
Outcomes of initial therapy for synchronous brain metastases from small cell lung cancer: a single-institution retrospective analysis
Introduction
Small cell lung cancer (SCLC) is a high-grade neuroendocrine malignancy that generally has a poor prognosis and a high incidence of brain metastases. Approximately 15% of patients have synchronous brain metastases at the time of initial SCLC diagnosis, and the prevalence of brain metastases increases to over 50% at 2 years (1,2). Whole brain radiation therapy (WBRT) has historically been the standard of care for SCLC brain metastases, but emerging data suggests that patients treated with upfront stereotactic radiosurgery (SRS) instead of WBRT have comparable overall survival (OS) outcomes (3,4). Surgery does not typically have a role in the management of SCLC brain metastases, although surgical resection can simultaneously give pathologic diagnosis and relief of neurologic symptoms. SCLC patients were excluded from the three major randomized trials evaluating the role of surgical resection of single brain metastases (5-7). The outcomes of patients following treatments can vary significantly. Using an institutional database, we sought to identify predictors of OS and brain progression-free survival (bPFS) in patients with synchronous brain metastases from SCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-641/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients treated for extensive-stage SCLC (ES-SCLC) at University Hospitals Cleveland Medical Center between 1998 and 2018 were identified using a University Hospitals Institutional Review Board (IRB) approved database (No. CHRV0021). There were minimal risks to the patients for study participation, thus obtaining consents was not required for this retrospective study. All patients with synchronous brain metastases diagnosed with initial staging head computed tomography (CT) or brain magnetic resonance imaging (MRI) scans, with or without extracranial metastases, were included in this retrospective analysis. OS was defined as the duration from date of pathologic diagnosis of SCLC to date of death and was censored at the date of last follow-up for survivors. bPFS was defined as the duration from date of pathologic diagnosis of SCLC to date of last follow-up, intracranial progression, or death (whichever occurred first). All available CT or brain MRI imaging and radiology reports were reviewed for bPFS. Our institution’s standard practice is to perform surveillance brain MRI with and without contrast every 3 months. Survival estimates were generated using the Kaplan-Meier method with log-rank test. Factors associated with OS and bPFS were analyzed using Cox proportional hazards model. Variables that were significant in univariate analyses (P<0.10) were included in multivariate Cox models to adjust for potential confounders. Modified Schoenfeld residuals were used to determine the validity of the proportional hazards assumption. All statistical analysis was performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) using the “survival” and “survminer” packages.
Results
Patient characteristics
We identified 836 ES-SCLC patients treated at our institution between 1998 and 2018, and 107 had synchronous brain metastases (Figure 1). The median follow-up was 9.0 months (interquartile range, 4.8–13.9 months). Table 1 shows patient characteristics and demographics for the entire cohort. At the time of SCLC diagnosis, the median age was 62.7 years (range, 34.2–86.7 years). Fifty-one (47.7%) were male and 56 (52.3%) were female. Seventy-two (67.3%) were Caucasian, 24 (22.4%) were African-American, and 11 (10.3%) had unknown race. Sixty-four (59.8%) had extracranial metastases in addition to brain metastases at initial diagnosis. The median number of brain metastases was three, and the median size of the largest brain metastasis for each patient was 2.0 cm (interquartile range, 1.0–3.3 cm). Fifty-nine (55.1%) had neurologic symptoms at presentation.
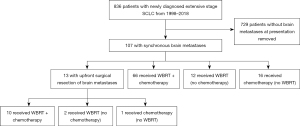
Table 1
| Characteristics/demographics | Values |
|---|---|
| Age (years), median [range] | 62.7 [34.2–86.7] |
| Sex, n (%) | |
| Male | 51 (47.7) |
| Female | 56 (52.3) |
| Race, n (%) | |
| Caucasian | 72 (67.3) |
| African-American | 24 (22.4) |
| Unknown | 11 (10.3) |
| T-stage, n (%) | |
| T1 | 23 (21.5) |
| T2 | 16 (15.0) |
| T3 | 17 (15.9) |
| T4 | 30 (28.0) |
| Tx | 8 (7.5) |
| Unknown | 13 (12.1) |
| N-stage, n (%) | |
| N0 | 4 (3.7) |
| N1 | 7 (6.5) |
| N2 | 50 (46.7) |
| N3 | 30 (28.0) |
| Nx | 1 (0.9) |
| Unknown | 15 (14.0) |
| Number of brain metastases, median [range] | 3 [1–innumerable] |
| Size of largest brain metastases (cm), median [range] |
2.0 [0.2–6.7] |
| No extracranial metastases, n (%) | 43 (40.2) |
| Neurological symptoms at diagnosis, n (%) | 59 (55.1) |
| WBRT, n (%) | 90 (84.1) |
| WBRT dose (Gy), median [range] | 30 [20–40] |
| Chemotherapy, n (%) | 93 (86.9) |
| Upfront craniotomy, n (%) | 13 (12.1) |
| Salvage radiation after WBRT, n (%) | 15 (14.0) |
WBRT, whole brain radiation therapy.
