
Efficacy of neoadjuvant chemo-immunotherapy in non-small cell lung cancer: a real-world, multicenter, retrospective study
Highlight box
Key findings
• The neoadjuvant chemo-immunotherapy is a feasible strategy for non-small cell lung cancer (NSCLC) with a favorable rate of pathologic response, modified resection and long-term survival.
• No clinical factor other than programmed death-ligand 1 (PD-L1) expression was predictive of the pathological response.
• Pathologic response may be effective surrogate endpoint for survival in NSCLC patients who received neoadjuvant chemo-immunotherapy.
What is known and what is new?
• Neoadjuvant chemo-immunotherapy has shown good efficacy for NSCLC in randomized controlled trials.
• The neoadjuvant chemo-immunotherapy is a feasible strategy for NSCLC in real clinical setting. Potential risk-benefit patients were identified. The relationship between pathological response and long-term survival was validated.
What is the implication, and what should change now?
• This study provides a reference for patient selection, efficacy evaluation and clinical application of neoadjuvant chemo-immunotherapy. It is feasible to modify the surgical procedure after neoadjuvant chemo-immunotherapy for selected patients.
Introduction
Lung cancer is one of the most common malignant tumors with the highest cancer-related mortality worldwide and about 85% are non-small cell lung cancer (NSCLC) (1). For stage IA–IIIA NSCLC patients, complete surgical excision is the most promising curative-intent therapeutic method (2). Nowadays, the overall survival (OS) of NSCLC patients who have received curative surgery is not optimistic because 30% to 55% of them suffer from postsurgical recurrence (3,4). Neoadjuvant therapy is expected to treat resectable and potential resectable NSCLC to downstage the tumor and achieve better surgical outcomes, especially for borderline operable NSCLC patients. However, the improvement of the 5-year survival rate for neoadjuvant chemotherapy is only 5% (5,6).
In recent years, the immune-checkpoint inhibitors (ICIs), especially programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors, have revolutionized the systemic treatment paradigm of advanced NSCLC with remarkable effectiveness and favorable safety and tolerability (7,8). Multiple phase II and III randomized controlled trials (RCTs) have also demonstrated that neoadjuvant immunotherapy could achieve better therapeutic effectiveness with manageable tolerability for resectable NSCLC, especially in combination with chemotherapy (5,9,10).
Though numerous studies have confirmed the promising application prospect of neoadjuvant chemo-immunotherapy for NSCLC, there are great differences in the efficacy of patients with different stages or different abundance of biomarkers. CheckMate 816 showed a pathologic complete response (pCR) benefit with chemo-immunotherapy across all stages (IB–IIIA), while the event-free survival (EFS) benefit for early stages (IB–II) is lower than locally advanced stage (IIIA) in subgroup analysis (9). Of course, this conclusion still needs to be verified by a longer follow-up. There is some evidence to support PD-L1 status as a predictive marker for treatment response and survival in neoadjuvant chemo-immunotherapy setting (9,11), however, PD-L1 and tumor mutation burden (TMB) were not significantly associated with survival in the NADIM trial (12). Investigation of the clinical prognostic factors associated with the therapeutic efficacy, such as pCR, major pathologic response (MPR), disease-free survival (DFS), and OS, could help us to identify the benefit-risk population for neoadjuvant chemo-immunotherapy.
Except the anti-oncology and safety outcomes, the benefits of the surgical approach and its extent are worth in-depth study for neoadjuvant chemo-immunotherapy. Given the high postoperative morbidity and mortality after complex and extensive surgery, such as pneumonectomy, bilobectomy, or sleeve lobectomy (13), efforts should be made to avoid these excisions for selected NSCLC. Nowadays, neoadjuvant chemo-immunotherapy has brought hope to reduce such excision. However, there is little research on whether neoadjuvant chemo-immunotherapy has any effect on long-term survival.
Therefore, we performed a retrospective study with a relatively large sample size which contained 158 NSCLC patients who received neoadjuvant chemo-immunotherapy prior to radical excision from two Chinese institutions, with particular attention paid to evaluating the safety, identifying benefit-risk population and surgery-related outcomes for neoadjuvant chemo-immunotherapy in real-world setting. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-17/rc).
Methods
Ethical statement
The retrospective cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by The Ethics Committee of Peking University People’s Hospital (approved number: 2021-PHB271-001) and The Second Affiliated Hospital of Air Force Medical University (approved number: K-202211-32). Informed consent was waived because this study was a noninterventional study.
