of PC and found that significant survival benefit of PC was only observed in carcinosarcoma patients. Our findings shed light on the important role of histological subtype when considering PC for PSC patients. Further studies evaluating the efficacy of PC based on the molecular profiles might help with individualized treatment in the future.
Acknowledgments
We acknowledged the effort of the National Cancer Institute and the SEER Program tumor registries in the creation of the SEER database.
Funding: This study was supported by the National Natural Science Foundation of China (82102968 to J.Z.).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-41/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-41/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-41/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery 2012;152:397-402. [Crossref] [PubMed]
- Chaft JE, Sima CS, Ginsberg MS, et al. Clinical outcomes with perioperative chemotherapy in sarcomatoid carcinomas of the lung. J Thorac Oncol 2012;7:1400-5. [Crossref] [PubMed]
- Hendriksen BS, Hollenbeak CS, Reed MF, et al. Perioperative chemotherapy is not associated with improved survival in stage I pleomorphic lung cancer. J Thorac Cardiovasc Surg 2019;158:581-591.e11. [Crossref] [PubMed]
- Maneenil K, Xue Z, Liu M, et al. Sarcomatoid Carcinoma of the Lung: The Mayo Clinic Experience in 127 Patients. Clin Lung Cancer 2018;19:e323-33. [Crossref] [PubMed]
- Gang J, Yan Q, Xiang S, et al. Clinicopathological characteristics and prognostic factors of pulmonary sarcomatoid carcinoma: a large population analysis. Ann Transl Med 2021;9:121. [Crossref] [PubMed]
- Yin J, Yang Y, Ma K, et al. Clinicopathological characteristics and prognosis of pulmonary pleomorphic carcinoma: a population-based retrospective study using SEER data. J Thorac Dis 2018;10:4262-73. [Crossref] [PubMed]
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Baldovini C, Rossi G, Ciarrocchi A. Approaches to Tumor Classification in Pulmonary Sarcomatoid Carcinoma. Lung Cancer (Auckl) 2019;10:131-49. [Crossref] [PubMed]
- Ung M, Rouquette I, Filleron T, et al. Characteristics and Clinical Outcomes of Sarcomatoid Carcinoma of the Lung. Clin Lung Cancer 2016;17:391-7. [Crossref] [PubMed]
- Lin Y, Yang H, Cai Q, et al. Characteristics and Prognostic Analysis of 69 Patients With Pulmonary Sarcomatoid Carcinoma. Am J Clin Oncol 2016;39:215-22. [Crossref] [PubMed]
- Lococo F, Rapicetta C, Cardillo G, et al. Pathologic Findings and Long-Term Results After Surgical Treatment for Pulmonary Sarcomatoid Tumors: A Multicenter Analysis. Ann Thorac Surg 2017;103:1142-50. [Crossref] [PubMed]
- Park JS, Lee Y, Han J, et al. Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology 2011;81:206-13. [Crossref] [PubMed]
- Gong T, Jia B, Chen C, et al. Clinical analysis of 78 pulmonary sarcomatoid carcinomas with surgical treatment. J Int Med Res 2022;50:3000605221128092. [Crossref] [PubMed]
- Karim NA, Schuster J, Eldessouki I, et al. Pulmonary sarcomatoid carcinoma: University of Cincinnati experience. Oncotarget 2018;9:4102-8. [Crossref] [PubMed]
- Abdallah HM, Martinez-Meehan D, Lutfi W, et al. Adjuvant chemotherapy for pulmonary sarcomatoid carcinoma: A retrospective analysis of the National Cancer Database. J Thorac Cardiovasc Surg 2022;163:1669-1681.e3. [Crossref] [PubMed]
