
Prolonged survival and novel prognostic factors in women with pleural mesothelioma treated with extended pleurectomy decortication
Highlight box
Key findings
• This study highlights that women diagnosed with epithelioid pleural mesothelioma exhibit a significant 5-year survival and benefit more from extended pleurectomy decortication as part of multimodality treatment than men.
What is known and what is new?
• Although previous retrospective and prospective studies have indicated no correlation between sex and prognosis in pleural mesothelioma, certain studies have demonstrated the enhanced significance of female survival.
• In this context, we examined the findings of one of the largest patient cohorts treated at a single institution, revealing that being female is associated with a better prognosis in epithelioid pleural mesothelioma.
What is the implication, and what should change now?
• The noteworthy 5-year survival rate in this extremely aggressive disease underscores the importance of multimodality treatment, which involves extended pleurectomy decortication in females with epithelioid pleural mesothelioma.
Introduction
Pleural mesothelioma (PM) is a highly aggressive rare tumor associated with an overall median survival of 12–18 months for unresectable patients treated with systemic therapies (1,2). There is a higher incidence of PM in males than females, which might be explained by the differences in occupational exposures. PM incidence declined somewhat for men from 2000 to 2015 but held steady for women, which may indicate other sources of exposure that are more difficult to detect, such as higher environmental exposure levels (3). Despite retrospective and prospective trials that showed no association between sex and prognosis (4-6), others have demonstrated the improved prognostic impact of the female sex on survival (7-11). The 5-year survival of 3,196 females with PM, constituting 22% of PM patients in the Surveillance, Epidemiology, and End Results (SEER) database, was 13.4% versus 4.5% for males (7). Herein, we examined the outcomes of a single high-volume PM program using intended extended pleurectomy decortication (ePD) as a component of a multimodal approach for female patients with PM. Prolonged overall survival (OS) in females following a multimodality approach is presented and potential novel prognostic factors are identified. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-797/rc).
Methods
Patients
This study is a retrospective analysis of a prospectively meticulously obtained database from the Division of Thoracic Surgery at Brigham and Women’s Hospital in Boston, encompassing all female patients who underwent intended ePD for PM over an 11-year period. All patients provided consent for participation in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Dana Farber Cancer Institute (No. 98-063), permitting this follow up and linking of data. The authors conducted a retrospective chart review to verify and enhance all prospective data previously gathered regarding preoperative, operative and perioperative care as well as the pathological and radiographic data. Outcome data and patient follow-up details were obtained by scrutinizing the visits via the outpatient clinics and when needed, by reaching out to referring physicians or patients.
Preoperative assessment
Before surgery, all patients were evaluated using chest radiograph (CXR), contrast-enhanced computed tomography of the chest (chest-CT), positron emission tomography-CT scan (PET-CT), magnetic resonance imaging of the chest (chest MRI), spirometry and ventilation perfusion scan. Neoadjuvant therapy was administered to the following groups: (I) patients with mediastinal nodal metastases detected during cervical mediastinoscopy or endo bronchial ultrasound; (II) patients showing ipsilateral extension through vital structures or chest wall; (III) a few of the patients who were not initially evaluated at our institution. Prior to surgery all these groups underwent restaging and needed to demonstrate treatment response.
ePD with intraoperative heated chemotherapy (IOHC)
Patients underwent evaluation by a multidisciplinary team with particular focus on input from the surgeons to assess resectability and ability to achieve the goal of macroscopic complete resection (MCR). The surgical objective was complete removal of parietal and visceral pleura along with excision of the diaphragm and pericardium if found to be involved with tumor either visibly or by frozen section analysis. Reconstruction of the diaphragm and pericardium as deemed necessary by the surgeon was carried out with permanent patches (GorTex with pericardial fenestrations). Lymph nodes were sampled from the mediastinum, hilum, intercostal and mammary vessel. Previous biopsy sites were excised, and the pleural cavity was irrigated with saline, water and peroxide. Additionally, the chest wall and suspected area of remaining visceral pleura were treated with argon beam painting. Subsequently, IOHC was performed using cisplatin at a concentration of 175 mg/m2 circulated at 42 °C for up to 60 minutes with renal protection measures as previously described (12,13). This approach previously shown to be safe has become a standard practice in our program for achieving local control (12). For patients who did not receive neo-adjuvant therapy and were found to have positive lymph nodes during ePD, advanced T stage, or non-epithelioid histology adjuvant therapy- postoperative chemotherapy (platinum-based chemotherapy and pemetrexed) as part of the multi-modality treatment is recommended following surgical resection.
