
Impact of lymph node dissection on cancer-specific survival in non-small cell lung cancer patients: a SEER database analysis
Highlight box
Key findings
• Our comprehensive analysis of 37,323 non-small cell lung cancer (NSCLC) patients from the Surveillance, Epidemiology, and End Results (SEER) database elucidates the pivotal role of lymph node dissection (LND) in enhancing cancer-specific survival, particularly among early-stage patients. The study’s key findings underscore the survival advantage associated with LND, revealing an optimal range of 24–32 lymph nodes for examination to maximize survival benefits. This novel insight challenges the prevailing notion that more extensive lymph node removal invariably leads to better outcomes, advocating instead for a balanced and nuanced approach.
What is known and what is new?
• Previously established knowledge in our field includes LND’s role in NSCLC management has been debated, with studies providing conflicting evidence regarding its efficacy.
• This manuscript contributes new insights by identifying an optimal lymph node examination range that correlates with improved survival rates, offering a more refined understanding of LND’s impact across different cancer stages. This precision in defining the beneficial extent of LND marks a significant advancement in the surgical.
What is the implication, and what should change now?
• The implications of our findings are multifaceted, suggesting a reevaluation of current surgical practices to incorporate the identified optimal lymph node examination range. This adjustment aims to enhance patient outcomes by tailoring LND strategies more precisely. Additionally, our study highlights the necessity for further research to explore the underlying mechanisms driving the survival benefits observed with specific extents of lymph node examination, fostering the development of more effective, evidence-based surgical protocols in NSCLC treatment.
Introduction
Non-small cell lung cancer (NSCLC) is the most prevalent form of lung cancer and is a significant cause of cancer-related mortality worldwide (1,2). The management of NSCLC often involves surgical resection, with the aim of removing the primary tumor and assessing the extent of cancer spread in the surrounding lymph nodes (3,4). This evaluation is critical for staging the disease and guiding treatment decisions. One surgical procedure frequently employed to assess and potentially remove cancer-affected lymph nodes is known as lymph node dissection (LND). LND involves the systematic removal of lymph nodes in the region surrounding the tumor, allowing for a thorough examination to determine the extent of cancer involvement. The decision to perform LND is based on various factors, including the stage of the cancer, the patient’s overall health, and clinical guidelines. It is, however, a procedure that has generated substantial debate and research within the medical community.
Several studies have been conducted to investigate the impact of LND on survival rates and disease-free survival of NSCLC patients (5-9). These findings, supported by their robust statistical methodologies, suggest a potential benefit associated with LND in NSCLC management. However, the research landscape is complex and conflicting. Not all studies have reached the same conclusions. For instance, single-center retrospective studies have reported no significant survival advantage associated with LND, suggesting that LND may not be necessary in all cases and can lead to increased morbidity and mortality. One study concludes that routine dissection of aortopulmonary zone and inferior mediastinal nodes is adequate for accurate staging in left-sided NSCLC patients, and the addition of station 4L LND (S4L-LND) does not improve survival but may increase the risk of postoperative complications (10). These discrepancies in research findings underscore the ongoing debate and uncertainty surrounding the role of LND in NSCLC management.
Despite the accumulated research, several knowledge gaps persist. It remains unclear which specific patient groups are most likely to benefit from LND, and what factors influence the procedure’s effectiveness. These factors may include patient demographics, tumor characteristics, and the extent of LND. Furthermore, the potential for LND to introduce complications and morbidity raises questions about its overall safety and applicability.
Our study aims to address these gaps by utilizing the Surveillance, Epidemiology, and End Results (SEER) database to investigate the impact of LND on cancer-specific survival and postoperative complications in NSCLC patients. By examining a large and diverse dataset, we seek to provide a more comprehensive evaluation of the effectiveness and safety of LND. Our research explores differences in the prognostic impact of LND among patients with varying cancer stages and the influence of factors such as race and socioeconomic status on the utilization of LND. Additionally, we determine the optimal range of lymph nodes to be removed during dissection to maximize survival benefits, shedding light on the number of nodes that significantly impacts outcomes. Furthermore, our study investigates the impact of LND in conjunction with adjuvant therapies, such as chemotherapy and radiation, on patient survival. Specifically, we aim to investigate the factors that influence the effectiveness of LND and identify which patients groups would benefit most from this procedure. We investigate the factors that influence the risk of these complications following LND, including the socioeconomic factors, stage of the cancer, the location of the tumor, and the extent of LND. This provides a comprehensive evaluation of the safety of LND in NSCLC patients. Addressing these gaps in knowledge is essential to provide more precise and evidence-based guidance for the management of NSCLC. It is imperative to clarify the circumstances under which LND is most beneficial and to offer insight into the safety of the procedure. Moreover, the impact of LND in conjunction with adjuvant therapies, such as chemotherapy and radiation, requires exploration. By filling these research gaps, our study aims to contribute to a better understanding of the impact of LND on NSCLC outcomes and to inform clinical decision-making for NSCLC patients undergoing surgical treatment. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-91/rc).
Methods
Data sources and methods
The primary data source for this study was the SEER database, a comprehensive and nationally representative database for cancer-related information in the United States (11). This study utilized the SEER-Stat version 8.3.6, which provides a wealth of data on cancer incidence, patient demographics, treatment modalities, and long-term survival outcomes. The robustness of the SEER database ensures that the findings of this study are grounded in a large and diverse patient population.
