
Identifying optimal surgical approach among T1N2–3M0 non-small cell lung cancer patients: a population-based analysis
Highlight box
Key findings
• Determining the survival advantages of surgery in T1N2–3M0 non-small cell lung cancer (NSCLC) patients and visually representing the benefits of surgical intervention.
What is known and what is new?
• Concurrent chemotherapy and radiotherapy are considered as the standard and viewed as potentially curative. The surgical benefit in T1N2–3M0 NSCLC patients remained a subject of considerable debate.
• We identified the survival benefits of different surgical approaches in T1N2–3M0 NSCLC patients, and provided a valuable tool for obtaining personalized survival estimates and more personalized treatment strategies.
What is the implication, and what should change now?
• Our predictive model enhances the understanding of the differential benefits associated with various surgical approaches and provides clinicians instructions for the personalized treatment of T1N2–3M0 NSCLC patients.
Introduction
Lung cancer continues to be the foremost contributor to cancer-related fatalities worldwide (1). Non-small cell lung cancer (NSCLC) is the predominant subtype, constituting roughly 80–85% of lung cancer cases (2). As health awareness among individuals improves and low-dose spiral computed tomography (CT) screening gains popularity among long-term smokers, the likelihood of detecting smaller lung cancers has significantly increased (3). However, despite the improved detection of small tumor sizes through CT screening, some patients present with lymph node metastasis at the time of diagnosis. For individuals with early-stage NSCLC (stages I–II), the standard approach involves surgery followed by adjuvant chemotherapy for those with large primary tumors or positive lymph nodes. The 5-year survival rate for this subgroup of patients ranges from approximately 50% to 75% (4).
Stage III NSCLC accounts for approximately 30% of patients diagnosed with NSCLC, which represents an intermediate phase between clearly resectable early-stage disease and metastatic involvement (5). The precise criteria for surgical treatment in this stage remain a subject of considerable debate, despite the completion of randomized controlled trials (RCTs) (6). Concurrent chemotherapy and radiotherapy are considered the standard of care for patients with satisfactory performance status and are viewed as potentially curative (7). Surgical resection can offer optimal local control and confer survival advantages beyond chemotherapy and radiation alone for suitable surgical candidates (8). A phase III RCT published by Albain et al. compared concurrent chemotherapy and radiotherapy followed by resection with standard concurrent chemotherapy and definitive radiotherapy without resection, revealing that progression-free survival was better in the surgery group than in the non-surgery group (9). Bott et al. also suggested that surgical resection as a part of multimodality therapy may be associated with improved OS in highly selected patients with stage IIIB NSCLC (10). Subsequently, a report published by Caglar et al. also suggested that stage III NSCLC patients who were candidates for resection appeared to achieve better outcomes following the induction of concurrent chemoradiation (11). But there is currently a paucity of conclusive studies regarding whether surgery benefits T1N2–3M0 patients and what the optimal surgical approach might be. In our study, we aimed to determine the survival advantages of surgery in T1N2–3M0 NSCLC patients, utilizing data from the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/), and to visually represent the benefits of surgical intervention. We present this article in accordance with the TRIPOD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-213/rc).
Methods
Patient selection
The SEER database is a national population-based reporting system that collects tumor-related data, including the incidence, treatment, mortality, and other demographics, covering around 28% of the US population and presenting information on the incidence of cancer in 18 regions across the US, which can help decrease incidence of tumors (12). The SEER database contains no identifiers and is publicly available for studies of cancer-related survival analysis. The patients diagnosed with NSCLC from 2000 to 2015 were identified from the SEER database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study population included patients with the following International Classification of Disease for Oncology Third Edition (ICD-O-3), morphology codes: 8010, 8012–8014, 8020–8022, 8050–8052, 8070–8078, 8140–8147, 8255, 8260, 8310, 8323, 8480, 8481, 8490, 8550, 8572. Patients diagnosed as stage T1N2–3M0 and histologically confirmed as having NSCLC were enrolled. The exclusion criteria were as follows: (I) patients without complete information concerning follow-up; (II) patients with a history of at least one previous malignancy; (III) not diagnosed by immunohistochemical pathology; and (IV) patients lacking information concerning primary lesion size (T), regional lymph node (N), or distant metastasis (M) stage and other clinically relevant information (Figure 1).

Variables
To make data analysis convenient, we transformed continuous variables into categorical variables. The extracted clinical information included sex, age, race, site, tumor laterality, grade, T stage, N stage, lymph node dissection (LND), histology, radiotherapy, chemotherapy, survival months, causes of death, and survival status. As for surgical approach, the resection of less than one lobe was defined as sublobectomy because some surgical procedures were unclear in the SEER database, or the number of cases was too small to analyze separately. The tumor-node-metastasis (TNM) stage was reclassified according to the American Joint Committee on Cancer (AJCC) 8th edition, based on tumor size, tumor collaborative stage (CS) extension, and the 6/7th edition N/M stages (13). Due to the absence of treatment sequence, we cannot determine radiotherapy and chemotherapy as neoadjuvant or adjuvant therapy. The time of the last follow-up was November 2020. Overall survival (OS) was defined as the interval between cancer diagnosis and death resulting from any cause or the last follow-up for patients still alive. Cancer-specific survival (CSS) was defined as the length of time between cancer diagnosis and death from NSCLC.
Development and validation of a nomogram
According to our exclusion criteria, the eligible patients after propensity score matching (PSM) were randomly divided into training and validation cohorts at a ratio of 7:3. The nomogram was developed using the training cohort of 801 patients and the validation cohort of 345 patients was used to validate the model. We performed a univariate Cox proportional hazard regression analysis to identify independent prognostic factors of OS and CSS. Significant factors in univariate analysis were included in the multivariate Cox proportional hazard regression analysis in order to acquire the hazard ratio (HR) and corresponding 95% confidential interval (CI) for each independent prognostic factor. The nomograms for predicting OS and CSS were developed using the risk factors calculated from the final multivariate Cox regression model.
The concordance index (C-index) was used to evaluate the performance for predicting the survival of this nomogram model, which indicates a measure of concordance. And it is similar to the area under the receiver operating characteristic (ROC) curve. The theoretical value of the C-index ranges from 0 to 1.0, and larger values of the C-index indicates better predictive performance (14). The calibration curves were plotted to assess the consistency between predicted survival probability and actual survival proportion in the training and validation cohort. A model that is perfectly calibrated would display a 45-degree curve. Discrimination and calibration were estimated by bootstrapping 1,000 times. Decision curve analysis (DCA) was also performed to assess the improved benefits and performance of the nomograms (15).
In the training cohort, we grouped patients into three risk subsets based on prognostic scores to evaluate the model’s discriminative ability. The cut-off values were determined using the X-tile software 3.6.1 (Copyright: Camp/Rimm; Yale University, New Haven, CT, USA). The cut-off values were also subsequently applied to the validation cohort. The difference in survival was assessed by calculating the respective log-rank P values. These data analyses were also performed using R Studio version 4.1.2 (RStudio, Boston, MA, USA). The R packages ‘survival’, ‘rms’, ‘riskRegression’, ‘survminer’, and ‘ggDCA’ were used for nomogram construction and evaluation. Furthermore, the R packages ‘DynNom’, ‘DNbuilder’, and ‘rsconnect’ were applied to develop a user-friendly web-based interface for our nomogram.
Statistical analysis
The patients were divided according to whether they received surgery versus non-surgery treatment of the primary tumor. The baseline characteristics of patients in the surgery group and the non-surgery group were described using frequencies and percentages. We performed PSM to balance potential bias and possible confounding interference between the two groups, with a caliper width of 0.008 (16). Patients in the two groups (surgery and non-surgery) were 1:1 matched using the nearest propensity score on the logit scale. Variables used for matching were sex, age, race, site, laterality, grade, T stage, N stage, LND, histology, radiotherapy, and chemotherapy. The difference of demographic data among the two groups were assessed for significance using the Student’s t-test or χ2 test and the Fisher’s exact test before and after PSM. The distinctions of OS and CSS were estimated by applying the Kaplan-Meier (K-M) method with the log-rank test. The Cox proportional hazards regression analyses with both univariate and multivariate Cox regression analyses were used to determine independent prognostic factors. HRs were calculated with 95% CIs. All data analyses were performed using R Studio version 4.1.2. A two-sided P value <0.05 was deemed significant.