Statistical analysis
We retrieved and examined patients’ data from medical records encompassing details such as patients’ demographics, age, preoperative histology, preoperative spirometry, neo-adjuvant treatment, extent of resection, use of IOHC, hospitalization length, postoperative complications, postoperative histology, adjuvant therapy and follow up intervals. Patient mortality was recorded in the registry based on notifications found in the electronic medical records, through scrutiny of the Social Security Death Index or obituaries. Both OS and length of stay were calculated from the date of surgery. Tumor characteristics included histology, TNM and laterality. Survival analysis was based on Descriptive statistics, Kaplan-Meier estimators, and Cox proportional hazards model. Univariable associations between patients and disease characteristics were assessed using Wilcoxon rank-sum tests and Fisher’s exact tests. Kaplan-Meier estimators were utilized to compare OS across different groups, and cox models were used to evaluate the relationship between OS and potential prognostic factors. Two-sided P values below 0.05 were considered significant.
Results
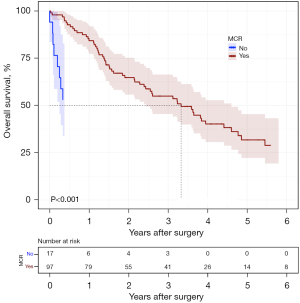
Between 2007 to 2017, a total of 454 consecutive patients underwent thoracotomy with intended ePD for PM applying a uniform protocol. Thirteen surgeons were involved while two of them performed almost ninety percent of the cases. There were 273 (60.1%) with epithelioid subtype, 340 (74.9%) males, median age was 69 years and MCR was achieved in 382 (84.1%). We excluded from survival analysis two patients (from overseas) due to lack of postoperative follow up data. One hundred and fourteen patients were females (25.1%). Among them 81 (71.0%) with epithelioid histology 31 (27.2%) with biphasic histology and just 2 (1.8%) with sarcomatoid histology. In this cohort, females tended to be significantly younger and had a higher forced expiratory volume in 1 second (FEV1) than males (Table 1). Additionally, epithelioid histology was more common in females, while sarcomatoid histology was very rare in this group. There was no substantial difference in the proportion of patients receiving neoadjuvant therapy, adjuvant therapy, IOHC, MCR and pathologic stage between males and females (Table 1). In a multivariable analysis, factors that were found to be associated with patient OS in 454 patients included female sex, epithelioid histology, MCR, IOHC treatment, age, early T stage and receipt of adjuvant chemotherapy (Table 2). Interestingly, smoking history of patients with PM was not identified as a negative prognostic factor as indicated in Appendix 1. Importantly, females had significantly longer survival compared to males independent of cofounders mentioned above (Figure 1), and the difference between males and females with PM was maintained in the MCR group as well (Figure 2). Survival outcomes such as progression-free survival (PFS) and relapse-free survival (RFS) which might be more sensitive to the isolated effect of surgery on PM patients were analyzed. PFS (time from surgery to disease progression or death, whichever comes first) was significantly longer in females (Figure S1). The median PFS for females was 455 days versus 310 for males [hazard ratio (HR): 1.5, P=0.001]. Median RFS of 468 in females was significantly longer compared to 323 days in males (HR: 1.4, P=0.002). Within the total group of 114 female patients 69 (60.3%) had right sided operations, 81 (71.0%) had epithelioid histology with a median age was 65 years (range, 29–84 years, mean 63.4 years). Additionally, 31 (27.2%) received neoadjuvant treatment. IOHC was administered to 90 (78.9%) patients while adjuvant chemotherapy was given to 72 (73.5%) patients. The median hospital stay lasted for 13 days (range, 1–63 days). The observed mortality rates at 30 and 90 days were 3.5% and 6.1% respectively. The median survival in this cohort was 38 months with a 5-year survival of 28.2%.