Inclusion and exclusion criteria
The selection of patients for this investigation was guided by a stringent set of inclusion and exclusion criteria designed to focus the study on a specific subset of the NSCLC patient population. Inclusion criteria included patients with pathologically confirmed NSCLC, diagnosed between 2004 and 2017, and those who underwent primary tumor resection as their treatment modality. Furthermore, only individuals for whom NSCLC constituted their first primary malignancy were included. This selection ensures that the study focuses on newly diagnosed NSCLC cases and those who underwent surgical intervention.
Exclusion criteria were applied to maintain data integrity and clinical relevance. Patients with missing critical information such as primary tumor position, Tumour, Node, Metastasis (TNM) stage, treatment details, surgical status, regional nodes examined, or survival month were excluded to preserve data completeness. Additionally, patients with T0 local disease and those diagnosed with concurrent primary cancer prior to or simultaneously with their NSCLC diagnosis were excluded to ensure the study’s focus on distinct NSCLC cases.
These inclusion and exclusion criteria were meticulously applied to ensure that the analysis is based on a well-defined and homogenous cohort, enhancing the internal validity and clinical relevance of the findings.
Statistical methods
The statistical analysis plan for this study comprises a comprehensive approach, including:
Descriptive statistics
Descriptive statistics was employed to provide a comprehensive summary of the study population, encompassing key demographic characteristics, tumor attributes, and the distribution of LND procedures.
Univariate analysis (12)
A univariate analysis explored the unadjusted relationships between individual variables and cancer-specific survival rates. This analysis identified potential factors that may exert a significant influence on patient outcomes.
Multivariate analysis [Cox proportional hazards (PH) model] (13)
A multivariate Cox proportional hazards analysis was conducted, taking into account the potential confounding variables. This sophisticated statistical model enabled the assessment of the independent impact of LND on cancer-specific survival rates, while controlling for other pertinent factors. Furthermore, to address potential confounding factors particularly prevalent among the patient cohort with advanced-stage disease, who comprise the largest portion of our study population, the multivariate Cox PH analysis was meticulously designed. This design accounted for the stage of the disease to differentiate the effect of LND on survival outcomes. While it is recognized that lymphadenectomy in the context of distant-stage disease is well documented, including these patients enables a more thorough understanding of the intervention’s role across the complete spectrum of the disease. This comprehensive approach facilitates the identification of stage-specific therapeutic benefits and informs nuanced treatment strategies that cater to individual patient profiles.
Sensitivity analysis
To assess the robustness of the results and address potential sources of bias or confounding variables, a sensitivity analysis was performed, adding an additional layer of rigor to the study’s findings.
Moreover, this research was carried out in strict adherence to ethical standards, aligning with the principles articulated in the Declaration of Helsinki (as revised in 2013).
Results
Baseline characteristics
In this study, a total of 37,323 patients were included. The median survival time was 19.58 months [95% confidence interval (CI): 19, 20], with 85% of the patients having experienced death during the follow-up period. Most patients were aged between 45 and 79 years old, with 37% aged between 45–64 years, and 46% aged between 65–79 years. There were more male patients (56%) than female patients (44%). White patients comprised the majority of the study population (81%), followed by Black patients (12%), and Asian or Pacific Islander patients (6.3%). Most of the patients were married (51%), while 18% were widowed. Most of the patients were diagnosed with distant stage cancer (63%), while only 11% of the patients had localized cancer. The majority of the patients (64%) had undifferentiated or anaplastic Grade IV tumors. The primary origin of the cancer was on the right side in 57% of the cases, followed by the left side in 39% of the cases. The most common surgical site was the upper lobe of the lung (54%). Most of the patients (84%) did not have regional lymph nodes removed or aspirated. The mean number of times the lymph nodes were examined was 8.35 (SD =18.53) (see Table 1).