As part of their initial therapy, 90 (84.1%) patients received WBRT with a median dose of 30 Gy (range, 20–40 Gy), and 93 (86.9%) received chemotherapy. Seventy-six (71.0%) received both chemotherapy and WBRT, 17 (15.9%) received chemotherapy without WBRT, and 14 (13.1%) received WBRT without chemotherapy. Of the 76 patients who received both chemotherapy and WBRT, 30 (39.5%) received chemotherapy first, 33 (43.4%) received WBRT first, and 13 (17.1%) received chemotherapy and WBRT concurrently (defined as starting WBRT within 14 days of the start of the first cycle of chemotherapy). Nine patients received immunotherapy in addition to chemotherapy. Thirteen of 107 patients (12.1%) underwent upfront craniotomy and brain metastasis resection; 10 of these patients went on to receive both chemotherapy and WBRT, one had chemotherapy alone, and two had WBRT alone.
Performance status data was available for 66 out of 107 patients (62%), including 12 out of the 13 patients (92%) who received upfront craniotomy. Of the 66 patients with recorded performance status, 18 (27%) had Karnofsky performance status (KPS) <70, while 48 (73%) had KPS ≥70. Five craniotomy patients had KPS <70, while seven had KPS ≥70.
Prognostic factors for OS and bPFS
For the entire cohort, median OS was 9.0 months (interquartile range, 4.2–13.8 months), and median bPFS was 7.3 months (interquartile range, 3.5–11.1 months) (Figure 2). There was no significant association between OS and year of diagnosis (Figure S1). Table 2 shows prognostic factors for OS and bPFS on univariate and multivariate analysis. On univariate analysis of OS, lower age [hazard ratio (HR) =1.03, P=0.004], fewer brain metastases (HR =1.01, P=0.07), receipt of both chemotherapy and WBRT (HR =0.38, P<0.001), upfront craniotomy (HR =0.41, P<0.001), and having no extracranial metastases at initial diagnosis (HR =0.67, P=0.056) were significant favorable predictors. On multivariate analysis, fewer brain metastases (HR =1.01, P=0.04), receipt of both chemotherapy and WBRT (HR =0.53, P=0.02), and upfront craniotomy (HR =0.44, P=0.02) remained significant factors for OS. For univariate analysis of bPFS, lower age (HR =1.02, P=0.03), fewer brain metastases (HR =1.01, P=0.052), receipt of both chemotherapy and WBRT (HR =0.44, P<0.001), and upfront craniotomy (HR =0.55, P=0.058) were significant favorable predictors. On multivariate analysis, fewer brain metastases (HR =1.01, P=0.03) and receipt of both chemotherapy and WBRT (HR =0.47, P=0.005) remained significant favorable predictors for bPFS.