Table 1
| Variable | Females (n=114) | Males (n=340) | P value |
|---|---|---|---|
| Age, years | <0.001 | ||
| Median [IQR] | 65 [29–84] | 70 [39–86] | |
| Mean | 63.4 | 69.3 | |
| Histology | 0.006 | ||
| Epithelioid | 81 (71.0%) | 192 (56.5%) | |
| Biphasic | 31 (27.2%) | 119 (35.0%) | |
| Sarcomatoid | 2 (1.8%) | 29 (8.5%) | |
| FEV1 ≥80% | 52 (45.6%) | 96 (28.7%) | 0.001 |
| Neoadjuvant therapy | 31 (27.2%) | 89 (26.3%) | 0.84 |
| IOHC | 90 (78.9%) | 258 (76.1%) | 0.53 |
| MCR | 97 (85.1%) | 285 (83.8%) | 0.74 |
| Adjuvant therapy | 72 (73.5%) | 226 (72.9%) | 0.91 |
| Tumor stage | 0.99 | ||
| T1 | 25 (21.9%) | 73 (21.5%) | |
| T2 | 29 (25.4%) | 85 (25.0%) | |
| T3 | 43 (37.7%) | 131 (38.5%) | |
| T4 | 17 (15.0%) | 51 (15.0%) | |
| Nodal stage | 0.24 | ||
| N0 | 73 (64.0%) | 223 (65.6%) | |
| N1 | 39 (34.2%) | 116 (34.1%) | |
| N2 | 2 (1.8%) | 1 (0.3%) | |
| AJCC 8th edition pathologic stage | 0.86 | ||
| 1A | 21 (18.4%) | 52 (15.3%) | |
| 1B | 44 (38.6%) | 139 (41.0%) | |
| 2 | 13 (11.4%) | 47 (13.9%) | |
| 3A | 16 (14.0%) | 49 (14.5%) | |
| 3B | 20 (17.6%) | 52 (15.3%) | |
Missing data: FEV1 (6 males), neoadjuvant therapy (1 male), IOHC (1 male), adjuvant therapy (16 females, 30 males), pathologic stage (1 male). IQR, interquartile range; FEV1, forced expiratory volume in 1 second; IOHC, intraoperative heated chemotherapy; MCR, macroscopic complete resection; AJCC, American Joint Committee on Cancer.
Table 2
| Variable | Category | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | |||
| Sex | Female | Ref | – | Ref | – | |
| Male | 1.69 | <0.001 | 1.5 | 0.006 | ||
| Age | ≤69 | Ref | – | Ref | – | |
| >69 | 1.64 | <0.001 | 1.4 | 0.005 | ||
| Laterality | Right | Ref | – | Ref | – | |
| Left | 0.94 | 0.61 | 0.92 | 0.51 | ||
| MCR | − | Ref | – | Ref | – | |
| + | 0.4 | <0.001 | 0.38 | <0.001 | ||
| Histology | Epithelioid | Ref | – | Ref | ||
| Biphasic | 1.79 | <0.001 | 1.9 | <0.001 | ||
| Sarcomatoid | 3.20 | <0.001 | 2.3 | <0.002 | ||
| Tumor stage | T1 | Ref | – | Ref | – | |
| T2 | 1.5 | 0.01 | 1.77 | 0.003 | ||
| T3 | 1.98 | <0.001 | 2.22 | <0.001 | ||
| T4 | 3.4 | <0.001 | 3.18 | <0.002 | ||
| Nodal stage | N0 | Ref | – | Ref | – | |
| N1 | 1.1 | 0.89 | 1.2 | 0.08 | ||
| N2 | 2.17 | 0.27 | 1.7 | 0.60 | ||
| IOHC | − | Ref | – | Ref | – | |
| + | 0.46 | <0.001 | 0.84 | 0.30 | ||
| Adjuvant chemotherapy | − | Ref | – | Ref | – | |
| + | 0.48 | <0.001 | 0.38 | <0.002 | ||
| Neoadjuvant chemotherapy | − | Ref | – | Ref | – | |
| + | 1.16 | 0.22 | 0.94 | 0.68 | ||
| FEV1 | <80% | Ref | – | Ref | – | |
| ≥80% | 0.59 | 0.001 | 0.85 | 0.26 | ||
Ref, reference; MCR, macroscopic complete resection; IOHC, intraoperative heated chemotherapy; FEV1, forced expiratory volume in 1 second.