Study population
NSCLC patients who received neoadjuvant chemo-immunotherapy between January 2019 and June 2022 in Peking University People’s Hospital and The Second Affiliated Hospital of Air Force Medical University were eligible for this study. The main criteria for neoadjuvant chemo-immunotherapy were T3–4 and N2 diseases. In addition, we also included early-stage patients whose tumors were close to the main bronchus and were seeking neoadjuvant therapy to reduce the extent of surgical resection. All patients who underwent neoadjuvant immunotherapy were reviewed by the multi-disciplinary team which included oncologists, radiologists, and thoracic surgeons. EGFR mutations and ALK rearrangements status analysis were not recommended routinely before treatment, while they were performed in some patients based on individual surgeon recommendations and patient wishes. Patients with EGFR mutations and ALK rearrangements were not excluded from neoadjuvant chemo-immunotherapy and our analysis. In general, the included criteria for this study were: (I) diagnosed as resectable or potentially resectable primary NSCLC, regardless of EGFR and ALK status; (II) clinical stage IB to IIIB (American Joint Committee on Cancer, eighth edition) based on preoperative staging examination; (III) received two to four cycles of neoadjuvant PD-1/PD-L1 immune checkpoint inhibitors combined with platinum-based chemotherapy; (IV) received radical resection and systemic lymph node dissection. The exclusion criteria were: (I) N3 lymph node metastasis; (II) wedge resection of the primary tumor; (III) previous lung cancer; (IV) previous or concurrent other malignant tumors; and (V) involved in any clinical trials. Patients with missing values were excluded from the analysis.
According to the National Comprehensive Cancer Network guidelines, all the enrolled patients underwent routine pretreatment examinations, including contrast-enhanced chest computed tomography (CT) to evaluate the primary tumor; positron emission tomography-CT (PET-CT) or combination of abdomen CT, brain magnetic resonance imaging (MRI) and radionuclide bone scanning to evaluate tumor staging; fiberoptic bronchoscopy or subcutaneous needle biopsy and endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) to obtain pathologic tumor diagnosis and staging. All patients received two to four cycles of ICI combined with platinum-based chemotherapy in 21-day cycles according to the international consensus. The efficacy of neoadjuvant chemo-immunotherapy was evaluated after every two cycles according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Comprehensive preoperative evaluation which included the aforementioned radiological examinations (PET-CT or combination of chest CT, abdominal CT, brain MRI and radionuclide bone scanning) and fiberoptic bronchoscopy were performed to confirm the resectability of the tumor. The neoadjuvant treatment cycles were decided based on comprehensive consideration of patients’ tolerance to drugs and imaging remission of lesions, and eventually decided by the multi-disciplinary team. Surgical approach, and surgical extent were also determined by the multi-disciplinary team. To investigate the change of surgical approach after neoadjuvant therapy, the extent of primary surgical resection prior to neoadjuvant treatment was evaluated by two experienced thoracic surgeons according to the outcomes of radiological examination and fiberoptic bronchoscopy prior to neoadjuvant regimen. Modified surgery referred to decreased complexity or reduced surgical extent for central tumor expected to undergo complex surgery, including pneumonectomy to sleeve lobectomy, bilobectomy, or lobectomy; bilobectomy to lobectomy; sleeve lobectomy to lobectomy. The suspicious residual disease, such as bronchial or vascular margin, were excluded by frozen section evaluation.
Pathologic evaluation
Surgical specimens, including primary tumors and dissected lymph nodes, were evaluated by two senior histopathologists. Pathological response was assessed by calculating the mean percentage of viable tumor cells. The pCR was defined as the absence of residual viable tumor cells in the primary tumor and dissected lymph nodes. The MPR was defined as ≤10% residual viable tumor.
Follow-up and endpoints
Follow-up information was obtained from the medical records, postoperative visits, and telephone calls. The loss of follow-up was recorded according to the last follow-up status. The follow-up data included not only survival information to assess the therapeutic effect but also adverse events to evaluate the safety of the neoadjuvant regimen. The primary endpoint was pCR. The secondary endpoints were objective response rate (ORR), MPR, progression-free survival (PFS), OS, and safety. Safety included immunotherapy-related adverse events, 30-day mortality, and complications during the first 30 days after surgery. Postoperative complications were graded according to the Clavien-Dindo classification (14).
Statistical analysis
Statistical analysis was carried out with the R version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org). The Kaplan-Meier method with a log-rank test was used to evaluate the survival differences. Continuous variables were presented as median [interquartile range (IQR)] or mean [standard deviation (SD)], and categorical variables were presented as percentages. The Shapiro-Wilk test was used to analyze the normal distribution of the continuous variables. Student’s t-test was used to compare variables with normal distributions. Non-normally distributed variables were compared using the Mann-Whitney U test. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test. To investigate the predictors of pCR and MPR, the odds ratio (OR) and its 95% confidence interval (CI) were estimated using a logistic regression model. Variables were included in multivariate analysis when P<0.2 in univariate analysis. Two-sided P<0.05 was considered statistically significant.