Results
The characteristics of patients before and after PSM
A total of 589,283 patients with NSCLC were identified in the SEER database spanning from 2000 to 2015, of whom 4,671 met the criteria for T1N2–3M0 NSCLC (Figure 1). Among these T1N2–3M0 NSCLC patients, the majority were male, aged 65 years or younger, of white race, and had tumors located on the right side and in the upper lobe. These tumors were predominantly of the adenocarcinoma subtype and were poorly differentiated. Among the eligible patients, only 2,034 (43.55%) underwent surgical treatment. Notably, there were significant differences in various factors including age, sex, race, tumor site, laterality, grade, N stage, LND, histology, and radiotherapy between the two treatment groups before performing PSM. Surgical intervention was more common among patients aged 65 years or younger and those with lower N stage. A higher proportion of female patients received surgical treatment. Additionally, compared to the non-surgery group, the surgery group had a higher prevalence of white race, adenocarcinoma histology, left-sided tumors, lower lobe location, and moderately differentiated tumors. Notably, the majority (88.89%) of patients who underwent surgery also had LND. Within the surgery group, 949 (46.66%) patients received radiotherapy. There was no statistically significant difference between the two treatment groups regarding the use of chemotherapy. These data reveal that the baseline characteristics of the two groups (surgery and non-surgery) were initially imbalanced (Table 1).
Table 1
| Characteristics | Total, n (%) | Non-surgery, n (%) | Surgery, n (%) | P value |
|---|---|---|---|---|
| All | 4,671 (100.00) | 2,637 (56.45) | 2,034 (43.55) | |
| Sex | <0.001 | |||
| Female | 2,295 (49.13) | 1,214 (46.04) | 1,081 (53.15) | |
| Male | 2,376 (50.87) | 1,423 (53.96) | 953 (46.85) | |
| Age (years) | <0.001 | |||
| ≤65 | 2,056 (44.02) | 1,019 (38.64) | 1,037 (50.98) | |
| >65 | 2,615 (55.98) | 1,618 (61.36) | 997 (49.02) | |
| Race | 0.02 | |||
| White | 3,793 (81.20) | 2,134 (80.93) | 1,659 (81.56) | |
| Black | 537 (11.50) | 328 (12.44) | 209 (10.28) | |
| Other | 341 (7.30) | 175 (6.64) | 166 (8.16) | |
| Site | <0.001 | |||
| Upper lobe | 2,998 (64.18) | 1,704 (64.62) | 1,294 (63.62) | |
| Middle lobe | 249 (5.33) | 141 (5.35) | 108 (5.31) | |
| Lower lobe | 1,274 (27.27) | 687 (26.05) | 587 (28.86) | |
| Main bronchus | 102 (2.18) | 88 (3.34) | 14 (0.69) | |
| Overlapping lesion | 48 (1.03) | 17 (0.64) | 31 (1.52) | |
| Laterality | <0.001 | |||
| Right | 2,790 (59.73) | 1,634 (61.96) | 1,156 (56.83) | |
| Left | 1,881 (40.27) | 1,003 (38.04) | 878 (43.17) | |
| Grade | <0.001 | |||
| Well | 305 (6.53) | 175 (6.64) | 130 (6.39) | |
| Moderate | 1,656 (35.45) | 786 (29.81) | 870 (42.77) | |
| Poor | 2,538 (54.34) | 1,563 (59.27) | 975 (47.94) | |
| Undifferentiated | 172 (3.68) | 113 (4.29) | 59 (2.90) | |
| T stage | 0.68 | |||
| T1a | 88 (1.88) | 46 (1.74) | 42 (2.06) | |
| T1b | 1,898 (40.63) | 1,079 (40.92) | 819 (40.27) | |
| T1c | 2,685 (57.48) | 1,512 (57.34) | 1,173 (57.67) | |
| N stage | <0.001 | |||
| N2 | 3,979 (85.19) | 2,010 (76.22) | 1,969 (96.80) | |
| N3 | 692 (14.81) | 627 (23.78) | 65 (3.20) | |
| LND | <0.001 | |||
| Yes | 2,268 (48.55) | 460 (17.44) | 1,808 (88.89) | |
| No | 2,403 (51.45) | 2,177 (82.56) | 226 (11.11) | |
| Histology | <0.001 | |||
| ADC | 2,676 (57.29) | 1,254 (47.55) | 1,422 (69.91) | |
| SCC | 1,211 (25.93) | 869 (32.95) | 342 (16.81) | |
| Other | 784 (16.78) | 514 (19.49) | 270 (13.27) | |
| Radiotherapy | <0.001 | |||
| Yes | 2,633 (56.37) | 1,684 (63.86) | 949 (46.66) | |
| No/unknown | 2,038 (43.63) | 953 (36.14) | 1,085 (53.34) | |
| Chemotherapy | 0.68 | |||
| Yes | 3,040 (65.08) | 1,709 (64.81) | 1,331 (65.44) | |
| No/unknown | 1,631 (34.92) | 928 (35.19) | 703 (34.56) |
NSCLC, non-small cell lung cancer; PSM, propensity score matching; LND, lymph node dissection; ADC, adenosquamous carcinoma; SCC, squamous cell carcinoma.
After implementing 1:1 PSM, a total of 1,146 T1N2–3M0 NSCLC patients, treated with or without surgery, were included in the analysis. Following PSM, baseline characteristics, including sex, age, race, tumor site, laterality, grade, T stage, N stage, LND, histology, radiotherapy, and chemotherapy, were all well-balanced (P>0.05). Furthermore, we observed that the majority of T1N2–3M0 NSCLC patients were male, aged >65 years, of white race, with right-sided upper lobe tumors, and predominantly had adenocarcinoma histology and poorly differentiated tumors. Most of the tumors measured 2–3 cm. More than half of the patients had N2-positive status and received both chemotherapy and radiotherapy. The detailed information is presented in Table 2.