The objective of achieving MCR was met in 97 (85.1%). The tumor staging breakdown for this cohort was as follows: T1 was present in 23 (23.7%) patients, T2 in 27 (27.8%), T3 in 39 (40.2%) and T4 in 8 (8.3%). Lymph node status indicated N0 in 63 (65%) of patients and N1 in 34 (35%) of patients. Based on the American Joint Commission on Cancer (AJCC) 8th edition the pathological stage distribution was as follows: stage 1A, 20.6%; 1B, 43.3%; 2, 11.35%; 3A 16.5%; 3B, 8.25%. The median survival of the patients undergoing PD with MCR was longer compared to the group of patients in whom MCR was not achievable. Median survival and 5-year survival in 85% of females in whom MCR was achieved was 40.5 months [95% confidence interval (CI): 29.1–53.7] and 31.8% (95% CI: 22.2–45.4%) respectively in contrast to median survival of 4.6 months in the non-MCR group (Figure 3). The median and 5-year survivals for patients with epithelioid histology and MCR were 44.4 months and 36.4% respectively.
Univariate analysis (Table 3) showed significant difference in female patient OS based on age as a continuous factor (P=0.03), epithelioid histology (P=0.009, Figure 4), MCR (P<0.001), early T status (P=0.03), adjuvant therapy (P=0.006) and IOHC (P=0.03). Neoadjuvant treatment, N status and preoperative percentage FEV1 were not associated with OS.
Table 3
| Variable | Category | Median OS, months | Survival (IQR), months | Univariate | |
|---|---|---|---|---|---|
| HR (95% CI) | P value | ||||
| Histology | Epithelioid | 40.5 | 17.3–87.4 | 2 (1.1–3.04) | 0.009 |
| Biphasic | 17 | 7.6–48 | Ref | – | |
| Sarcomatoid | 0.9 | 0.9–NR | – | – | |
| Age | – | – | – | 1.02 (1.002–1.05) | 0.03 |
| FEV1 | ≥80% | 40.5 | 21.2–87.4 | 0.72 (0.45–1.14) | 0.16 |
| <80% | 23.2 | 11.8–78.7 | Ref | ||
| Tumor stage | T1/T2 | 44.1 | 18.3–87.4 | Ref | – |
| T3/T4 | 31 | 11.3–56 | 1.6 (1–2.6) | 0.03 | |
| Nodal stage | N0 | 39.2 | 17.3–78.7 | 0.81 (0.77–2.0) | 0.30 |
| N1&N2 | 22.4 | 8.6–59.1 | Ref | – | |
| IOHC | − | 19.1 | 3.4–NR | Ref | – |
| + | 40.5 | 17–78.7 | 0.8 (0.32–0.9) | 0.03 | |
| Adjuvant chemotherapy | − | 10.1 | 4.1–88.2 | Ref | – |
| + | 44.1 | 23.2–66.3 | 0.5 (0.3–0.86) | 0.006 | |
| MCR | − | 4.5 | 2.4–36.8 | Ref | – |
| + | 40.5 | 14.7–78.7 | 0.3 (0.2–0.56) | <0.001 | |
OS, overall survival; Ref, reference; IQR, interquartile range; HR, hazard ratio; CI, confidence interval; FEV1, forced expiratory volume in 1 second; IOHC, intraoperative heated chemotherapy; NR, not reached; MCR, macroscopic complete resection.