Results
Characteristics of the patients
As shown in Figure S1, 158 patients eventually met our inclusion and exclusion criteria and were divided into early-stage group (stage IB–IIB) and locally advanced-stage group (stage IIIA–IIIB). Patients’ baseline characteristics are presented in Table 1. The patient cohort consisted predominantly of males (143, 90.5%), with a high prevalence of former or current smokers (123, 77.8%) and exhibiting central type (119, 75.3%) and squamous cell carcinoma (115, 72.8%). PD-L1 expression levels at diagnosis were examined in 73 individuals. Forty-one patients (25.9%) received two cycles of neoadjuvant treatment, while 80 (50.6%) had three cycles, and the left 37 (23.4%) had four cycles. Immunotherapy and chemotherapy drugs and treatment regimens are shown in Table S1. In general, no significant difference was identified between early-stage and locally advanced-stage groups. Adjuvant treatment was received by 22.8% of the patients (Table S2). The rate of adjuvant therapy was significantly lower in patients who received four cycles of neoadjuvant therapy than in those who received three cycles of neoadjuvant therapy (0.8% vs. 30.0%, P=0.04). While there was no significant difference in the proportion of adjuvant therapy in other pairwise comparisons (two cycles vs. three cycles: 17.1% vs. 30.0%, P=0.18; two cycles vs. four cycles: 17.1% vs. 10.8%, P=0.64).
Table 1
| Variables | All patients (N=158) | Stage IB–IIB (N=34) | Stage IIIA–IIIB (N=124) | P |
|---|---|---|---|---|
| Age (years), median [IQR] | 62 [56, 66] | 63 [57, 67] | 61 [55, 66] | 0.47 |
| Gender, N (%) | ||||
| Male | 143 (90.5) | 33 (97.1) | 110 (88.7) | 0.25 |
| Female | 15 (9.5) | 1 (2.9) | 14 (11.3) | |
| Smoking status, N (%) | ||||
| Current or ever | 123 (77.8) | 29 (85.3) | 94 (75.8) | 0.34 |
| Never | 35 (22.2) | 5 (14.7) | 30 (24.2) | |
| Tumor location, N (%) | ||||
| Central | 119 (75.3) | 29 (85.3) | 90 (72.6) | 0.19 |
| Peripheral | 39 (24.7) | 5 (14.7) | 34 (27.4) | |
| Histology, N (%) | ||||
| Squamous | 115 (72.8) | 25 (73.5) | 90 (72.6) | 0.54 |
| Adenocarcinoma | 38 (24.1) | 7 (20.6) | 31 (25.0) | |
| Adenosquamous | 5 (3.2) | 2 (5.9) | 3 (2.4) | |
| Tumor PD-L1 TPS, N (%) | ||||
| <1% | 18 (11.4) | 6 (17.6) | 12 (9.7) | 0.49 |
| 1–49% | 26 (16.5) | 4 (11.8) | 22 (17.7) | |
| ≥50% | 29 (18.4) | 5 (14.7) | 24 (19.4) | |
| Unknown | 85 (53.8) | 19 (55.9) | 66 (53.2) | |
| Treatment cycles, N (%) | ||||
| Two | 41 (25.9) | 9 (26.5) | 32 (25.8) | 0.32 |
| Three | 80 (50.6) | 14 (41.2) | 66 (53.2) | |
| Four | 37 (23.4) | 11 (32.4) | 26 (21.0) |
NSCLC, non-small cell lung cancer; IQR, interquartile range; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
Surgery summary
As shown in Table 2, the median interval time between the last neoadjuvant therapy and surgery was 37 (IQR, 31–43) days. The mean operation duration and median blood loss were 166.7 (SD 52.9) minutes and 100 (IQR, 50–200) mL, respectively. Video-assisted thoracoscopic surgery (VATS) was performed in 89 (56.3%) patients. Fifty-eight out of 96 (60.4%) central NSCLC patients expected to undergo complex surgery underwent the aforementioned modified surgery. The proportion of surgical modification was significantly higher in locally advanced-stage patients (52/77, 67.5%) than in early-stage patients (6/19, 31.6%, P=0.009). Other aspects of the modified surgery have been reported previously (15).