Table 2
| Characteristics | Total, n (%) | Non-surgery, n (%) | Surgery, n (%) | P value |
|---|---|---|---|---|
| All | 1,146 (100.00) | 573 (50.00) | 573 (50.00) | |
| Sex | 0.29 | |||
| Female | 553 (48.25) | 286 (49.91) | 267 (46.60) | |
| Male | 593 (51.75) | 287 (50.09) | 306 (53.40) | |
| Age (years) | 0.63 | |||
| ≤65 | 503 (43.89) | 256 (44.68) | 247 (43.11) | |
| >65 | 643 (56.11) | 317 (55.32) | 326 (56.89) | |
| Race | 0.15 | |||
| White | 934 (81.50) | 479 (83.60) | 455 (79.41) | |
| Black | 137 (11.95) | 63 (10.99) | 74 (12.91) | |
| Other | 75 (6.54) | 31 (5.41) | 44 (7.68) | |
| Site | 0.22 | |||
| Upper lobe | 725 (63.26) | 376 (65.62) | 349 (60.91) | |
| Middle lobe | 69 (6.02) | 27 (4.71) | 42 (7.33) | |
| Lower lobe | 316 (27.57) | 150 (26.18) | 166 (28.97) | |
| Main bronchus | 24 (2.09) | 14 (2.44) | 10 (1.75) | |
| Overlapping lesion | 12 (1.05) | 6 (1.05) | 6 (1.05) | |
| Laterality | 0.51 | |||
| Right | 698 (60.91) | 355 (61.95) | 343 (59.86) | |
| Left | 448 (39.09) | 218 (38.05) | 230 (40.14) | |
| Grade | 0.17 | |||
| Well | 95 (8.29) | 41 (7.16) | 54 (9.42) | |
| Moderate | 372 (32.46) | 201 (35.08) | 171 (29.84) | |
| Poor | 626 (54.62) | 303 (52.88) | 323 (56.37) | |
| Undifferentiated | 53 (4.62) | 28 (4.89) | 25 (4.36) | |
| T stage | 0.14 | |||
| T1a | 24 (2.09) | 8 (1.40) | 16 (2.79) | |
| T1b | 459 (40.05) | 222 (38.74) | 237 (41.36) | |
| T1c | 663 (57.85) | 343 (59.86) | 320 (55.85) | |
| N stage | 0.70 | |||
| N2 | 1027 (89.62) | 511 (89.18) | 516 (90.05) | |
| N3 | 119 (10.38) | 62 (10.82) | 57 (9.95) | |
| LND | 0.95 | |||
| Yes | 696 (60.73) | 349 (60.91) | 347 (60.56) | |
| No | 450 (39.27) | 224 (39.09) | 226 (39.44) | |
| Histology | 0.84 | |||
| ADC | 668 (58.29) | 339 (59.16) | 329 (57.42) | |
| SCC | 288 (25.13) | 141 (24.61) | 147 (25.65) | |
| Other | 190 (16.58) | 93 (16.23) | 97 (16.93) | |
| Radiotherapy | 0.15 | |||
| Yes | 649 (56.63) | 337 (58.81) | 312 (54.45) | |
| No/unknown | 497 (43.37) | 236 (41.19) | 261 (45.55) | |
| Chemotherapy | 0.19 | |||
| Yes | 774 (67.54) | 398 (69.46) | 376 (65.62) | |
| No/unknown | 372 (32.46) | 175 (30.54) | 197 (34.38) |
NSCLC, non-small cell lung cancer; PSM, propensity score matching; LND, lymph nodes dissection; ADC, adenosquamous carcinoma; SCC, squamous cell carcinoma.
Surgical treatment as an independent prognostic factor for survival in T1N2–3M0 NSCLC patients
In the univariate analysis, several factors including sex, age, race, grade, histology, surgery, radiotherapy, and chemotherapy exhibited significant associations with OS, as presented in Table 3. These same factors, with the exception of histology and radiotherapy, were also found to be significantly associated with CSS (Table 3). Following multivariate analysis, variables such as sex, age, race, grade, surgery, radiotherapy, and chemotherapy were confirmed to be independently associated with OS (Table 4). Specifically, individuals aged over 65 years, male, of white race, with poorly differentiated or undifferentiated tumors, and who did not undergo surgery or receive chemotherapy were identified as having a higher hazard of death due to lung cancer, as indicated by the results of multivariate analysis (Table 4). The site and laterality of the tumor, extent of lymph node removal, histological subtype, T stage, N stage, and radiotherapy were not found to have a significant impact on CSS. Regarding surgical procedures, it was observed that lobectomy was associated with the lowest risk of death, whereas other surgical approaches also demonstrated improvements in both OS and CSS among T1N2–3M0 NSCLC patients.
Table 3
| Variables | Univariate (OS) | Univariate (CSS) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex | |||||
| Male | 1 | 1 | |||
| Female | 0.74 (0.65–0.83) | <0.001 | 0.74 (0.65–0.85) | <0.001 | |
| Age (years) | |||||
| ≤65 | 1 | 1 | |||
| >65 | 1.34 (1.18–1.52) | <0.001 | 1.25 (1.09–1.44) | 0.001 | |
| Race | |||||
| White | 1 | 1 | |||
| Blank | 0.81 (0.66–0.98) | 0.03 | 0.84 (0.68–1.04) | 0.11 | |
| Other | 0.62 (0.48–0.81) | 0.001 | 0.63 (0.47–0.84) | 0.002 | |
| Site | |||||
| Lower lobe | 1 | 1 | |||
| Upper lobe | 0.93 (0.80–1.07) | 0.30 | 0.92 (0.79–1.08) | 0.32 | |
| Middle lobe | 0.95 (0.72–1.25) | 0.70 | 1.03 (0.77–1.38) | 0.85 | |
| Main bronchus | 1.22 (0.79–1.88) | 0.37 | 1.27 (0.79–2.03) | 0.32 | |
| Overlapping lesion | 1.50 (0.82–2.73) | 0.19 | 1.44 (0.74–2.80) | 0.28 | |
| Laterality | |||||
| Left | 1 | 1 | |||
| Right | 0.97 (0.86–1.10) | 0.64 | 0.97 (0.84–1.11) | 0.64 | |
| Grade | |||||
| Well | 1 | 1 | |||
| Moderate | 1.79 (1.38–2.33) | <0.001 | 1.64 (1.24–2.18) | <0.001 | |
| Poor | 1.89 (1.47–2.43) | <0.001 | 1.72 (1.31–2.25) | <0.001 | |
| Undifferentiated | 1.96 (1.35–2.84) | <0.001 | 1.88 (1.26–2.81) | 0.002 | |
| T stage | |||||
| T1a | 1 | 1 | |||
| T1b | 1.19 (0.75–1.89) | 0.45 | 1.32 (0.77–2.25) | 0.31 | |
| T1c | 1.38 (0.87–2.17) | 0.17 | 1.55 (0.91–2.63) | 0.11 | |
| N stage | |||||
| N2 | 1 | 1 | |||
| N3 | 1.10 (0.90–1.35) | 0.34 | 1.17 (0.94–1.45) | 0.15 | |
| Surgery | |||||
| Non-surgery | 1 | 1 | |||
| Sublobectomy | 0.71 (0.59–0.85) | <0.001 | 0.65 (0.52–0.80) | <0.001 | |
| Lobectomy | 0.53 (0.46–0.60) | <0.001 | 0.48 (0.41–0.56) | <0.001 | |
| LND | |||||
| No | 1 | 1 | |||
| Yes | 0.90 (0.79–1.02) | 0.09 | 0.94 (0.82–1.08) | 0.39 | |
| Histology | |||||
| ADC | 1 | 1 | |||
| SCC | 1.25 (1.08–1.45) | 0.003 | 1.15 (0.98–1.36) | 0.09 | |
| Other | 1.00 (0.84–1.20) | 0.96 | 1.03 (0.85–1.24) | 0.77 | |
| Radiotherapy | |||||
| Yes | 1 | 1 | |||
| No | 1.18 (1.04–1.34) | 0.008 | 1.14 (1.00–1.31) | 0.056 | |
| Chemotherapy | |||||
| Yes | 1 | 1 | |||
| No | 1.31 (1.15–1.50) | <0.001 | 1.20 (1.04–1.39) | 0.01 | |
OS, overall survival; CSS, cancer-specific survival; NSCLC, non-small cell lung cancer; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; LND, lymph node dissection; ADC, adenosquamous carcinoma; SCC, squamous cell carcinoma.