Postoperative complications were categorized as major including deep venous thrombosis, Chyle leak, empyema, pneumonia, Horner’s syndrome, pulmonary embolism, vocal cord paralysis/paresis, surgical site infection, cerebral vascular disease/transient ischemic attack, acute respiratory distress syndrome, seizures, myocardial infarction, epidural abscess, and hemothorax and minor such as prolonged air leak- over 14 days, atrial fibrillation, urinary tract infection, delirium, ileus, and urinary retention (14). These complications were defined as occurring within 90 days of the surgery. Out of the patients, 36 patients (31.6%) did not experience any complications (neither major nor minor), 26 (22.8%) had minor but not major complications, 21 (18.4%) had both major and minor complications and 31 (27.2%) had major complications without minor. The most frequently observed complications were prolonged air leak (33.3%), deep venous thrombosis (17.5%) pneumonia (8.8%) and Chyle leak (8.8%). Data regarding postoperative complications are compiled in Table S1.
Discussion
We present herein an analysis of one of the most extensive patient cohorts treated at a single institution, consisting of females who underwent intended ePD for PM with the goal of achieving MCR. The data is derived from a continuous large dataset covering a 11-year experience in the modern practice conducted by a small group of experienced surgeons at an established center and not an amalgam of selected retrospective patients over various institutions and time periods. This study outlines the perioperative results and long-term survival of 114 consecutive patients who underwent radical lung sparing surgery with intent to perform MCR of whom 97 (85.1%) had a successful and complete resection. Females constitute 25% percent of intended ePD operated in our institution from 2007–2017. The females’ proportions with pleural PM herein is in accordance with its incidence in the literature (3,15). The low incidence rate of PM in females compares to males is attributed to differences in occupational exposure, where most of asbestos workers were males. Interestingly the incidence of women with mesothelioma has not changed during the last decades despite the ban on asbestos containing products in the US during the last decades. Females are characterized by higher percent of epithelioid mesothelioma subtype and lower rate of non-epithelioid histology and specifically rare cases of sarcomatoid subtype compared to males.
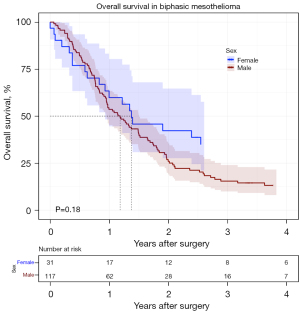
Importantly, by applying multivariate analysis, significant prolonged survival in females compare to males is seen in the entire group of 454 patients, independent of cofounders such as histology subtype, age, FEV1, MCR, T status and adjuvant therapy. Lower rate of survival in males compared to females is not unique to PM and seen in other malignancies such as anorectal, melanoma, salivary gland, thyroid cancer and more. Certain variances in cancer occurrence between sexes can be attributed to the actions of circulating sex hormones, whereas other distinctions are independent of estrogen, testosterone, or progesterone levels. Rather, these differences stem from sexual differentiation, a process that involves genetic and epigenetic mechanisms (16). In a recent study conducted by our group (17), utilizing transcriptomic analysis with gene expression microarrays, it was observed that high expression of RAS like estrogen regulated growth inhibitor gene (RERG) was found to be linked with longer survival after surgery in females with PM. Although previous research has reported correlation between estrogen receptor beta and survival (18), in the RERG paper no association between survival and estrogen receptor protein or gene was identified. Remarkably the results seen in this set of pleurectomy decortication are either comparable or superior to a substantial cohort of 145 females, who underwent extra pleural pneumonectomy (EPP) at the same institution during a longer duration and mostly in earlier years (1987–2010) (8). The median survival of intended ePD patients with epithelioid subgroup in our study is 40.5 months compared to 30 months in the EPP