Table 1
| Characteristics | Values (N=37,323) | 95% CI |
|---|---|---|
| Survival (months) | 19.58 (28.49) | 19–20 |
| Death | ||
| Yes | 31,650/37,323 (85%) | 84–85% |
| No | 5,673/37,323 (15%) | 15–16% |
| Age (years) | ||
| 45–64 years | 13,695/37,323 (37%) | 36–37% |
| 65–79 years | 17,118/37,323 (46%) | 45–46% |
| Greater than 80 years | 5,694/37,323 (15%) | 15–16% |
| Less than 45 years | 816/37,323 (2.2%) | 2.0–2.3% |
| Gender | ||
| Female | 16,555/37,323 (44%) | 44–45% |
| Male | 20,768/37,323 (56%) | 55–56% |
| Race | ||
| White | 30,058/37,323 (81%) | 80–81% |
| Black | 4,621/37,323 (12%) | 12–13% |
| Asian or Pacific Islander | 2,338/37,323 (6.3%) | 6.0–6.5% |
| American Indian/Alaska Native | 250/37,323 (0.7%) | 0.59–0.76% |
| Unknown | 56/37,323 (0.2%) | 0.11–0.20% |
| Marital status | ||
| Married (including common law) | 18,962/36,851 (51%) | 51–52% |
| Divorced | 4,704/36,851 (13%) | 12–13% |
| Separated | 0/36,851 (0%) | 0.00–0.01% |
| Single (never married) | 5,146/36,851 (14%) | 14–14% |
| Widowed | 6,519/36,851 (18%) | 17–18% |
| Unmarried or domestic partner | 35/36,851 (<0.1%) | 0.07–0.13% |
| Unknown | 1,485/36,851 (4.0%) | 3.8–4.2% |
| Missing | 472 | – |
| T category | ||
| T1 | 5,409/37,323 (14%) | 14–15% |
| T2 | 10,113/37,323 (27%) | 27–28% |
| T3 | 2,846/37,323 (7.6%) | 7.4–7.9% |
| T4 | 13,609/37,323 (36%) | 36–37% |
| TX | 5,346/37,323 (14%) | 14–15% |
| N category | ||
| N0 | 10,344/37,259 (28%) | 27–28% |
| N1 | 2,977/37,259 (8.0%) | 7.7–8.3% |
| N2 | 14,796/37,259 (40%) | 39–40% |
| N3 | 5,367/37,259 (14%) | 14–15% |
| NX | 3,775/37,259 (10%) | 9.8–10% |
| M category | ||
| M0 | 16,389/37,323 (44%) | 43–44% |
| M1 | 18,875/37,323 (51%) | 50–51% |
| MX | 2,059/37,323 (5.5%) | 5.3–5.8% |
| Stage | ||
| Distant | 23,364/37,323 (63%) | 62–63% |
| Localized | 4,283/37,323 (11%) | 11–12% |
| Regional | 8,299/37,323 (22%) | 22–23% |
| Unknown/unstaged | 1,377/37,323 (3.7%) | 3.5–3.9% |
| Grade | ||
| Well-differentiated; Grade I | 676/37,323 (1.8%) | 1.7–2.0% |
| Moderately differentiated; Grade II | 11,805/37,323 (32%) | 31–32% |
| Poorly differentiated; Grade III | 741/37,323 (2.0%) | 1.8–2.1% |
| Undifferentiated; anaplastic; Grade IV | 23,955/37,323 (64%) | 64–65% |
| Unknown | 146/37,323 (0.4%) | 0.33–0.46% |
| Laterality | ||
| Bilateral, single primary | 419/37,323 (1.1%) | 1.0–1.2% |
| Left—origin of primary | 14,385/37,323 (39%) | 38–39% |
| Not a paired site | 20/37,323 (<0.1%) | 0.03–0.08% |
| Only one side—side unspecified | 169/37,323 (0.5%) | 0.39–0.53% |
| Paired site, but no information concerning laterality | 1,199/37,323 (3.2%) | 3.0–3.4% |
| Right—origin of primary | 21,131/37,323 (57%) | 56–57% |
| Surgery site | ||
| Main bronchus | 1,654/37,323 (4.4%) | 4.2–4.6% |
| Upper lobe, lung | 20,332/37,323 (54%) | 54–55% |
| Middle lobe, lung | 1,512/37,323 (4.1%) | 3.9–4.3% |
| Lower lobe, lung | 8,474/37,323 (23%) | 22–23% |
| Overlapping lesion of lung | 391/37,323 (1.0%) | 0.95–1.2% |
| Lung, NOS | 4,960/37,323 (13%) | 13–14% |
| Lymph node dissection | ||
| Biopsy or aspiration of regional lymph node | 2,123/37,323 (5.7%) | 5.5–5.9% |
| No regional lymph nodes removed or aspirated | 31,190/37,323 (84%) | 83–84% |
| Removal of regional lymph nodes | 3,392/37,323 (9.1%) | 8.8–9.4% |
| Unknown | 618/37,323 (1.7%) | 1.5–1.8% |
| Conjoint treatment | ||
| Conjoint treatment | 1,214/37,323 (3.3%) | 3.1–3.4% |
| Conjoint with chemo only | 687/37,323 (1.8%) | 1.7–2.0% |
| Conjoint with radio only | 255/37,323 (0.7%) | 0.60–0.77% |
| RLN without conjoint treatment | 22,882/37,323 (61%) | 61–62% |
| No RLN | 12,285/37,323 (33%) | 32–33% |
| Number of times lymph node examined | 8.35 (18.53) | 8.2–8.5 |
Data are presented as mean (SD) or n/N (%). CI, confidence interval; NOS, not otherwise specified; RLN, recurrent laryngeal nerve; SD, standard deviation.
Impact of LND on cancer-specific survival
The univariate analysis showed the hazard ratios and their corresponding confidence intervals and P values for various variables in relation to lung cancer-specific survival in patients with NSCLC (Table 2). The analysis indicated that age, gender, race, marital status, T category, N category, M category, stage, grade, laterality, surgery site, LND, and conjoint treatment were all significant predictors of lung cancer-specific survival in patients with NSCLC.