Table 2
| Variables | All patients | Stage IB–IIB | Stage IIIA–IIIB | P |
|---|---|---|---|---|
| Clinical response | 0.86 | |||
| Complete response | 10 (6.3) | 2 (5.9) | 8 (6.5) | |
| Partial response | 101 (63.9) | 20 (58.8) | 81 (65.3) | |
| Stable disease | 44 (27.8) | 11 (32.4) | 33 (26.6) | |
| Progressive disease | 3 (1.9) | 1 (2.9) | 2 (1.6) | |
| Interval time to surgery (day), median [IQR] | 37 [31, 43] | 35 [30, 42] | 38 [32, 43] | 0.46 |
| Type of operation, N (%) | 0.005 | |||
| VATS | 89 (56.3) | 26 (76.5) | 63 (50.8) | |
| Thoracotomy | 50 (31.6) | 3 (8.8) | 47 (37.9) | |
| Convert to thoracotomy | 19 (12.0) | 5 (14.7) | 14 (11.3) | |
| Surgical approach, N (%) | 0.04 | |||
| Lobectomy | 95 (60.1) | 26 (76.5) | 69 (55.6) | |
| Sleeve lobectomy | 21 (13.3) | 4 (11.8) | 17 (13.7) | |
| Bilobectomy | 20 (12.7) | 4 (11.8) | 16 (12.9) | |
| Pneumonectomy | 22 (13.9) | 0 | 22 (17.7) | |
| Operative duration (min), mean (SD) | 166.7 (52.9) | 154.6 (56.6) | 170.0 (51.6) | 0.13 |
| Blood loss (mL), median [IQR] | 100 [50, 200] | 100 [50, 200] | 135 [50, 200] | 0.28 |
| Pathologic response, N (%) | 0.06 | |||
| pCR | 62 (39.2) | 19 (55.9) | 43 (34.7) | 0.04a |
| MPR | 95 (60.1) | 23 (67.6) | 72 (58.1) | 0.41b |
| Non-MPR | 63 (39.9) | 11 (32.4) | 52 (41.9) | |
| Change in pN stage, N (%) | <0.001 | |||
| N0 to N0 | 31 (19.6) | 20 (58.8) | 11 (8.9) | |
| N1 to N1 | 5 (3.2) | 4 (11.8) | 1 (0.8) | |
| N1 to N0 | 19 (12.0) | 6 (17.6) | 13 (10.5) | |
| N2 to N2 | 18 (11.4) | 0 | 18 (14.5) | |
| N2 to N1 | 8 (5.1) | 0 | 8 (6.5) | |
| N2 to N0 | 69 (43.7) | 0 | 69 (55.6) | |
| Progress | 8 (5.1) | 4 (11.8) | 4 (3.2) | |
| Modified surgery, N (%) | 0.01 | |||
| Yes | 58 (36.7) | 6 (17.6) | 52 (41.9) | 0.009 |
| No | 38 (24.1) | 13 (38.2) | 25 (20.2) | |
| Uninvolved | 62 (39.2) | 15 (44.1) | 47 (37.9) |
a, comparison of pCR and non-pCR; b, comparison of MPR and non-MPR. NSCLC, non-small cell lung cancer; IQR, interquartile range; VATS, video-assisted thoracoscopic surgery; SD, standard deviation; pCR, pathologic complete response; MPR, major pathologic response.
Efficacy
According to the RECIST 1.1 criteria, 10 (6.3%) patients achieved complete response (CR), 101 (63.9%) with partial response (PR), 44 (27.8%) with stable disease (SD), and three (1.9%) with radiographic progressive disease (PD). Ninety-five (60.1%) patients achieved MPR, including 62 (39.2%) patients with pCR. As shown in Figure 1, all ten patients with clinical CR achieved pCR; all three patients with PD were judged to be pathologically non-MPR; 15 (34.1%) of 44 patients with SD and 37 (36.6%) of 101 patients with PR achieved pCR. The postoperative pathological downstaging of tumor-node-metastasis (TNM) stage and N stage is shown in Figure S2. None of the eight patients with known EGFR mutations achieved MPR.

Multivariate regression analysis revealed that no clinical factors such as age, gender, smoking history, tumor type, initial tumor size, N2 lymph node metastasis, neoadjuvant cycles adverse reactions, and radiological response (CR and PR) were associated with pCR or MPR. Meanwhile, patients with high PD-L1 tumor proportion score (TPS) level were more likely to achieve MPR (Tables 3,4).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Age (≥65 years) | 1.10 | 0.55–2.19 | 0.78 | ||||
| Gender (female) | 0.21 | 0.05–0.98 | 0.04 | ||||
| Smoke history (yes) | 3.24 | 1.31–7.97 | 0.01 | 1.85 | 0.46–9.33 | 0.41 | |
| Tumor size (≥4 cm) | 0.58 | 0.29–1.14 | 0.11 | 0.33 | 0.10–1.03 | 0.05 | |
| Tumor location (peripheral) | 0.96 | 0.46–2.01 | 0.90 | ||||
| Histological type (non-SCC) | 0.76 | 0.37–1.56 | 0.45 | ||||
| cN stage (N2) | 0.52 | 0.27–1.01 | 0.05 | 0.42 | 0.14–1.24 | 0.12 | |
| Neo-adjuvant cycle (≥C3) | 0.88 | 0.43–1.82 | 0.73 | ||||
| PD-L1 (high) | 3.17 | 1.15–8.75 | 0.02 | 4.13 | 1.38–13.58 | 0.01 | |
| irAE (yes) | 0.56 | 0.19–1.66 | 0.29 | ||||
| Radiographic remission (ORR) | 1.57 | 0.76–3.22 | 0.22 | ||||
Gender was not included in the multivariate analysis to avoid overfitting given the paucity of data (only 2/13 patients achieved pCR). pCR, pathologic complete response; OR, odds ratio; CI, confidence interval; SCC, squamous cell carcinomas; PD-L1, programmed death-ligand 1; irAE, immune-related adverse events; ORR, objective response rate.