group. Reduced short-term mortality, improved quality of life and comparable if not superior long-term survival associated with lung sparing operation compared to EPP have prompted many centers including ours to change the surgical procedure from EPP to ePD during the last two decades (19,20). Interestingly, although occasionally, female sex has been noted as a favorable prognostic factor for patients with PM (21,22), we noticed no significant difference in survival between males and females with non-epithelioid mesothelioma (Figure 5). In our recent published biphasic mesothelioma paper we demonstrated that 40% of the patients had preoperative diagnosis of epithelioid histology which turned out to be biphasic histology after ePD (23). We assume that this rate of histological subtype misdiagnosis is even higher and comprises many non-surgical published series that led to the determination that sex is not a prognostic factor in PM. This paper highlights the prognostic factors associated with extended survival in females who underwent pleurectomy decortication for PM such as epithelioid histology, early age, adjuvant chemotherapy, early T status, IOHC and achieving MCR. Importantly it was observed that the cohort that underwent MCR resection had significantly improved survival outcomes compared to non-MCR resections. In addition, women who underwent partial or no resection did not fare well, and none reached 4-year survival. The significance of attaining MCR and control of micro metastasis is underscored and supported by the National Comprehensive Cancer Network and International Mesothelioma Interest Group as integral components of PM multimodality treatment. In our paper IOHC was found to increase OS in females with PM who underwent ePD. In 2013, Sugarbaker and colleagues conducted a study involving 103 low-risk groups of patients who underwent cytoreductive surgery and IOHC compared to those who underwent cytoreductive surgery alone demonstrating benefits to patients receiving IOHC. Their research revealed that hyperthermic intraoperative pleural cisplatin chemotherapy prolonged the time to recurrence and improved survival among low-risk patients with malignant PM undergoing surgical MCR (24). The effectiveness and safety of surgical resection and IOHC were evaluated in phase I clinical trial (25) but further investigation through a prospective randomized phase II clinical trial is necessary to elucidate the benefits of these features. It is important to approach the interpretation of our findings regarding IOHC cautiously as a significant proportion of patients in our study were skewed towards receiving IOHC.
The retrospective design of this study comparing the OS between two groups is one of its limitations. Furthermore, while the study benefits from the large cohort derived from a single institution the findings may not be generalizable and vary in other institutions. However, this may be a strength of the present study because it ensures a more consistent approach to the surgical procedure, follow-up and management compared with retrospective multi-institutional studies.
Conclusions
The findings of significant real 5-year survival in this highly aggressive disease based on one of the most extensive patient cohorts at a single institution, advocates for an aggressive multimodality approach including ePD in women with epithelioid mesothelioma. This study confirms that women with epithelioid PM benefit more from treatment with ePD as part of multimodal approach than men and enjoy prolonged median survival of almost 4 years with a low 30- and 90-day mortality. The demonstrated outcomes should provide a new benchmark for comparison in forthcoming studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-797/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-797/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-797/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-797/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Dana Farber Cancer Institute (No. 98-063). All patients provided consent for participation in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375-86. [Crossref] [PubMed]