Table 4
| Variables | Multivariate (OS) | Multivariate (CSS) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex | |||||
| Male | 1 | 1 | |||
| Female | 0.74 (0.65–0.84) | <0.001 | 0.74 (0.64–0.85) | <0.001 | |
| Age (years) | |||||
| ≤65 | 1 | 1 | |||
| >65 | 1.31 (1.15–1.49) | <0.001 | 1.25 (1.09–1.44) | <0.001 | |
| Race | |||||
| White | 1 | 1 | |||
| Blank | 0.88 (0.72–1.07) | 0.20 | 0.91 (0.73–1.13) | 0.40 | |
| Other | 0.64 (0.49–0.84) | 0.001 | 0.65 (0.48– 0.87) | <0.001 | |
| Grade | |||||
| Well | 1 | 1 | |||
| Moderate | 1.77 (1.35–2.30) | <0.001 | 1.58 (1.19–2.09) | <0.001 | |
| Poor | 2.01 (1.55–2.60) | <0.001 | 1.73 (1.31–2.26) | <0.001 | |
| Undifferentiated | 2.29 (1.53–3.41) | <0.001 | 1.84 (1.23–2.76) | <0.001 | |
| Surgery | |||||
| Non-surgery | 1 | 1 | |||
| Sublobectomy | 0.65 (0.54–0.78) | <0.001 | 0.61 (0.49–0.75) | <0.001 | |
| Lobectomy | 0.50 (0.44–0.58) | <0.001 | 0.47 (0.41–0.55) | <0.001 | |
| Histology | |||||
| ADC | 1 | ||||
| SCC | 1.05 (0.90–1.22) | 0.52 | |||
| Other | 0.84 (0.69–1.02) | 0.08 | |||
| Radiotherapy | |||||
| Yes | 1 | ||||
| No | 1.20 (1.05–1.37) | 0.008 | |||
| Chemotherapy | |||||
| Yes | 1 | 1 | |||
| No | 1.32 (1.15–1.52) | <0.001 | 1.28 (1.10–1.48) | <0.001 | |
OS, overall survival; CSS, cancer-specific survival; NSCLC, non-small cell lung cancer; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; LND, lymph node dissection; ADC, adenosquamous carcinoma; SCC, squamous cell carcinoma.
Impact of tumor resection on survival outcomes in T1N2–3M0 NSCLC patients
In the KM analyses and log-rank tests conducted on a matched patient population, as illustrated in Figure S1 and Figure 2, patients who underwent either lobectomy or sublobectomy exhibited significantly prolonged OS and CSS compared to those who did not receive surgery, both before and after PSM. After PSM, the median CSS time for patients who underwent lobectomy was 42 months (95% CI: 32.32–51.68), whereas for patients who did not receive surgery, it was only 18 months (95% CI: 16.14–19.86) (P<0.001). The median OS time for patients who underwent lobectomy was 34 months (95% CI: 28.91–39.09), whereas for those who did not undergo surgery, it was only 16 months (95% CI: 14.28–17.72) (P<0.001). In summary, the median OS times for patients with no surgery, sublobectomy, or lobectomy were 16, 24, and 34 months, respectively, and the median CSS times were 18, 29, and 42 months, respectively.
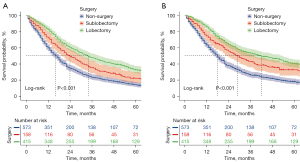
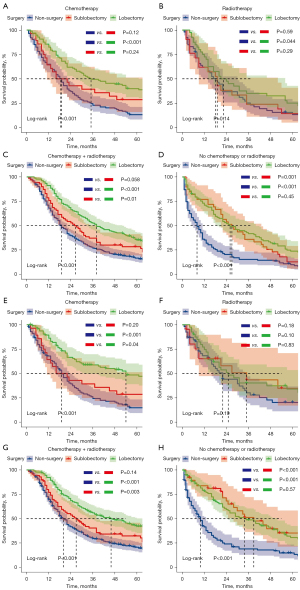
To further determine the protective effect of surgical procedures on OS and CSS, we conducted subgroup analysis among different age groups, N stages, and treatment categories after PSM. Across various age groups, the surgery group consistently demonstrated better prognoses than the non-surgery group for both OS and CSS (Figure 3A-3D), with the exception of patients aged >65 years. However, it is noteworthy that within all age groups, the trend favored lobectomy over sublobectomy in terms of survival benefits. When considering different N-stage categories, the surgery group exhibited improved prognoses compared to the non-surgery group for both OS and CSS in all cases. In the N2 subgroup analysis, we found that the lobectomy group presented improved OS compared with the sublobectomy group (Figure 4A), and the lobectomy group also exhibited better OS compared with the sublobectomy group in the N3 subgroup (Figure 4B). The CSS subgroup analysis showed that the sublobectomy group presented a similar survival compared with the lobectomy group in N2-positive patients (Figure 4C), whereas the lobectomy group exhibited improved prognosis compared with the sublobectomy group in N3-positive patients (Figure 4D). In terms of treatment regimens, the surgery group presented better OS and CSS than the non-surgery group except for the patients who received radiotherapy alone. The difference in OS and CSS outcomes was not significant between the sublobectomy and non-surgery groups in patients who received chemotherapy alone or chemotherapy plus radiotherapy, although the trend favored sublobectomy for both OS and CSS. In the KM analyses of OS, lobectomy did not provide improved survival compared with sublobectomy in patients with chemotherapy (Figure 5A). For patients in the radiotherapy group, the surgery group could not achieve better survival than the non-surgery group (Figure 5B). However, the lobectomy provided improved survival compared to sublobectomy in patients with chemotherapy plus radiotherapy (Figure 5C). For the patients without radiotherapy and chemotherapy, the lobectomy exhibited similar survival compared with the sublobectomy (Figure 5D). In the KM analyses of CSS, lobectomy achieved better CSS than sublobectomy in patients who underwent single chemotherapy (Figure 5E), whereas surgery did not improve the prognosis compared with the non-surgery group in patients with single radiotherapy (Figure 5F). The outcomes of sublobectomy and non-surgery were comparable whereas lobectomy showed superior survival outcome compared with sublobectomy for patients underwent chemotherapy plus radiotherapy (Figure 5G). For the patients without radiotherapy and chemotherapy, the lobectomy and sublobectomy group achieved better CSS than non-surgery group and the similar results of lobectomy and sublobectomy are displayed in Figure 5H. Overall, these findings provide valuable insights into the benefits of surgery for T1N2–3M0 NSCLC patients, taking into account various clinical factors and treatment modalities.


Nomogram to visualize the benefits of different surgical approaches in T1N2-3M0 NSCLC patients
A total of 1,146 T1N2–3M0 NSCLC patients were randomly assigned to the training set (n=801) and the validation set (n=345) in a 7:3 ratio. In comparing the training and validation cohorts, the demographic variables were insignificant (Table 5) (all P>0.05). Within the training cohort, there were 707 recorded events, specifically patient deaths, and out of these, 583 patients succumbed to cancer. The mean follow-up duration for these patients was 38.18 months, with a range spanning from 1 to 217 months. In univariate analysis, sex, age, race, grade, surgery, histology, and chemotherapy were significantly associated with OS. These factors were also significantly associated with CSS except for the factor of histology and chemotherapy (Table 6). Given the recognized impact of chemotherapy on patient prognosis in previous literature, we included this factor in the subsequent multivariate analysis. After the multivariate analysis, the most significant variables for the development of the nomogram model for OS were identified as sex, age, grade, surgery, and chemotherapy, as depicted in Figure 6A and Table 7. For the nomogram model for CSS, the significant variables included sex, grade, surgery, and chemotherapy, as shown in Figure 6B and Table 7.