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Age (≥65 years) | 0.94 | 0.48–1.88 | 0.87 | ||||
| Gender (female) | 0.55 | 0.19–1.59 | 0.26 | ||||
| Smoke history (yes) | 1.58 | 0.74–3.37 | 0.23 | ||||
| Tumor size (≥4 cm) | 1.03 | 0.52–2.03 | 0.92 | ||||
| Tumor location (peripheral) | 1.69 | 0.78–3.65 | 0.18 | 2.98 | 0.76–13.78 | 0.13 | |
| Histological type (non-SCC) | 0.49 | 0.24–0.99 | 0.04 | 0.43 | 0.11–1.61 | 0.21 | |
| cN stage (N2) | 0.54 | 0.28–1.05 | 0.06 | 0.48 | 0.14–1.61 | 0.24 | |
| Neo-adjuvant cycle (≥C3) | 1.64 | 0.80–3.36 | 0.17 | 0.84 | 0.20–3.34 | 0.81 | |
| PD-L1 (high) | 5.26 | 1.70–16.28 | 0.004 | 6.98 | 2.12–28.22 | 0.003 | |
| irAE (yes) | 0.81 | 0.30–2.18 | 0.67 | ||||
| Radiographic remission (ORR) | 3.65 | 1.79–7.45 | <0.001 | 3.16 | 0.91–11.94 | 0.07 | |
MPR, major pathologic response; OR, odds ratio; CI, confidence interval; SCC, squamous cell carcinomas; PD-L1, programmed death-ligand 1; irAE, immune-related adverse events; ORR, objective response rate.
The median follow-up time from diagnosis was 27.1 (IQR, 22.0–34.6) months with six (3.8%) patients were lost. The median duration of PFS and OS was not reached (Figure S3). The 2-year PFS and OS were 80.6% (95% CI: 74.5–87.3%) and 89.5% (95% CI: 84.5–94.7%), respectively. During the follow-up period, postoperative recurrence occurred in 25 (15.8%) patients, of whom eight (5.1%) had local recurrence and 17 (10.8%) had distant metastasis. Notably, one patient who reached pCR without adjuvant treatment was found to have intracranial metastasis 13 months after surgery. Seventeen (10.8%) patients died, including nine from tumor progression, four from perioperative complications, one from severe side effects (immune myocarditis) of postoperative immunotherapy, one from pulmonary infection, and two from other causes. The patient who died from immune myocarditis received three cycles of neoadjuvant chemo-immunotherapy and achieved pCR. Immune myocarditis occurred one month after the first cycle of adjuvant chemo-immunotherapy.
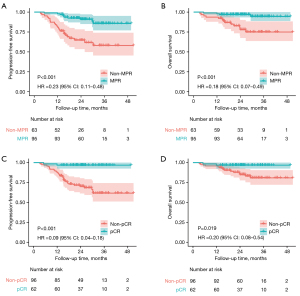
As for subgroup analysis, both PFS and OS in pCR and MPR groups were significantly higher than those in non-pCR and non-MPR groups (Figure 2). Moreover, there was no statistically significant difference on PFS or OS between early-stage group and locally advanced-stage group, and no significant association was found between PD-L1 TPS level and PFS or OS (Figure S4).
Safety and surgical complications
As shown in Figure S1, among 177 patients who were assessed for eligibility, 19 (10.7%) patients without radical resection were excluded. Of these, five cases (2.8%) were because the tumor was deemed unresectable or the surgical risk was too high; three (1.7%) were denied surgery because of poor lung function or other serious underlying diseases; two (1.1%) because of severe immunotherapy-associated pneumonia. In three cases (1.7%), the reason was that the patients met the operation conditions but they explicitly refused the operation. In six cases (3.4%), only wedge resection or palliative resection was performed considering the patient’s physical condition and the patient’s wish. Twenty-four patients (15.2%) experienced grade 3 or worse immune-related adverse events, including immune-related pneumonitis, myelosuppression, and skin lesions.