Table 2
| Variable | Level | Hazard ratio | 95% CI | P value |
|---|---|---|---|---|
| Age | 45–64 years | Reference | ||
| 65–79 years | 0.99 | 0.96–1.01 | 0.30 | |
| Greater than 80 years | 1.06 | 1.03–1.1 | <0.001 | |
| Less than 45 years | 0.89 | 0.82–0.96 | <0.001 | |
| Gender | Female | Reference | ||
| Male | 1.14 | 1.12–1.17 | <0.001 | |
| Race | White | Reference | ||
| Black | 1.03 | 0.99–1.06 | 0.11 | |
| Asian or Pacific Islander | 0.92 | 0.88–0.97 | <0.001 | |
| American Indian/Alaska Native | 0.89 | 0.77–1.02 | 0.10 | |
| Unknown | 1.03 | 0.99–1.06 | <0.001 | |
| Marital status | Married (including common law) | Reference | ||
| Divorced | 1.05 | 1.02–1.09 | <0.001 | |
| Single (never married) | 1.08 | 1.04–1.12 | <0.001 | |
| Widowed | 1.02 | 0.99–1.06 | 0.14 | |
| Unmarried or domestic partner | 1.33 | 0.93–1.9 | 0.12 | |
| T category | T1 | Reference | ||
| T2 | 0.95 | 0.9–1 | <0.001 | |
| T3 | 1.62 | 1.56–1.68 | <0.001 | |
| T4 | 1.85 | 1.76–1.95 | <0.001 | |
| TX | 2.32 | 2.24–2.41 | <0.001 | |
| N category | N0 | Reference | ||
| N1 | 1.48 | 1.41–1.54 | <0.001 | |
| N2 | 1.75 | 1.7–1.8 | <0.001 | |
| N3 | 1.88 | 1.82–1.95 | <0.001 | |
| NX | 1.72 | 1.66–1.8 | <0.001 | |
| M category | M0 | Reference | ||
| M1 | 2.30 | 2.24–2.35 | <0.001 | |
| MX | 1.47 | 1.4–1.55 | <0.001 | |
| Stage | Distant | Reference | ||
| Localized | 0.29 | 0.28–0.3 | <0.001 | |
| Regional | 0.50 | 0.48–0.51 | <0.001 | |
| Unknown/unstaged | 0.59 | 0.55–0.62 | <0.001 | |
| Grade | Well-differentiated; Grade I | Reference | ||
| Moderately differentiated; Grade II | 1.69 | 1.54–1.85 | <0.001 | |
| Poorly differentiated; Grade III | 1.74 | 1.54–1.96 | <0.001 | |
| Undifferentiated; anaplastic; Grade IV | 1.78 | 1.62–1.95 | <0.001 | |
| Unknown | 0.96 | 0.77–1.19 | 0.71 | |
| Laterality | Bilateral, single primary | Reference | ||
| Left—origin of primary | 0.71 | 0.64–0.79 | <0.001 | |
| Not a paired site | 0.77 | 0.48–1.26 | 0.30 | |
| Only one side—side unspecified | 0.80 | 0.67–0.97 | 0.02 | |
| Paired site, but no information concerning laterality | 0.89 | 0.79–1 | 0.06 | |
| Right—origin of primary | 0.70 | 0.63–0.78 | <0.001 | |
| Surgery site | Main bronchus | Reference | ||
| Upper lobe, lung | 0.77 | 0.73–0.81 | <0.001 | |
| Middle lobe, lung | 0.78 | 0.72–0.84 | <0.001 | |
| Lower lobe, lung | 0.83 | 0.79–0.88 | <0.001 | |
| Overlapping lesion of lung | 0.98 | 0.87–1.1 | 0.73 | |
| Lung, NOS | 1.04 | 0.98–1.1 | 0.23 | |
| Lymph node dissection | No regional lymph nodes removed or aspirated | Reference | ||
| Biopsy or aspiration of regional lymph node | 0.84 | 0.8–0.89 | <0.001 | |
| Removal of regional lymph nodes | 0.38 | 0.37–0.4 | <0.001 | |
| Unknown | 0.93 | 0.85–1.01 | 0.07 | |
| Conjoint treatment | Conjoint treatment | Reference | ||
| Conjoint with chemo only | 0.81 | 0.72–0.9 | <0.001 | |
| Conjoint with radio only | 1.40 | 1.2–1.64 | <0.001 | |
| RLN without conjoint treatment | 1.95 | 1.82–2.08 | <0.001 | |
| No RLN | 1.82 | 1.7–1.95 | <0.001 |
NSCLC, non-small cell lung cancer; CI, confidence interval; NOS, not otherwise specified; RLN, recurrent laryngeal nerve.
In terms of tumor characteristics, T category, N category, and M category were all significant predictors of lung cancer-specific survival, with higher T, N, and M categories corresponding to higher hazard ratios. Stage was also a significant predictor, with patients with localized and regional stage having lower hazard ratios than those with distant stage. Grade was also significant, with higher grade corresponding to higher hazard ratios. ‘Laterality’ refers to the side of the lung (left or right) where the primary tumor is located, which impacts survival predictions. Tumors on the left and right sides may lead to differences in treatment strategies and prognoses due to anatomical differences, the feasibility of surgical treatment, and variations in lymphatic drainage patterns. Additionally, tumor laterality and specific locations within the lung were significant predictors of outcomes. Tumors located in the left lung and in the upper and middle lobes were associated with lower hazard ratios compared to tumors in the main bronchus or the lower lobes. These differences could be attributed to anatomical variations, differences in lymphatic drainage and blood supply, impacts on pulmonary function, and variations in surgical accessibility and effectiveness.
LND was also a significant predictor, with removal of regional lymph nodes corresponding to a lower hazard ratio than no regional lymph nodes removed or aspirated. Finally, conjoint treatment was a significant predictor, with patients receiving conjoint treatment with chemo only having a lower hazard ratio than those receiving recurrent laryngeal nerve (RLN) without conjoint treatment or no RLN. RLN in this study stands for regional lymph nodes. The removal or biopsy of regional lymph nodes during surgery is a key step in assessing tumor spread and guiding subsequent treatment plans. We have paid special attention to the management of RLN within the context of conjoint treatment, including chemotherapy and radiotherapy, and their impacts on survival rates. On the other hand, patients receiving conjoint treatment with radio only had a higher hazard ratio than those receiving RLN without conjoint treatment or no RLN.