Table 5
| Characteristics | Training cohort, n (%) | Validation cohort, n (%) | P value |
|---|---|---|---|
| Number of cases | 801 | 345 | |
| Sex | 0.52 | ||
| Female | 381 (47.57) | 172 (49.86) | |
| Male | 420 (52.43) | 173 (50.14) | |
| Age (years) | 0.36 | ||
| ≤65 | 344 (42.95) | 159 (46.09) | |
| >65 | 457 (57.05) | 186 (53.91) | |
| Race | 0.91 | ||
| White | 652 (81.40) | 282 (81.74) | |
| Black | 95 (11.86) | 42 (12.17) | |
| Other | 54 (6.74) | 21 (6.09) | |
| Site | 0.45 | ||
| Upper lobe | 516 (64.42) | 209 (60.58) | |
| Middle lobe | 49 (6.12) | 20 (5.80) | |
| Lower lobe | 214 (26.72) | 102 (29.57) | |
| Main bronchus | 16 (2.00) | 8 (2.32) | |
| Overlapping lesion | 6 (0.75) | 6 (1.74) | |
| Laterality | 0.08 | ||
| Right | 474 (59.18) | 224 (64.93) | |
| Left | 327 (40.82) | 121 (35.07) | |
| Grade | 0.39 | ||
| Well | 59 (7.37) | 36 (10.43) | |
| Moderate | 262 (32.71) | 110 (31.88) | |
| Poor | 442 (55.18) | 184 (53.33) | |
| Undifferentiated | 38 (4.74) | 15 (4.35) | |
| T stage | 0.65 | ||
| T1a | 18 (2.25) | 6 (1.74) | |
| T1b | 326 (40.70) | 133 (38.55) | |
| T1c | 457 (57.05) | 206 (59.71) | |
| N stage | 0.95 | ||
| N2 | 717 (89.51) | 310 (89.86) | |
| N3 | 84 (10.49) | 35 (10.14) | |
| LND | 0.51 | ||
| Yes | 481 (60.05) | 215 (62.32) | |
| No | 320 (39.95) | 130 (37.68) | |
| Histology | 0.33 | ||
| ADC | 456 (56.93) | 212 (61.45) | |
| SCC | 210 (26.22) | 78 (22.61) | |
| Other | 135 (16.85) | 55 (15.94) | |
| Radiotherapy | 0.69 | ||
| Yes | 450 (56.18) | 199 (57.68) | |
| No/unknown | 351 (43.82) | 146 (42.32) | |
| Chemotherapy | 0.24 | ||
| Yes | 532 (66.42) | 242 (70.14) | |
| No/unknown | 269 (33.58) | 103 (29.86) |
LND, lymph node dissection; ADC, adenosquamous carcinoma; SCC, squamous cell carcinoma.
Table 6
| Variables | Univariate (OS) | Univariate (CSS) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex | |||||
| Male | 1 | 1 | |||
| Female | 0.69 (0.60–0.80) | <0.001 | 0.70 (0.59–0.82) | <0.001 | |
| Age (years) | |||||
| ≤65 | 1 | 1 | |||
| >65 | 1.23 (1.06–1.43) | 0.007 | 1.36 (1.19–1.56) | <0.001 | |
| Race | |||||
| White | 1 | 1 | |||
| Blank | 0.89 (0.7–1.11) | 0.30 | 0.89 (0.69–1.15) | 0.38 | |
| Other | 0.62 (0.46–0.85) | 0.003 | 0.64 (0.45–0.9) | 0.01 | |
| Site | |||||
| Lower lobe | 1 | 1 | |||
| Upper lobe | 0.93 (0.78–1.1) | 0.73 | 0.95 (0.78–1.14) | 0.57 | |
| Middle lobe | 0.96 (0.69–1.34) | 0.72 | 1.03 (0.72–1.47) | 0.87 | |
| Main bronchus | 1.46 (0.86–2.47) | 0.99 | 1.63 (0.95–2.83) | 0.08 | |
| Overlapping lesion | 2.43 (1.08–5.49) | 0.46 | 2.40 (0.99–5.86) | 0.054 | |
| Laterality | |||||
| Left | 1 | 1 | |||
| Right | 1.02 (0.87–1.18) | 0.84 | 1.04 (0.88–1.22) | 0.67 | |
| Grade | |||||
| Well | 1 | 1 | |||
| Moderate | 1.85 (1.33–2.57) | <0.001 | 1.65 (1.16–2.35) | 0.005 | |
| Poor | 2.1 (1.53–2.89) | <0.001 | 1.83 (1.30–2.58) | 0.001 | |
| Undifferentiated | 2.72 (1.74–4.27) | <0.001 | 2.53 (1.56–4.1) | <0.001 | |
| T stage | |||||
| T1a | 1 | 1 | |||
| T1b | 1.10 (0.65–1.84) | 0.73 | 1.21 (0.66–2.22) | 0.53 | |
| T1c | 1.28 (0.77–2.15) | 0.35 | 1.46 (0.8–2.65) | 0.22 | |
| N stage | |||||
| N2 | 1 | 1 | |||
| N3 | 0.96 (0.75–1.23) | 0.75 | 1.02 (0.78–1.33) | 0.89 | |
| Surgery | |||||
| Non-surgery | 1 | 1 | |||
| Sublobectomy | 0.68 (0.54–0.85) | 0.001 | 0.61 (0.48–0.79) | <0.001 | |
| Lobectomy | 0.57 (0.48–0.67) | <0.001 | 0.51 (0.42–0.61) | <0.001 | |
| LND | |||||
| No | 1 | 1 | |||
| Yes | 0.90 (0.77–1.04) | 0.15 | 0.98 (0.83–1.16) | 0.80 | |
| Histology | |||||
| ADC | 1 | 1 | |||
| SCC | 1.32 (1.11–1.57) | 0.002 | 1.18 (0.97–1.43) | 0.10 | |
| Other | 1.10 (0.89–1.35) | 0.38 | 1.14 (0.92–1.43) | 0.24 | |
| Radiotherapy | |||||
| Yes | 1 | 1 | |||
| No | 1.16 (1–1.34) | 0.056 | 1.11 (0.94–1.31) | 0.21 | |
| Chemotherapy | |||||
| Yes | 1 | 1 | |||
| No | 1.25 (1.07–1.45) | 0.006 | 1.16 (0.98–1.38) | 0.09 | |
OS, overall survival; CSS, cancer-specific survival; NSCLC, non-small cell lung cancer; HR, hazard ratio; CI, confidence interval; LND, lymph node dissection; ADC, adenosquamous carcinoma; SCC, squamous cell carcinoma.
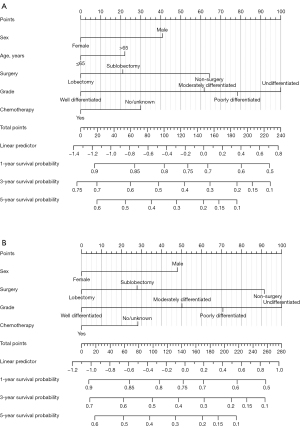
Table 7
| Variables | Multivariate (OS) | Multivariate (CSS) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex | |||||
| Male | 1 | 1 | |||
| Female | 0.70 (0.60–0.81) | <0.001 | 0.68 (0.58–1.80) | <0.001 | |
| Age (years) | |||||
| ≤65 | 1 | 1 | |||
| >65 | 1.22 (1.05–1.43) | 0.01 | 1.14 (0.97–1.35) | 0.12 | |
| Race | |||||
| White | 1 | 1 | |||
| Blank | 1.00 (0.79–1.26) | 0.99 | 0.98 (0.76–1.27) | 0.88 | |
| Other | 0.62 (0.45–0.86) | 0.003 | 0.62 (0.44–0.87) | 0.007 | |
| Grade | |||||
| Well | 1 | 1 | |||
| Moderate | 1.70 (1.21–2.38) | 0.002 | 1.49 (1.04–2.12) | 0.03 | |
| Poor | 2.02 (1.46–2.81) | <0.001 | 1.72 (1.22–2.43) | 0.002 | |
| Undifferentiated | 2.71 (1.67–4.40) | <0.001 | 2.20 (1.35–3.59) | 0.002 | |
| Surgery | |||||
| Non-surgery | 1 | 1 | |||
| Sublobectomy | 0.66 (0.52–0.83) | <0.001 | 0.60 (0.47–0.78) | <0.001 | |
| Lobectomy | 0.56 (0.47–0.66) | <0.001 | 0.50 (0.42–0.60) | <0.001 | |
| Histology | |||||
| ADC | 1 | ||||
| SCC | 1.09 (0.91–1.30) | 0.37 | |||
| Other | 0.89 (0.71–1.12) | 0.33 | |||
| Radiotherapy | |||||
| Yes | |||||
| No | |||||
| Chemotherapy | |||||
| Yes | 1 | 1 | |||
| No | 1.30 (1.10–1.52) | 0.002 | 1.21 (1.01–1.44) | 0.04 | |
OS, overall survival; CSS, cancer-specific survival; NSCLC, non-small cell lung cancer; HR, hazard ratio; CI, confidence interval; LND, lymph node dissection; ADC, adenosquamous carcinoma; SCC, squamous cell carcinoma.