Perioperative complications are statistically analyzed and shown in Table S3. A total of 32 (20.3%) patients experienced grade 2–5 postoperative complications, and there was no significant difference between the different neoadjuvant treatment cycles (21.9% for two cycles, 20% for three cycles and 18.9% for four cycles, P=0.94). The most frequent complications were excessive hemorrhage (>400 mL, 18, 11.4%), pneumonia (7, 4.4%), chylothorax (3, 1.9%), and respiratory failure (3, 1.9%). Four (2.5%) patients died within the first 30 days after surgery, three from pneumonia and one from acute heart attack accompanying respiratory failure.
Discussion
We presented 158 cases of NSCLC patients who had received neoadjuvant chemo-immunotherapy prior to radical excision from two Chinese academic institutions. Compared to most previous studies on neoadjuvant therapy for lung cancer (5,9), more locally advanced-stage NSCLC (54.3% IIIA and 24.3% IIIB) were enrolled in our cohort. Stage IIIB or above is generally considered unsuitable for surgical treatment. However, the results of chemo-immunotherapy RCTs have revolutionized the therapeutic landscape for locally advanced-stage NSCLC patients (stage III) with promising effectiveness, favorable safety, and tolerability (9,16).
Deng and colleagues reported that 60.1% (31/51) of clinical IIIB patients successfully converted from initial unresectable to radical resection after neoadjuvant chemo-immunotherapy with favorable DFS compared to those without surgery (17). However, the involvement of N3 lymph nodes (supraclavicular and contralateral mediastinal lymph nodes), bulky N2 lymph nodes and direct invasion to heart, great vessels, diaphragm, trachea, or carina after neoadjuvant therapy were still regarded as unresectable disease (18). In our study, of the 42 patients with stage IIIB disease, 40 achieved stage IIIA or better after neoadjuvant therapy. This series provides evidence that surgery after neoadjuvant chemo-immunotherapy, even in those with later-stage disease is effective, with tolerable perioperative mortality and complication rates.
The pCR and MPR were identified as potential surrogate endpoints for survival in NSCLC patients who received neoadjuvant therapy. For those who achieved MPR, it was reported that there was a two-time improvement of 5-year OS (85.0% vs. 40.0%, P<0.001) compared to the absence of MPR (19). However, historical data showed that pCR and MPR rates were relatively low in the era of neoadjuvant chemotherapy, with a pCR rate of 4% and an MPR rate of 20%, respectively (20). There was no doubt that the combination of chemotherapy and immunotherapy increased the pathological responses, and multiple RCTs showed that the pCR rate could range from 18% to 63%, and 36.9% to 85% for MPR rate (20). Consistent with previous trials, our study demonstrated promising anti-oncology outcomes resulting in a pCR rate of 39.2% and an MPR rate of 60.1%, respectively. Meanwhile, we demonstrated that there was no local recurrence or distant metastasis among the patients who achieved pCR. And the 2-year PFS and OS rates were significantly higher for patients who achieved pCR and MPR, which reinforced the relevance of the pathological response as a potential survival surrogate.
Nowadays, there is still controversy on the clinical predictive factors for therapeutic effect of patients who received neoadjuvant chemo-immunotherapy (5,9). Indeed, although in some trials, PD-L1, biomarker in adjuvant immunotherapy, was found to have the potential to predict radiologic and pathologic response of neoadjuvant chemo-immunotherapy (11,21), PD-L1 and TMB were not significantly associated with survival in the NADIM trial (12). In the CheckMate 816 trial, although the EFS benefit of neoadjuvant chemo-immunotherapy was observed in all PD-L1 subgroups, the benefits were greater in the PD-L1 ≥50% group [hazard ratio (HR), 0.24] and PD-L1 ≥1% group (HR 0.41) compared with PD-L1 <1% group (HR, 0.85) (9). In the whole group of our study, we found that no clinical factor could predict the pCR or MPR status but PD-L1 staining levels. Patients with high PD-L1 staining levels seemed to have better PFS and OS (HR 0.44 & 0.42). Larger scale cohorts and longer follow-up time are still required to confirm the clinical predictive factors for pathological response and survival outcomes.
Most of the earlier neoadjuvant studies (such as NADIM and SAKK16/14) specifically focused on advanced stage disease. However, CheckMate 816 showed a consistent pCR benefit with chemo-immunotherapy across all stages (IB–IIIA). Although the EFS benefit was not evident in lower stages (IB–IIB) compared with IIIA in subgroup analysis (9). Our study showed that pCR was more likely to be achieved in early-stage patients compared to locally advanced patients, while MPR rates were not significantly different. The EFS and OS curves also did not show significant differences between the early-stage and locally advanced-stage patients. Although there are potential differences of the benefits in patients with different stages for neoadjuvant chemo-immunotherapy, the relevant results need to be confirmed by further follow-up because of the low incidence of outcome events in early stage.