The multivariate Cox PH analysis for lung cancer-specific survival in patients with NSCLC revealed that several variables were significantly associated with survival (Table 3). The variables that had a statistically significant impact on survival were age, sex, race, marital status, T category, N category, M category, stage, grade, laterality, surgery site, LND during surgery, and conjoint treatment. Older age, male sex, Black or American Indian/Alaska Native race, unmarried or domestic partnership, advanced T, N, M categories, higher stage, poorly or undifferentiated grade, left or right origin of primary, and no RLN were associated with increased hazard ratios, indicating a higher risk of death from lung cancer.
Table 3
| Variables | Level | Hazard ratio | 95% CI | P value |
|---|---|---|---|---|
| Age | 45–64 years | Reference | ||
| 65–79 years | 1.07 | 1.04–1.10 | <0.001 | |
| Greater than 80 years | 1.21 | 1.17–1.26 | <0.001 | |
| Less than 45 years | 0.89 | 0.82–0.96 | 0.003 | |
| Sex | Female | Reference | ||
| Male | 1.17 | 1.15–1.20 | <0.001 | |
| Race | White | Reference | ||
| Black | 0.98 | 0.95–1.02 | 0.26 | |
| Asian or Pacific Islander | 0.79 | 0.76–0.83 | <0.001 | |
| American Indian/Alaska Native | 0.93 | 0.81–1.07 | 0.29 | |
| Unknown | 0.51 | 0.36–0.72 | <0.001 | |
| Marital status | Married (including common law) | Reference | ||
| Divorced | 1.14 | 1.10–1.18 | <0.001 | |
| Single (never married) | 1.08 | 1.04–1.12 | <0.001 | |
| Widowed | 1.11 | 1.08–1.15 | <0.001 | |
| Unmarried or domestic partner | 1.37 | 0.96–1.96 | 0.08 | |
| Unknown | 0.98 | 0.92–1.03 | 0.41 | |
| T category | T1 | Reference | ||
| T2 | 1.32 | 1.27–1.38 | <0.001 | |
| T3 | 1.42 | 1.34–1.50 | <0.001 | |
| T4 | 1.47 | 1.41–1.53 | <0.001 | |
| TX | 1.36 | 1.29–1.43 | <0.001 | |
| N category | N0 | Reference | ||
| N1 | 1.24 | 1.19–1.30 | <0.001 | |
| N2 | 1.33 | 1.29–1.37 | <0.001 | |
| N3 | 1.28 | 1.23–1.34 | <0.001 | |
| NX | 1.23 | 1.17–1.29 | <0.001 | |
| M category | M0 | Reference | ||
| M1 | 1.5 | 1.44–1.55 | <0.001 | |
| MX | 1.24 | 1.16–1.32 | <0.001 | |
| Stage | Stage I | Reference | ||
| Stage II | 0.57 | 0.54–0.61 | <0.001 | |
| Stage III | 0.76 | 0.73–0.80 | <0.001 | |
| Stage IV | 0.63 | 0.58–0.69 | <0.001 | |
| Grade | Well-differentiated; Grade I | Reference | ||
| Moderately differentiated; Grade II | 1.28 | 1.16–1.40 | <0.001 | |
| Poorly differentiated; Grade III | 1.4 | 1.24–1.57 | <0.001 | |
| Undifferentiated; anaplastic; Grade IV | 1.2 | 1.09–1.32 | <0.001 | |
| Unknown | 0.89 | 0.72–1.11 | 0.30 | |
| Laterality | Bilateral, single primary | Reference | ||
| Left—origin of primary | 1.23 | 1.10–1.36 | <0.001 | |
| Not a paired site | 1.06 | 0.65–1.73 | 0.81 | |
| Only one side—side unspecified | 0.97 | 0.81–1.18 | 0.78 | |
| Paired site, but no information concerning laterality | 1.18 | 1.05–1.33 | 0.005 | |
| Right—origin of primary | 1.2 | 1.08–1.34 | <0.001 | |
| Surgery site | Main bronchus | Reference | ||
| Upper lobe, lung | 0.89 | 0.84–0.94 | <0.001 | |
| Middle lobe, lung | 0.88 | 0.82–0.95 | 0.001 | |
| Lower lobe, lung | 0.93 | 0.88–0.99 | 0.44 | |
| Overlapping lesion of lung | 0.95 | 0.85–1.08 | 0.02 | |
| Lung, NOS | 0.96 | 0.90–1.02 | 0.15 | |
| Lymph node dissection during surgery | No regional lymph nodes removed or aspirated | Reference | ||
| Biopsy or aspiration of regional lymph node | 0.85 | 0.81–0.89 | <0.001 | |
| Removal of regional lymph nodes | 0.43 | 0.39–0.46 | <0.001 | |
| Unknown | 0.94 | 0.86–1.02 | 0.14 | |
| Conjoint treatment | Conjoint treatment | Reference | ||
| Conjoint with chemo only | 0.87 | 0.77–0.97 | 0.01 | |
| Conjoint with radio only | 1.49 | 1.27–1.74 | <0.001 | |
| RLN without conjoint treatment | 0.83 | 0.75–0.93 | <0.001 | |
| No RLN | 0.65 | 0.58–0.72 | <0.001 |
NSCLC, non-small cell lung cancer; CI, confidence interval; NOS, not otherwise specified; RLN, recurrent laryngeal nerve.