Each of these variables was assigned a point score ranging from 0 to 100. In both the OS and CSS nomograms, the grade of tumor differentiation had the most substantial contribution to the prognosis, being assigned a maximum score of 100, followed by the type of surgical procedure performed. Notably, the impact of the surgical procedure was more pronounced in the nomogram model for CSS compared to OS, with a score of 91. It is worth highlighting that lobectomy had a substantial influence on the prediction of survival for both CSS and OS, followed by sublobectomy. Each factor can obtain a corresponding point by drawing a line straight upward to the “point axis”. The individual risk scores were calculated by summing up the score of each variable. The probabilities of survival at 1-, 3-, and 5-year were easily determined by locating their corresponding point on the survival scale.
Model performance and validation of the nomogram
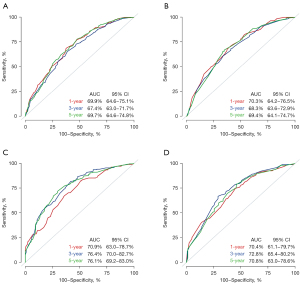
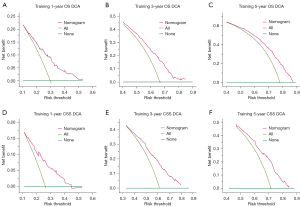
In the training cohort, the C-indexes for the established nomogram were 0.699 (95% CI: 0.646–0.751), 0.674 (95% CI: 0.630–0.717), and 0.697 (95% CI: 0.646–0.748) for predicting 1-, 3-, and 5-year OS, respectively, as depicted in Figure 7A. In the validation cohort, these C-indexes were 0.703 (95% CI: 0.642–0.765), 0.683 (95% CI: 0.636–0.729), and 0.694 (95% CI: 0.641–0.747), respectively, as shown in Figure 7B. For predicting 1-, 3-, and 5-year CSS, the C-indexes in the training cohort were 0.709 (95% CI: 0.630–0.787), 0.764 (95% CI: 0.700–0.827), and 0.761 (95% CI: 0.692–0.830), respectively (Figure 7C). In the validation cohort, these C-indexes were 0.704 (95% CI: 0.611–0.797), 0.728 (95% CI: 0.654–0.802), and 0.708 (95% CI: 0.630–0.786), as displayed in Figure 7D. The calibration plots at 1-, 3-, and 5-year OS showed excellent consistency in the training cohort (Figure 8A) and acceptable consistency in the validation cohort (Figure 8B) between the predicted survival probability and actual observation. Similar results could be seen in the calibration plots at 1-, 3-, and 5-year CSS in the training cohort (Figure 8C) and the validation cohort (Figure 8D). Additionally, DCA revealed that our nomogram model offered practical and wide ranges of threshold probabilities regardless of OS (Figure 9A-9C) and CSS (Figure 9D-9F). This further confirmed the clinical applicability and performance of our nomogram in predicting patient prognosis.

Risk-stratifying ability of the nomogram
Based on the total predictive risk scores, we subcategorized the training cohort into three risk groups, including low-, middle-, and high-risk groups, with the optimal cut-off values developed from X-tile software. Detailed subgroups of CSS were 0–91.5, 98.0–189.5, and 190.5–267.5, and OS were 0–124.0, 125.0–197.0, and 205.0–235.0 (Figure S2). The same stratification method was subsequently applied to the validation cohort. The survival curves for OS showed significant differences between any two adjacent groups in the training cohort and the validation cohort (P<0.0001; Figure 10A,10B). Significant distinctions in the survival curves for CSS were also observed between different risk groups in the training cohort and the validation cohort (P<0.0001; Figure 10C,10D).

Webserver development for the nomogram
For the sake of user convenience, we have developed a user-friendly website that facilitates the easy calculation of individualized survival probabilities for T1N2–3M0 NSCLC patients. To obtain personalized survival estimates, users simply need to input specific clinical variables pertaining to the patient in question, along with a desired prediction time frame in months. Additionally, the website generates corresponding survival plots for the provided case. The public online version of our nomogram is accessible via the following links: https://shanghaisuzhousclcnomogrampredictability.shinyapps.io/Nomogram/ and https://shanghaisuzhousclcnomogrampredictability.shinyapps.io/Nomogramcss/. We have made these websites freely available for clinicians and users, eliminating the need for any password input. It is important to note that this tool may offer clinicians instructions for survival counseling and treatment strategy making conveniently, but we should apply it to clinical practice cautiously before its predictive capacity has been validated in prospective, large-sample RCTs.
Discussion
Stage III NSCLC patients constitute a highly diverse group with varying survival outcomes, primarily due to the significant heterogeneity observed in factors such as tumor size, the extent of lymph node involvement, and the level of lymph node engagement. The choice between surgical intervention with adjuvant chemoradiotherapy and concurrent chemoradiotherapy alone has typically been made on a case-by-case basis. Consequently, determining the optimal treatment approach for T1N2–3M0 NSCLC patients has been a challenging task. Moreover, the debate regarding the potential benefits of lobectomy for stage III NSCLC patients has persisted without strong supporting evidence. Furthermore, there has been a lack of attention given to sublobectomy as a treatment option for this specific patient population. Some previous RCTs have indicated that surgery yields equivalent survival outcomes when compared to non-surgical approaches for stage III NSCLC patients who have undergone chemotherapy or chemoradiotherapy (9,17). However, a retrospective study conducted by Caglar et al., have suggested that stage III NSCLC patients who are eligible for resection may experience improved outcomes following induction concurrent chemoradiotherapy (11). Other retrospective studies have also reported a positive association between surgery and enhanced survival (18-20). To comprehensively investigate the role of surgery in T1N2–3M0 NSCLC patients, we conducted this study using a large cohort of patients, utilizing data from the SEER database. Additionally, we developed a predictive nomogram model to visualize the potential survival benefits of surgery. This model can aid in providing survival counseling for patients and clinicians, informing the design of clinical trials, guiding postoperative strategies, and contributing to the advancement of precision medicine for T1N2–3M0 NSCLC patients.
There is existing literature supporting the use of radical anatomical segmentectomy, particularly in elderly patients with limited cardiopulmonary function (21,22). In specific patient subsets, segmentectomy has been shown to be oncologically equivalent to lobectomy while offering the advantage of better preservation of pulmonary function (23). Our study revealed that both sublobectomy and lobectomy were associated with improved rates of OS and CSS. Subgroup analyses based on factors such as age, N stage, and the administration of radiotherapy or chemotherapy consistently supported these findings. Notably, a multicenter retrospective study led by Behera et al., based on the National Cancer Database, found that chemoradiotherapy followed by lobectomy or pneumonectomy was linked to superior survival outcomes compared to chemoradiotherapy alone, which aligns with the results of our subgroup analysis (4). However, there is currently no definitive study elucidating the survival advantages of different surgical approaches in T1N2–3M0 NSCLC patients. Our study was the first to demonstrate that sublobectomy confers a survival benefit when compared to the non-surgery group. Therefore, sublobectomy may still be a viable consideration for stage T1N2–3M0 NSCLC patients, particularly those of advanced age or with compromised cardiopulmonary function, even though lobectomy offers a more favorable survival prognosis. Nonetheless, further validation through multicenter RCTs is necessary to confirm the benefits of both lobectomy and sublobectomy for these patients.
The guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO) recommend definitive concurrent chemoradiation and immunotherapy as the initial therapy for N3-stage NSCLC (24,25). A recent study also showed that immunotherapy-based treatments in the neoadjuvant period could downstage initially unresectable NSCLC, converting into resectable disease and improve the prognosis in patients who received surgery (26). For patients confirmed to have N2 disease, upfront surgical resection has traditionally been considered infeasible because the tumor is unresectable and is not recommended (4). However, there is limited literature available on the outcomes of surgery for these patients. In cases where patients exhibit microscopic or minimal nodal involvement, induction therapy followed by surgical resection is recommended (4). Notably, the study by Caglar et al. indicated that the rate of local recurrence for IIIA–IIIB patients who received chemoradiotherapy or chemoradiotherapy followed by surgery was 50% and 7%, respectively (11). Similarly, Raman et al. found that surgery is associated with comparable or slightly worse short-term survival but improved long-term survival compared to chemoradiation in selected patients with N3 NSCLC (27). A retrospective study conducted by Fu et al. reported that patients with stage IIIA–N2 NSCLC treated with upfront surgery followed by adjuvant therapy showed promising long-term outcomes (28). Despite these recommendations and findings, the rate of surgery for T1N2–3M0 NSCLC patients remains low. In our study, only 29.8% of T1N2–3M0 NSCLC patients underwent surgical treatment, indicating that a minority of these specific patients could potentially benefit from surgery. The management of T1N2–3M0 NSCLC patients requires a multidisciplinary approach involving medical oncologists, thoracic surgeons, and radiation oncologists to assess the potential benefits of surgery and the resectability of the tumor (29). Given the heterogeneity within the T1N2–3M0 NSCLC stage, characterized by variations in the extent and location of nodal involvement, the decision to pursue surgery should be made carefully and on a case-by-case basis.
In our study, another intriguing finding was that sublobectomy did not significantly improve OS or CSS compared to the non-surgery group in patients with N3-positive disease. A prospective study of stage III NSCLC by Grunenwald et al. indicated that patients with N2 and N3 disease exhibited similar rates of mediastinal node sterilization, which suggests that patients with N3 disease may have tumor behavior more aligned with N2 rather than T4 disease and could potentially benefit from multimodal therapy, including surgery (30). Additionally, mounting evidence suggests that downstaging of mediastinal nodes is associated with improved prognosis (31,32). Therefore, lobectomy may be the preferred surgical approach for patients with N3-positive disease, even when the tumor size is less than 3 cm. However, for N2-positive patients with limited cardiopulmonary function, sublobectomy could still be a viable option.
Lung cancer is a highly heterogeneous disease, and its treatment should be tailored to the individual patient. Our study represents the first attempt to establish a prediction model for the long-term survival of T1N2–3M0 NSCLC patients and visualize the benefits of different treatment strategies specifically for this subset of patients. In our study, the calibration curve showed optimal agreement between predicted survival and actual observation, demonstrating good repeatability and reliability of this established model. Furthermore, these nomograms fit well in the validation cohort, which represent the universalized application of the models. Our nomogram models represented practical and wide ranges of threshold probabilities regardless of OS and CSS, although the C-index of our models failed to reach a high magnitude. It is noteworthy that when the validation dataset was stratified into different risk groups using the optimal cut-off values from the training cohort, significant differences in the survival curves were observed, which indicates the satisfactory discriminative ability of these models. According to the scoring system developed from our model, these models could offer a valuable tool for survival prediction, identifying high-risk patients with poor prognosis, improving precision medicine, and enhancing the prognosis of this unique patient group. Meanwhile, our model for different treatment modalities may not be suitable for direct use, as the decision on treatment involves multiple factors, not just these factors. And because of the limited sample size for constructing the model in our study, the relevant conclusions may not be applicable to all patients. But the relevant conclusions can provide some reference for treatment strategy making.
So far, there have been several published nomograms concerning survival prediction for N2/N3 positive NSCLC. Mao et al. developed and validated a nomogram that could provide an individual prediction of OS for stage IIIA–N2 NSCLC patients after surgery (33). Han et al. reported a clinicopathologic prediction model for the survival of patients with the N3 stage (34). These previous models were not exclusively designed for T1N2–3M0 NSCLC patients and did not include specific surgical approaches. Therefore, they may not be entirely suitable for predicting survival in this particular patient population. In our nomogram, we included a substantial number of T1N2–3M0 NSCLC patients from the SEER database containing approximately 28% of the United States (US) population (12), which could maintain the generalizability of our model. Furthermore, our model incorporates a comprehensive panel of clinicopathologic variables, including surgical approaches, to ensure accuracy and reliability. Notably, our model is the first to conduct the prediction of CSS, providing valuable insights into the most beneficial treatment modalities and a more precise estimation of survival probabilities for these patients. Independent validation of our model yielded an ideal C-index, demonstrating its generalizability and predictive accuracy.
Some common independent prognostic factors for locally advanced NSCLC, such as age, sex, and histology, have been included in several published models (35-37). Our model also identified age and sex as significant predictors, but histology did not emerge as a significant predictor. This discrepancy could be attributed to the smaller tumor sizes in our study population. The significance of histology reported by previous studies may be further validated in a subsequent multicenter study. In addition, tumor grade reflected the differentiated ability and malignant degree of cancer, which were significantly related to prognosis (37-39). The results of these studies reinforced the reliability of tumor grade in our model. After that, we found that radiotherapy could not provide improved prognosis, which is consistent with the results of the study reported by Zhu et al. (40). The prediction of different surgical approaches, including sublobectomy, in N2/3-positive NSCLC is an area that has not been extensively explored in the existing literature. Mao et al. reported a nomogram to predict the survival of stage IIIA–N2 NSCLC after surgery, in which this report contains the lobectomy and pneumonectomy, not including other surgical approaches (33). Liang et al. also established and validated a novel nomogram that can provide an individual prediction of OS for patients with resected NSCLC, in which surgical approaches involving wedge resection were included for analysis (35). These studies demonstrated that the surgical approaches included significant variables, which may further improve the accuracy and reliability of the prediction of survival benefits for patients with T1N2–3M0 NSCLC.
Nonetheless, it is essential to acknowledge the limitations of our current study. First and foremost, as a retrospective study, there is an inherent risk of population selection bias, and the ability to control for confounding factors may not be as rigorous as in prospective studies. Second, a major limitation arises from the absence of comprehensive information regarding chemotherapy and radiotherapy in the SEER database (41), including details on treatment regimens, courses of chemotherapy, cycles, doses, and radiotherapy methods. Additionally, we could not determine the sequence of chemotherapy and radiotherapy in relation to surgery, which could potentially impact the reliability of our study and the performance of our predictive model. In addition, we could not assess more pretreatment variables which may be related to the survival outcome and the reason why the patients did not receive the surgical treatment, including comorbidity, forced expiratory volume in 1 second (FEV1), diffusing capacity of the lung for carbon monoxide (DLCO), performance score, smoking status, body mass index (BMI), and surgical approach (e.g., video-assisted thoracic surgery or open surgery). Due to our reliance on the SEER database, we were unable to include these parameters in our analysis. Moreover, specific details about LND, including the regions dissected and the number of lymph nodes removed, were not available in the SEER database. Nevertheless, we believe that our study was conducted using clinically relevant factors that are accessible in the SEER database and benefited from a large sample size, offering valuable insights for clinical practice in T1N2–3M0 NSCLC patients. Our findings, particularly regarding the benefits of sublobectomy in stage T1N2–3M0 NSCLC and the visualization of survival advantages associated with different surgical approaches, represent an important contribution. However, to further validate our results and provide more robust clinical guidance before being recommended for clinical use, prospective large-sample RCTs with comprehensive data on clinicopathological variables, performance status, and detailed treatment regimens should be conducted. These trials would offer a more precise assessment of the outcomes observed in our study and enhance the reliability of clinical recommendations.
Conclusions
Our study demonstrates that surgical intervention could offer significant survival benefits for stage T1N2–3M0 NSCLC patients. Among the surgical approaches, lobectomy emerged as the superior option, providing improved OS and CSS compared to sublobectomy. However, for patients who may not be suitable candidates for lobectomy, sublobectomy may remain a valuable alternative that confers survival advantages. Our predictive model enhances the understanding of the differential benefits associated with various surgical approaches, thus serving as a valuable tool for informing survival discussions between patients and clinicians, guiding the design and monitoring of clinical trials, and facilitating the development of more personalized treatment strategies.