There is still a controversy about therapeutic effectiveness of immunotherapy in NSCLC with EGFR-sensitizing mutations. The tumor microenvironment (TME) of the NSCLC with EGFR mutations is mainly in non-inflammatory state which contains weak lymphocytic infiltration and low anti-tumor inflammatory response. Thus, this TME state would decrease the effectiveness of immunotherapy for NSCLC with EGFR mutations, theoretically (22). In the LCMC3 trial, two neoadjuvant cycles atezolizumab (a PD-L1 inhibitor) monotherapy was administered in resectable stage NSCLC. For patients without known EGFR or ALK alterations, 6.8% achieved pCR and 20.4% achieved MPR, while there was no MPR among the patients with EGFR mutations (23). However, Zhang and colleagues reported that the pCR rate was 11.1% (2/18) and the MPR rate was 44.4% (8/18) with neoadjuvant chemo-immunotherapy for NSCLC with EGFR mutation (24). Though, our study also suggested low pCR and MPR rates for patients with gene mutations after neoadjuvant chemo-immunotherapy. We believe that as the sample size increases, the clinical feasibility and value of combination therapy for patients with EGFR or ALK mutations would be identified by larger cohorts.
Complex surgery, such as pneumonectomy, bilobectomy, sleeve lobectomy, lobectomy with bronchoplasty or arterioplasty, are usually needed for patients with extensive invasion tumors (i.e., invasion of trachea, carina, main bronchus, fissure, or main pulmonary artery). However, it was reported that pneumonectomy was associated with worse long-term survival, quality of life and non-oncologic death than lobectomy because of high postoperative morbidity and mortality (10). Thus, there is an urgent need to investigate whether the surgical feasibility and surgical extent could be changed after neoadjuvant therapy.
Chen and colleagues first demonstrated that the feasibility and safety of using chemo-immunotherapy before sleeve lobectomy were similar to those of sleeve lobectomy alone in a small sample study (25). Wu and colleagues reported that pneumonectomy was avoided in 80% (20/25) NSCLC after chemo-immunotherapy (26). Combined with the above literature and our findings, we believe that it is feasible to perform modified resection for pulmonary preservation among selected cases, benefiting from remarkable tumor regression after neoadjuvant chemo-immunotherapy.
In the CheckMate 816 study, the 2-year EFS rate was 63.8% with nivolumab plus chemotherapy and 45.3% with chemotherapy alone, and a better median EFS was observed in the combined treatment group (31.6 vs. 20.8 months; HR, 0.65; 95% CI: 0.47–0.90). Zhang and colleagues reported in a retrospective analysis that the 2-year DFS rate in the PD-1 plus chemotherapy group was higher than that of the chemotherapy group (79.3% vs. 60.2%, P=0.04) (27). The 2-year PFS rate of our series in the real world is comparable to previous RCT studies and adds to the evidence for the clinical use of this treatment modality.
There are several limitations in our research. Firstly, the patient selection biases were inevitable for this was a real-world retrospective study and only NSCLC patients who have received radical surgery after neoadjuvant immuno-chemotherapy were enrolled. Secondly, though patients from two centers were included in the current study, larger scale cohorts should be needed to verify our results. Thirdly, the follow-up duration was relatively short, the long-term OS should be investigated in further study. In addition, as a one-arm study, comparing with neoadjuvant chemotherapy was not performed, so the pathological benefit of immunotherapy should be interpreted with caution. Finally, clinical factors were taken into consideration to identify the predictive factors for pCR and MPR, the TME and molecular characteristics were not involved in the current study.
Conclusions
The neoadjuvant chemo-immunotherapy is a feasible strategy for NSCLC with a favorable rate of pCR/MPR, modified resection and PFS. No clinical factor other than PD-L1 expression was predictive of the pathological response. pCR/MPR may be effective surrogate endpoints for survival in NSCLC patients who received neoadjuvant chemo-immunotherapy.
Acknowledgments
We thank Wenhui Ren from the Academic Research Office of Peking University People’s Hospital for providing thoughtful insights on statistical methods.