- Cen Y, Yang C, Ren J, et al. Additional chemotherapy improves survival in stage II-III pulmonary sarcomatoid carcinoma patients undergoing surgery: a propensity scoring matching analysis. Ann Transl Med 2021;9:24. [Crossref] [PubMed]
- Sun L, Dai J, Chen Y, et al. Pulmonary Sarcomatoid Carcinoma: Experience From SEER Database and Shanghai Pulmonary Hospital. Ann Thorac Surg 2020;110:406-13. [Crossref] [PubMed]
- Surveillance Research Program, National Cancer Institute SEER*Stat software, version 8.4.2. Available online: www.seer.cancer.gov/seerstat
- Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005;40:90-7. [Crossref] [PubMed]
- Jiang X, Liu Y, Chen C, et al. The value of biomarkers in patients with sarcomatoid carcinoma of the lung: molecular analysis of 33 cases. Clin Lung Cancer 2012;13:288-96. [Crossref] [PubMed]
- Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. World J Surg Oncol 2013;11:252. [Crossref] [PubMed]
- Wang Y, Cao Y, Liu J. The role of prognostic nutritional index in the management of pulmonary sarcomatoid carcinoma. Clin Sarcoma Res 2020;10:26. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Correa AM, et al. "Sarcomatoid" carcinomas of the lung: a clinicopathological study of 86 cases with a new perspective on tumor classification. Hum Pathol 2017;63:14-26. [Crossref] [PubMed]
- Terra SB, Aubry MC, Yi ES, et al. Immunohistochemical study of 36 cases of pulmonary sarcomatoid carcinoma--sensitivity of TTF-1 is superior to napsin. Hum Pathol 2014;45:294-302. [Crossref] [PubMed]
- Vieira T, Antoine M, Ruppert AM, et al. Blood vessel invasion is a major feature and a factor of poor prognosis in sarcomatoid carcinoma of the lung. Lung Cancer 2014;85:276-81. [Crossref] [PubMed]
- Pelosi G, Gasparini P, Cavazza A, et al. Multiparametric molecular characterization of pulmonary sarcomatoid carcinoma reveals a nonrandom amplification of anaplastic lymphoma kinase (ALK) gene. Lung Cancer 2012;77:507-14. [Crossref] [PubMed]
- Chang YL, Wu CT, Shih JY, et al. EGFR and p53 status of pulmonary pleomorphic carcinoma: implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann Surg Oncol 2011;18:2952-60. [Crossref] [PubMed]
- Kaira K, Horie Y, Ayabe E, et al. Pulmonary pleomorphic carcinoma: a clinicopathological study including EGFR mutation analysis. J Thorac Oncol 2010;5:460-5. [Crossref] [PubMed]
- Pelosi G, Scarpa A, Manzotti M, et al. K-ras gene mutational analysis supports a monoclonal origin of biphasic pleomorphic carcinoma of the lung. Mod Pathol 2004;17:538-46. [Crossref] [PubMed]
- Holst VA, Finkelstein S, Colby TV, et al. p53 and K-ras mutational genotyping in pulmonary carcinosarcoma, spindle cell carcinoma, and pulmonary blastoma: implications for histogenesis. Am J Surg Pathol 1997;21:801-11. [Crossref] [PubMed]
- Hames ML, Chen H, Iams W, et al. Correlation between KRAS mutation status and response to chemotherapy in patients with advanced non-small cell lung cancer☆. Lung Cancer 2016;92:29-34. [Crossref] [PubMed]
- Schrock AB, Li SD, Frampton GM, et al. Pulmonary Sarcomatoid Carcinomas Commonly Harbor Either Potentially Targetable Genomic Alterations or High Tumor Mutational Burden as Observed by Comprehensive Genomic Profiling. J Thorac Oncol 2017;12:932-42. [Crossref] [PubMed]
- Terra SB, Jang JS, Bi L, et al. Molecular characterization of pulmonary sarcomatoid carcinoma: analysis of 33 cases. Mod Pathol 2016;29:824-31. [Crossref] [PubMed]
- Kim S, Kim MY, Koh J, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015;51:2698-707. [Crossref] [PubMed]