Acknowledgments
The authors appreciate the efforts of the SEER program in establishing the SEER database.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-213/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-213/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-213/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Gao F, Li N, Xu Y, et al. Evaluation of Postoperative Radiotherapy Effect on Survival of Resected Stage III-N2 Non-small Cell Lung Cancer Patients. Front Oncol 2020;10:1135. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Behera M, Steuer CE, Liu Y, et al. Trimodality Therapy in the Treatment of Stage III N2-Positive Non-Small Cell Lung Cancer: A National Cancer Database Analysis. Oncologist 2020;25:e964-75. [Crossref] [PubMed]
- Kaniski F, Enewold L, Thomas A, et al. Temporal patterns of care and outcomes of non-small cell lung cancer patients in the United States diagnosed in 1996, 2005, and 2010. Lung Cancer 2017;103:66-74. [Crossref] [PubMed]
- Van Schil PE, De Waele M, Hendriks JM, et al. Surgical treatment of stage III non-small cell lung cancer. Eur J Cancer 2009;45:106-12. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Goldstraw P, Mannam GC, Kaplan DK, et al. Surgical management of non-small-cell lung cancer with ipsilateral mediastinal node metastasis (N2 disease). J Thorac Cardiovasc Surg 1994;107:19-27; discussion 27-8.
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Bott MJ, Patel AP, Crabtree TD, et al. Role for Surgical Resection in the Multidisciplinary Treatment of Stage IIIB Non-Small Cell Lung Cancer. Ann Thorac Surg 2015;99:1921-8. [Crossref] [PubMed]
- Caglar HB, Baldini EH, Othus M, et al. Outcomes of patients with stage III nonsmall cell lung cancer treated with chemotherapy and radiation with and without surgery. Cancer 2009;115:4156-66. [Crossref] [PubMed]
- Doll KM, Rademaker A, Sosa JA. Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg 2018;153:588-9. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-24.
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565-74. [Crossref] [PubMed]
- Shiba K, Kawahara T. Using Propensity Scores for Causal Inference: Pitfalls and Tips. J Epidemiol 2021;31:457-63. [Crossref] [PubMed]
- Johnstone DW, Byhardt RW, Ettinger D, et al. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2002;54:365-9. [Crossref] [PubMed]
- Toyooka S, Kiura K, Takemoto M, et al. Long-term outcome of induction chemoradiotherapy with docetaxel and cisplatin followed by surgery for non-small-cell lung cancer with mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg 2012;14:565-9. [Crossref] [PubMed]
- Koshy M, Fedewa SA, Malik R, et al. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non-small-cell lung cancer. J Thorac Oncol 2013;8:915-22. [Crossref] [PubMed]
- Kim S, Lim JU, Kang HS, et al. The association between clinical parameters and resectability in stage III non-small cell lung cancer, and a combination of N2 lymph node burden and lung immune prognostic index score as a potential biomarker. Transl Lung Cancer Res 2023;12:79-95. [Crossref] [PubMed]
- Zhang L, Li M, Yin R, et al. Comparison of the oncologic outcomes of anatomic segmentectomy and lobectomy for early-stage non-small cell lung cancer. Ann Thorac Surg 2015;99:728-37. [Crossref] [PubMed]
- Yang CF, Kumar A, Gulack BC, et al. Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis. J Thorac Cardiovasc Surg 2016;151:1380-8. [Crossref] [PubMed]
- Echavarria MF, Cheng AM, Velez-Cubian FO, et al. Comparison of pulmonary function tests and perioperative outcomes after robotic-assisted pulmonary lobectomy vs segmentectomy. Am J Surg 2016;212:1175-82. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines® Insights: Non-Small Cell Lung Cancer, Version 2.2023. J Natl Compr Canc Netw 2023;21:340-50. [Crossref] [PubMed]
- Hendriks LE, Kerr KM, Menis J, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:358-76. [Crossref] [PubMed]
- Deng H, Liu J, Cai X, et al. Radical Minimally Invasive Surgery After Immuno-chemotherapy in Initially-unresectable Stage IIIB Non-small cell Lung Cancer. Ann Surg 2022;275:e600-2. [Crossref] [PubMed]
- Raman V, Jawitz OK, Yang CJ, et al. Outcomes of surgery versus chemoradiotherapy in patients with clinical or pathologic stage N3 non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1680-1692.e2. [Crossref] [PubMed]
- Fu F, Sun W, Bai J, et al. Long-Term Outcomes of Selected Patients with IIIA-N2 Non-small Cell Lung Cancer Receiving Upfront Surgical Resection. Ann Surg Oncol 2023;30:8261-70. [Crossref] [PubMed]
- Swaminath A, Vella ET, Ramchandar K, et al. Surgery after chemoradiotherapy in patients with stage III (N2 or N3, excluding T4) non-small-cell lung cancer: a systematic review. Curr Oncol 2019;26:e398-404. [Crossref] [PubMed]
- Grunenwald DH, André F, Le Péchoux C, et al. Benefit of surgery after chemoradiotherapy in stage IIIB (T4 and/or N3) non-small cell lung cancer. J Thorac Cardiovasc Surg 2001;122:796-802. [Crossref] [PubMed]
- Ziel E, Hermann G, Sen N, et al. Survival Benefit of Surgery after Chemoradiotherapy for Stage III (N0-2) Non-Small-Cell Lung Cancer Is Dependent on Pathologic Nodal Response. J Thorac Oncol 2015;10:1475-80. [Crossref] [PubMed]
- Jeremić B, Casas F, Dubinsky P, et al. Surgery for Stage IIIA Non-Small-cell Lung Cancer: Lack of Predictive and Prognostic Factors Identifying Any Subgroup of Patients Benefiting From It. Clin Lung Cancer 2016;17:107-12. [Crossref] [PubMed]
- Mao Q, Xia W, Dong G, et al. A nomogram to predict the survival of stage IIIA-N2 non-small cell lung cancer after surgery. J Thorac Cardiovasc Surg 2018;155:1784-1792.e3. [Crossref] [PubMed]
- Han C, Wu Y, Sun X, et al. Outcome of Non-small Cell Lung Cancer Patients With N3 Stage: Survival Analysis of Propensity Score Matching With a Validated Predictive Nomogram. Front Surg 2021;8:666332. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Rocco G, Nason K, Brunelli A, et al. Management of stage IIIA (N2) non-small cell lung cancer: A transatlantic perspective. J Thorac Cardiovasc Surg 2016;151:1235-8. [Crossref] [PubMed]
- Oberije C, De Ruysscher D, Houben R, et al. A Validated Prediction Model for Overall Survival From Stage III Non-Small Cell Lung Cancer: Toward Survival Prediction for Individual Patients. Int J Radiat Oncol Biol Phys 2015;92:935-44. [Crossref] [PubMed]
- Tian D, Pei Y, Zheng Q, et al. Effect of visceral pleural invasion on the prognosis of patients with lymph node negative non-small cell lung cancer. Thorac Cancer 2017;8:97-105. [Crossref] [PubMed]
- Sun Z, Aubry MC, Deschamps C, et al. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: an analysis of 5018 hospital- and 712 population-based cases. J Thorac Cardiovasc Surg 2006;131:1014-20. [Crossref] [PubMed]
- Zhu M, Li S, Yuan L, et al. The high-risk features and effect of postoperative radiotherapy on survival for patients with surgically treated stage IIIA-N2 non-small cell lung cancer. World J Surg Oncol 2023;21:238. [Crossref] [PubMed]
- Park HS, Lloyd S, Decker RH, et al. Limitations and biases of the Surveillance, Epidemiology, and End Results database. Curr Probl Cancer 2012;36:216-24. [Crossref] [PubMed]