Among these variables, LND during surgery had a significant impact on survival. The hazard ratio for no regional lymph nodes removed or aspirated was 1 (reference), while the hazard ratios for biopsy or aspiration of regional lymph node and removal of regional lymph nodes were 0.85 (95% CI: 0.81–0.89; P<0.001) and 0.43 (95% CI: 0.39–0.46; P<0.001), respectively, indicating a survival benefit. Contrary to initial expectations, an analysis mentioned that an increase in stage is associated with a lower hazard ratio, implying better survival outcomes. This counterintuitive finding may be attributed to factors such as selection bias for surgical candidates, the comprehensive treatment approaches for higher-stage patients, more aggressive surveillance and follow-up care, and stage migration due to advances in diagnostic accuracy. These aspects highlight the complexity of treating lung cancer and underscore the necessity of a nuanced understanding of how staging, treatment interventions, and patient characteristics interplay to influence survival outcomes. While the beneficial impact of LND on survival was clear, the analysis was adjusted for confounding factors, including the predominance of patients with distant-stage cancer. By integrating the stage of the disease into the model, the analysis provided insights into the therapeutic value of LND even in advanced stages, which is critical for developing balanced and evidence-based clinical guidelines.
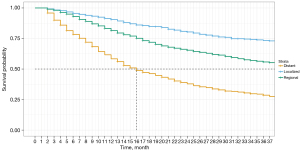
The data visualizations presented in this study demonstrated the impact of LND on cancer-specific survival among different cohorts of NSCLC patients. Specifically, Figure 1 depicts a Kaplan-Meier curve based on statistics from a multivariate Cox PH model, which indicates that patients who underwent the removal of regional lymph nodes and the number of regional lymph nodes removal over 4 had a significantly higher probability of survival than those who did not undergo this procedure. The figure shows the impact on 3-year cancer-specific survival, highlighting the crucial role that LND plays in improving patient outcomes.
To investigate the survival advantages of LND in NSCLC patients across different cancer stages, we analyzed the data and created Figures 2-5. The analysis indicated that patients with non-metastatic NSCLC, particularly those presenting with a localized stage and classified as T1 and N1, showed the most significant survival benefits from LND. It is important to note that while the inclusion of M1 (a designation for distant metastasis) in this context may suggest a contradiction, it can be postulated that this reflects a subset of patients where the primary tumor characteristics (such as T1 and N1) are indicative of a localized disease process, but isolated metastases (M1) were also present. In such cases, comprehensive LND may still offer a survival advantage, potentially due to the removal of metastatic deposits within accessible regional nodes, or it may reflect a survival benefit in a specific patient cohort that warrants further investigation. In Figure 6, we observed that the addition of adjuvant chemotherapy to LND resulted in the greatest short-term survival benefits for patients. However, LND alone had a higher survival probability over the long term (after 2 years).




Impact of lymph node examination during dissection on cancer-specific survival
We performed restricted cubic spline method to explore the relationship between a continuous variable of the number of lymph nodes examined and a binary outcome of lung cancer-specific mortality. In this way, we can use it to explore the optimal range of lymph node dissection for achieving the greatest survival benefit. This method allows us to model the relationship between LND and survival as a smooth curve with several knots, instead of assuming a linear relationship. The choice of 3–5 knots in the analysis serves to smooth the data and capture the complex relationship between the continuous variable (the number of lymph nodes) and the binary outcome (lung cancer-specific survival). These knots allow the data to flexibly shape the curve to better accommodate potential nonlinear relationships. This is because the impact of lymph node count on survival may not be linear but rather exhibiting as a specific range, which is why knots were utilized.
We then plotted the results of the analysis to visualize the relationship between the number of lymph nodes examined and lung cancer-specific survival. The plot showed a U-shaped curve, suggesting that examining too few or too many lymph nodes could be detrimental to survival, while examining an intermediate number of lymph nodes could result in the greatest survival benefit.
Data analysis identified 24–32 as the optimal number of lymph nodes to examine during dissection for enhanced survival benefits in NSCLC patients. This range, supported by a U-shaped curve, aligns with the highest survival probability, indicating its strong association with better patient outcomes (Figure 7). Such findings can inform surgical strategies, particularly emphasizing the importance of extensive LND in early-stage patients to potentially improve prognosis. This suggests that among early-stage lung cancer patients, LND may have a more pronounced survival benefit. This observation warrants further research and exploration to understand the biological and clinical mechanisms underlying this phenomenon. Several potential explanations can be considered to elucidate why early-stage patients experience greater benefits. Firstly, early-stage patients typically exhibit smaller tumor burdens and fewer affected lymph nodes, making LND potentially more feasible and effective in reducing the spread of tumor cells. Additionally, for early-stage patients, LND may aid in more accurately staging the disease, thereby guiding more effective treatment strategies. Lastly, there may be biological differences, with early-stage lung cancer possibly displaying a more favorable response to LND. However, this observation requires further research for validation and a more comprehensive exploration of why early-stage patients derive greater benefits from LND. This will contribute to guiding personalized treatment strategies to maximize the chances of survival for NSCLC patients.

Discussion
This study provides critical insights into the impact of LND on NSCLC patients, with a focus on cancer-specific survival rates and postoperative complications. The findings of this research illuminate critical aspects of LND in NSCLC patients, carrying substantial implications for clinical practice and guiding future research directions.