Funding: This work was supported by Clinical Medicine Plus X - Young Scholars Project, Peking University, the Fundamental Research Funds for the Central Universities (No. PKU2023LCXQ001); CAMS Innovation Fund for Medical Sciences (CIFMS; No. 2022-I2M-C&T-B-120); Research Unit of Intelligence Diagnosis and Treatment in Early Non-small Cell Lung Cancer, Chinese Academy of Medical Sciences (No. 2021RU002); National Natural Science Foundation of China (No. 92059203).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-17/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-17/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-17/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-17/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by The Ethics Committee of Peking University People’s Hospital (approved number: 2021-PHB271-001) and The Second Affiliated Hospital of Air Force Medical University (approved number: K-202211-32). Informed consent was waived because this study was a noninterventional study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Pfannschmidt J, Muley T, Bülzebruck H, et al. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer 2007;55:371-7. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Taylor MD, Nagji AS, Bhamidipati CM, et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg 2012;93:1813-20; discussion 1820-1. [Crossref] [PubMed]
- Friedlaender A, Naidoo J, Banna GL, et al. Role and impact of immune checkpoint inhibitors in neoadjuvant treatment for NSCLC. Cancer Treat Rev 2022;104:102350. Erratum in: Cancer Treat Rev 2022;104:102358. [Crossref] [PubMed]
- Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Rodríguez-Abreu D, Powell SF, Hochmair M, et al. Final analysis of KEYNOTE-189: Pemetrexed-platinum chemotherapy (chemo) with or without pembrolizumab (pembro) in patients (pts) with previously untreated metastatic nonsquamous non-small cell lung cancer (NSCLC). J Clin Oncol 2020;38:9582. [Crossref]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J Clin Oncol 2021;39:2339-49. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Ulas EB, Dickhoff C, Schneiders FL, et al. Neoadjuvant immune checkpoint inhibitors in resectable non-small-cell lung cancer: a systematic review. ESMO Open 2021;6:100244. [Crossref] [PubMed]
- Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. [Crossref] [PubMed]
- Provencio M, Serna-Blasco R, Nadal E, et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol 2022;40:2924-33. Erratum in: J Clin Oncol 2022;40:3785. [Crossref] [PubMed]
- Yun J, Choi YS, Hong TH, et al. Nononcologic Mortality after Pneumonectomy Compared to Lobectomy. Semin Thorac Cardiovasc Surg 2022;34:1122-31. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Hui B, Wang X, Wang X, et al. Organ preservation strategies after neoadjuvant chemoimmunotherapy in resectable non-small cell lung cancer: a multicenter retrospective cohort study. Int J Surg 2023;109:2286-92. [Crossref] [PubMed]
- Powell SF, Rodríguez-Abreu D, Langer CJ, et al. Outcomes With Pembrolizumab Plus Platinum-Based Chemotherapy for Patients With NSCLC and Stable Brain Metastases: Pooled Analysis of KEYNOTE-021, -189, and -407. J Thorac Oncol 2021;16:1883-92. [Crossref] [PubMed]
- Deng H, Liu J, Cai X, et al. Radical Minimally Invasive Surgery After Immuno-chemotherapy in Initially-unresectable Stage IIIB Non-small cell Lung Cancer. Ann Surg 2022;275:e600-e602. [Crossref] [PubMed]
- Yao Y, Tang D, Gao W, et al. Neoadjuvant Immuno-Chemotherapy: A New Perspective for Stage III NSCLC? Front Surg 2022;9:843987. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]
- Lee JM, Tsuboi M, Brunelli A. Surgical Perspective on Neoadjuvant Immunotherapy in Non-Small Cell Lung Cancer. Ann Thorac Surg 2022;114:1505-15. [Crossref] [PubMed]
- Jiang J, Wang Y, Gao Y, et al. Neoadjuvant immunotherapy or chemoimmunotherapy in non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res 2022;11:277-94. [Crossref] [PubMed]
- Yang Y, Zhang X, Gao Y, et al. Research Progress in Immunotherapy of NSCLC With EGFR-Sensitive Mutations. Oncol Res 2022;29:63-74. [Crossref] [PubMed]
- Carbone D, Lee J, Kris M, et al. Oa06.06 Clinical/Biomarker data for neoadjuvant atezolizumab in resectable stage ib-iiib nsclc: primary analysis in the Lcmc3 study. J Thorac Oncol 2021;16:S115-6. [Crossref]
- Zhang C, Chen HF, Yan S, et al. Induction immune-checkpoint inhibitors for resectable oncogene-mutant NSCLC: A multicenter pooled analysis. NPJ Precis Oncol 2022;6:66. [Crossref] [PubMed]
- Chen Y, Zhang L, Yan B, et al. Feasibility of sleeve lobectomy after neo-adjuvant chemo-immunotherapy in non-small cell lung cancer. Transl Lung Cancer Res 2020;9:761-7. [Crossref] [PubMed]
- Wu J, Hou L, E H, et al. Real-world clinical outcomes of neoadjuvant immunotherapy combined with chemotherapy in resectable non-small cell lung cancer. Lung Cancer 2022;165:115-23. [Crossref] [PubMed]
- Zhang B, Xiao H, Pu X, et al. A real-world comparison between neoadjuvant chemoimmunotherapy and chemotherapy alone for resectable non-small cell lung cancer. Cancer Med 2023;12:274-86. [Crossref] [PubMed]