- Alpert N, van Gerwen M, Taioli E. Epidemiology of mesothelioma in the 21(st) century in Europe and the United States, 40 years after restricted/banned asbestos use. Transl Lung Cancer Res 2020;9:S28-38. [Crossref] [PubMed]
- Tanrikulu AC, Abakay A, Kaplan MA, et al. A clinical, radiographic and laboratory evaluation of prognostic factors in 363 patients with malignant pleural mesothelioma. Respiration 2010;80:480-7. [Crossref] [PubMed]
- Francart J, Vaes E, Henrard S, et al. A prognostic index for progression-free survival in malignant mesothelioma with application to the design of phase II trials: a combined analysis of 10 EORTC trials. Eur J Cancer 2009;45:2304-11. [Crossref] [PubMed]
- Yan TD, Boyer M, Tin MM, et al. Extrapleural pneumonectomy for malignant pleural mesothelioma: outcomes of treatment and prognostic factors. J Thorac Cardiovasc Surg 2009;138:619-24. [Crossref] [PubMed]
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Determinants of Survival in Malignant Pleural Mesothelioma: A Surveillance, Epidemiology, and End Results (SEER) Study of 14,228 Patients. PLoS One 2015;10:e0145039. [Crossref] [PubMed]
- Wolf AS, Richards WG, Tilleman TR, et al. Characteristics of malignant pleural mesothelioma in women. Ann Thorac Surg 2010;90:949-56; discussion 956. [Crossref] [PubMed]
- Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957-65. [Crossref] [PubMed]
- Van Gerwen M, Alpert N, Wolf A, et al. Prognostic factors of survival in patients with malignant pleural mesothelioma: an analysis of the National Cancer Database. Carcinogenesis 2019;40:529-36. [Crossref] [PubMed]
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Women with malignant pleural mesothelioma have a threefold better survival rate than men. Ann Thorac Surg 2014;98:1020-4. [Crossref] [PubMed]
- Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561-7. [Crossref] [PubMed]
- Chang MY, Sugarbaker DJ. Innovative therapies: intraoperative intracavitary chemotherapy. Thorac Surg Clin 2004;14:549-56. [Crossref] [PubMed]
- Burt BM, Cameron RB, Mollberg NM, et al. Malignant pleural mesothelioma and the Society of Thoracic Surgeons Database: an analysis of surgical morbidity and mortality. J Thorac Cardiovasc Surg 2014;148:30-5. [Crossref] [PubMed]
- Taioli E, Wolf A, Alpert N, et al. Malignant pleural mesothelioma characteristics and outcomes: A SEER-Medicare analysis. J Surg Oncol 2023;128:134-41. [Crossref] [PubMed]
- Rubin JB, Lagas JS, Broestl L, et al. Sex differences in cancer mechanisms. Biol Sex Differ 2020;11:17. [Crossref] [PubMed]
- De Rienzo A, Coleman MH, Yeap BY, et al. Association of RERG Expression with Female Survival Advantage in Malignant Pleural Mesothelioma. Cancers (Basel) 2021;13:565. [Crossref] [PubMed]
- Pinton G, Brunelli E, Murer B, et al. Estrogen receptor-beta affects the prognosis of human malignant mesothelioma. Cancer Res 2009;69:4598-604. [Crossref] [PubMed]
- Lapidot M, Bueno R. Pleurectomy decortication is the preferred surgical procedure in pleural mesothelioma. Transl Lung Cancer Res 2023;12:190-2. [Crossref] [PubMed]
- Klotz LV, Hoffmann H, Shah R, et al. Multimodal therapy of epithelioid pleural mesothelioma: improved survival by changing the surgical treatment approach. Transl Lung Cancer Res 2022;11:2230-42. [Crossref] [PubMed]
- Friedberg JS, Simone CB 2nd, Culligan MJ, et al. Extended Pleurectomy-Decortication-Based Treatment for Advanced Stage Epithelial Mesothelioma Yielding a Median Survival of Nearly Three Years. Ann Thorac Surg 2017;103:912-9. [Crossref] [PubMed]
- Lapidot M, Gill RR, Mazzola E, et al. Pleurectomy Decortication in the Treatment of Malignant Pleural Mesothelioma: Encouraging Results and Novel Prognostic Implications Based on Experience in 355 Consecutive Patients. Ann Surg 2022;275:1212-20. [Crossref] [PubMed]
- Lapidot M, Mazzola E, Bueno R. Outcomes of pleurectomy decortication in patients with biphasic mesothelioma. J Thorac Cardiovasc Surg 2022;164:1340-1348.e3. [Crossref] [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [Crossref] [PubMed]
- Burt BM, Richards WG, Lee HS, et al. A Phase I Trial of Surgical Resection and Intraoperative Hyperthermic Cisplatin and Gemcitabine for Pleural Mesothelioma. J Thorac Oncol 2018;13:1400-9. [Crossref] [PubMed]