Prognostic impact of LND across different cancer stages
Our study categorizes ‘early-stage’ NSCLC patients as those with localized disease, reflecting T1 and N1 classifications. However, it is crucial to address an apparent inconsistency where M1 status, indicative of distant metastases, has been associated with early-stage disease. This inclusion stems from a subset of patients with primary tumors characteristic of early-stage (T1, N1) but also present with singular metastatic sites (M1), which may still be amenable to surgical intervention and lymphadenectomy. This condition is sometimes referred to as oligometastatic disease and represents a particular clinical scenario distinct from widespread metastatic disease typically associated with advanced stages. To avoid confusion and ensure precise communication of our findings, we will revise the text to clarify that patients with M1 disease are not categorized as early-stage, but rather, they represent a specific group where targeted surgical approaches, including lymphadenectomy, may still provide survival benefits. Further research is warranted to delineate the survival implications in this unique patient population thoroughly. Our findings align with the meta-analysis by Deng et al. (14), which reported improved overall survival with LND in NSCLC patients. However, while Deng et al. did not analyze the impact of LND on different cancer stages, our study demonstrates that the survival benefits are particularly pronounced in early-stage NSCLC patients. This suggests a nuanced relationship between LND and cancer stage, which has not been extensively explored in prior literature. Our analysis has demonstrated that the prognostic impact of LND on NSCLC patients varies across different cancer stages. Patients with localized NSCLC, particularly those with early-stage tumors, seem to derive the most significant survival benefits from LND. In contrast, the impact on patients with distant-stage cancer is less pronounced. This suggests that the decision to perform LND should be influenced by the specific stage of the disease, emphasizing the need for personalized treatment approaches based on TNM staging. Including advanced-stage disease in our analysis allowed for a comprehensive examination of LND’s impact across all stages. Despite the acknowledged efficacy of lymphadenectomy in early-stage NSCLC, its role in advanced disease has been less clear. By analyzing a full spectrum of disease stages, we aimed to clarify the survival benefits in the context of distant-stage diagnoses. While the largest part of our study population had a distant-stage diagnosis, representing 63% with a mortality rate of 85%, we accounted for this potential bias. The study design and statistical analysis, especially the use of multivariate Cox PH models, aimed to control for confounding factors, ensuring that the observed benefits of LND are not overestimated for advanced-stage patients. This careful consideration ensures that the study’s conclusions are reliable and applicable to the wider NSCLC patient population.
Factors influencing the use of LND
Furthermore, our study uncovers disparities in the utilization of LND based on demographic factors. Race and socioeconomic status have emerged as significant influencers of the likelihood of undergoing LND. These findings have profound implications for ensuring equitable access to potentially life-saving procedures and necessitate measures to address and rectify these disparities.
Extent of LND and survival outcomes
Notably, the extent of LND, gauged by the number of lymph nodes removed, emerges as a critical determinant of survival outcomes in NSCLC patients undergoing primary tumor resection. Our study, in line with the previous research conducted by Saji et al. (15), highlights an optimal range of lymph nodes, when examined during dissection, is associated with the most substantial survival benefit. This information serves as invaluable guidance for clinical decision-making, ultimately optimizing patient outcomes. Contrary to the conventional belief that more lymph nodes cleared results in better outcomes, our study introduces a nuanced perspective. Notably, Professor Jianxing He and Professor Wenhua Liang’s 2017 multicenter study indicated a positive correlation between examining up to 16 lymph nodes and improved long-term survival (16). Our study delineates that the survival benefit does not extend indefinitely with more lymph nodes; instead, it plateaus and potentially declines when more than 32 lymph nodes are dissected. This suggests that excessive lymph node removal may be detrimental, thus necessitating a balanced approach to lymphadenectomy. These findings offer valuable guidance for clinical decision-making, emphasizing the need for future research to further refine the optimal range of LND to maximize survival advantages, as the previous recommendation was 10, while our study suggests a range of 24–32. Previous research indicates that performing systematic LND (SLND) involving at least 11 lymph nodes from at least five stations is essential for both assessing lymph node involvement and accurately staging tumors, particularly in the context of recurrent-free survival, with the need for further validation in larger cohorts (17).
Location of lymph node metastasis
The previous research focused on subcarinal node involvement in upper lobe NSCLC patients, evaluating its frequency and impact on outcomes to determine the validity of omitting subcarinal LND. Their findings indicated that subcarinal node metastases were rare, particularly among squamous cell carcinoma patients, and these metastases predicted an extremely poor outcome. This suggests that it is valid to omit subcarinal node dissection in upper lobe NSCLC patients, especially in clinical N0 squamous cell carcinoma patients (18). In the broader context of the discussion on the location of lymph node metastasis, this research emphasizes the importance of considering the specific location of metastatic lymph nodes when making treatment decisions. Tailoring treatment strategies based on the location of metastasis is essential for enhancing patient outcomes, as seen in the rare occurrence of subcarinal node involvement in upper lobe NSCLC patients.
Impact of adjuvant therapy in conjunction with LND
Combining adjuvant therapies, such as chemotherapy and radiation, with LND has demonstrated varying effects on survival. The previous study investigated the impact of neoadjuvant radiation (N-RT) versus adjuvant radiation (A-RT) in the treatment of locally advanced NSCLC. It revealed that the survival benefit of N-RT is influenced by factors such as age, nodal status, and type of surgery, with a notable benefit in patients who do not undergo LND during surgery and in patients younger than 70 years of age (19). Our results indicate that specific patient subgroups may benefit more from these combined treatments. The choice of adjuvant therapy should be carefully considered in light of individual patient characteristics and tumor profiles.
Incidence of postoperative complications
Previous research indicates that the addition of S4L-LND do not improve survival, but might increase the risk of postoperative complications (10). While our study did not provide data on the incidence of postoperative complications, it is important to acknowledge the potential risks associated with LND. Balancing the potential benefits of LND with the risk of postoperative complications is a complex decision that should be made with a comprehensive understanding of each patient’s clinical situation.
While this investigation offers valuable insights into the impact of LND on NSCLC patients, it is essential to recognize and acknowledge its inherent limitations. These include the retrospective nature of the study, which may introduce biases, and the utilization of the SEER database, which, like any extensive database, may contain instances of missing or inaccurate data. Nevertheless, this research represents a substantial contribution to the field of NSCLC management, offering a rigorous and methodologically sound analysis of the impact of LND on both cancer-specific survival rates and postoperative complications, thereby contributing to the body of knowledge in this critical area of oncology.
Conclusions
This comprehensive study, involving 37,323 NSCLC patients, meticulously examined the intricate relationship between LND and lung cancer-specific survival. The median survival time of 19.58 months, coupled with a mortality rate of 85%, emphasized the critical need for nuanced treatment approaches. Baseline characteristics highlighted demographic and clinical factors influencing patient outcomes. Our findings underscore the prognostic impact of LND across various stages, with early-stage NSCLC patients experiencing more substantial survival benefits. Demographic disparities in LND utilization, particularly influenced by race and socioeconomic status, call for targeted interventions to ensure equitable access to life-saving procedures. The extent of LND emerged as a pivotal factor, with optimal survival benefits observed within the range of 24–32 lymph nodes examined during dissection. This challenges conventional beliefs, emphasizing the delicate balance required for maximal survival advantages. The location of lymph node metastasis, especially in upper lobe NSCLC patients, emphasizes the importance of tailoring treatment strategies based on metastatic location. Furthermore, the study delineated the combined impact of adjuvant therapies with LND, revealing varying effects across patient subgroups. Consideration of individual patient characteristics and tumor profiles is crucial in choosing appropriate adjuvant therapies. Limitations, including the retrospective nature of the study and reliance on SEER data, warrant caution in generalizing findings. However, the study’s implications are clear: personalized approaches to LND, guided by cancer stage, demographics, and optimal examination ranges, are essential for optimizing NSCLC patient outcomes.
In conclusion, these insights contribute to the evolving landscape of personalized treatment strategies, offering potential avenues to enhance overall survival rates in NSCLC patients. Future research endeavors should focus on prospective studies to validate these findings and explore long-term impacts, guiding the refinement of personalized treatment approaches and addressing disparities in LND utilization.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-91/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-91/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-91/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016;5:288-300. [Crossref] [PubMed]
- De Leyn P, Lardinois D, Van Schil P, et al. European trends in preoperative and intraoperative nodal staging: ESTS guidelines. J Thorac Oncol 2007;2:357-61. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Yang MZ, Hou X, Li JB, et al. Impact of L4 lymph node dissection on long-term survival in left-side operable non-small-cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2020;57:1181-8. [Crossref] [PubMed]
- Yang MZ, Tan ZH, Li JB, et al. Impact of the Number of Harvested Lymph Nodes on Long-Term Survival in Node-Negative Non-Small-Cell Lung Cancer: Based on Clinical Stage But Not Pathological Stage. Clin Lung Cancer 2023;24:e226-35. [Crossref] [PubMed]
- Peng L, Huang KL, Shang QW, et al. The prognostic value of 4L lymph node dissection in left-side operable non-small-cell lung cancer: a meta-analysis. Updates Surg 2024;76:23-32. [Crossref] [PubMed]
- Gallina FT, Marinelli D, Tajè R, et al. Analysis of predictive factors of unforeseen nodal metastases in resected clinical stage I NSCLC. Front Oncol 2023;13:1229939. [Crossref] [PubMed]
- Takamori S, Komiya T, Powell E. Clinical impact of number of lymph nodes dissected on postoperative survival in node-negative small cell lung cancer. Front Oncol 2022;12:962282. [Crossref] [PubMed]
- Wo Y, Li H, Zhang Y, et al. The impact of station 4L lymph node dissection on short-term and long-term outcomes in non-small cell lung cancer. Lung Cancer 2022;170:141-7. [Crossref] [PubMed]
- National Cancer Institute: Surveillance, Epidemiology, and End Results. Available online: http://www.seer.cancer.gov/
- Proctor M, Farquhar C. Diagnosis and management of dysmenorrhoea. BMJ 2006;332:1134-8. [Crossref] [PubMed]
- Bradburn MJ, Clark TG, Love SB, et al. Survival analysis part II: multivariate data analysis--an introduction to concepts and methods. Br J Cancer 2003;89:431-6. [Crossref] [PubMed]
- Deng HY, Li D, Qiu XM, et al. Dissection of 4L lymph node for left-sided non-small cell lung cancer: a meta-analysis. ANZ J Surg 2021;91:E696-E702. [Crossref] [PubMed]
- Saji H, Tsuboi M, Yoshida K, et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J Thorac Oncol 2011;6:1865-71. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Tantraworasin A, Saeteng S, Siwachat S, et al. Impact of lymph node management on resectable non-small cell lung cancer patients. J Thorac Dis 2017;9:666-74. [Crossref] [PubMed]
- Aokage K, Yoshida J, Ishii G, et al. Subcarinal lymph node in upper lobe non-small cell lung cancer patients: is selective lymph node dissection valid? Lung Cancer 2010;70:163-7. [Crossref] [PubMed]
- Jain AK, Horowitz DP, Chao CKS, et al. Factors Affecting the Impact of Neoadjuvant Radiation on Survival in Locally Advanced NSCLC: A SEER Database Analysis. Int J Radiat Oncol Biol Phys 2011;81:S163-4. [Crossref]