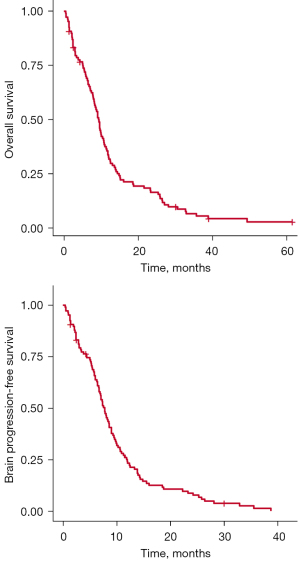
Table 2
| Patient variable | Values (n=107) | OS univariate | OS multivariate | bPFS univariate | bPFS multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Age (years), median | 62.7 | 1.03 (1.01–1.05) | 0.004 | 1.01 (0.99–1.03) |
0.41 | 1.02 (1.00–1.04) | 0.03 | 1.00 (0.98–1.03) |
0.92 | |||
| Sex, n (%) | ||||||||||||
| Male | 51 (47.7) | 1.35 (0.91–2.01) | 0.14 | – | – | 1.23 (0.83–1.82) | 0.30 | – | – | |||
| Female | 56 (52.3) | Ref | – | – | – | – | – | – | – | |||
| Race, n (%) | ||||||||||||
| Caucasian | 72 (67.3) | Ref | – | – | – | Ref | – | – | – | |||
| African-American | 24 (22.4) | 0.98 (0.60–1.60) | 0.93 | – | – | 0.98 (0.60–1.59) | 0.93 | – | – | |||
| Unknown | 11 (10.3) | 0.74 (0.38–1.45) | 0.38 | – | – | 0.74 (0.38–1.45) | 0.38 | – | – | |||
| Smoking status, n | – | – | ||||||||||
| Active | 82 | Ref | – | – | – | Ref | – | – | – | |||
| Never | 3 | 0.40 (0.095–1.68) | 0.21 | – | – | 0.40 (0.10–1.68) | 0.21 | – | – | |||
| Former | 21 | 1.01 (0.61–1.66) | 0.98 | – | – | 1.01 (0.61–1.66) | 0.98 | – | – | |||
| Unknown | 1 | – | – | – | – | – | – | – | – | |||
| T stage, n | ||||||||||||
| T1 | 23 | Ref | – | – | – | Ref | – | – | – | |||
| T2 | 16 | 0.94 (0.48–1.83) | 0.85 | – | – | 0.88 (0.44–1.75) | 0.71 | – | – | |||
| T3 | 17 | 1.47 (0.78–2.80) | 0.24 | – | – | 1.70 (0.89–3.25) | 0.11 | – | – | |||
| T4 | 30 | 0.91 (0.51–1.61) | 0.74 | – | – | 0.91 (0.52–1.58) | 0.73 | – | – | |||
| Tx | 8 | 0.88 (0.39–1.99) | 0.77 | – | – | 0.93 (0.42–2.09) | 0.87 | – | – | |||
| Unknown | 13 | – | – | – | – | – | – | – | – | |||
| N stage, n | ||||||||||||
| N0 | 4 | Ref | – | – | – | Ref | – | – | – | |||
| N1 | 7 | 1.00 (0.26–3.90) | >0.99 | – | – | 0.80 (0.20–3.12) | 0.75 | – | – | |||
| N2 | 50 | 0.64 (0.20–2.10) | 0.46 | – | – | 0.59 (0.18–1.93) | 0.39 | – | – | |||
| N3 | 30 | 0.97 (0.29–3.21) | 0.96 | – | – | 0.71 (0.21–2.38) | 0.58 | – | – | |||
| Nx | 1 | 1.63 (0.17–15.93) | 0.68 | – | – | 1.06 (0.11–10.31) | 0.96 | – | – | |||
| Unknown | 13 | – | – | – | – | – | – | – | – | |||
| Number of brain metastases, median | 3 | 1.01 (0.99–1.02) | 0.07 | 1.01 (1.00–1.02) |
0.04 | 1.01 (0.99–1.02) | 0.052 | 1.01 (1.00–1.02) |
0.03 | |||
| Size of largest brain metastases (cm), median | 2.0 | 0.90 (0.78–1.04) | 0.15 | – | – | 0.94 (0.81–1.09) | 0.40 | – | – | |||
| Chemotherapy + WBRT, n (%) | 76 (71.0) | 0.38 (0.24–0.60) | <0.001 | 0.53 (0.31–0.92) |
0.02 | 0.44 (0.29–0.68) | <0.001 | 0.47 (0.27–0.79) |
0.005 | |||
| Upfront craniotomy, n (%) | 13 (12.1) | 0.41 (0.22–0.79) | <0.001 | 0.44 (0.23–0.87) |
0.02 | 0.55 (0.30–1.02) | 0.058 | 0.58 (0.31–1.08) |
0.09 | |||
| No extracranial metastases, n (%) | 43 (40.2) | 0.67 (0.44–1.01) | 0.056 | 0.91 (0.58–1.43) |
0.68 | 0.74 (0.50–1.12) | 0.14 | – | – | |||
| Symptomatic brain metastases, n (%) | 59 (55.1) | 0.98 (0.66–1.45) | 0.90 | – | – | 1.16 (0.79–1.72) | 0.45 | – | – | |||
| Salvage RT after WBRT, n (%) | 15 (14.0) | 0.29 (0.15–0.53) | <0.0001 | – | – | 0.58 (0.33–1.01) | 0.054 | – | – | |||
OS, overall survival; bPFS, brain progression-free survival; HR, hazard ratio; CI, confidence interval; Ref, reference; WBRT, whole brain radiation therapy; RT, radiation therapy.
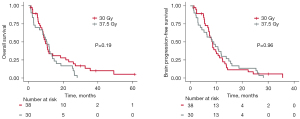
Patients who received both chemotherapy and WBRT had significantly improved OS and bPFS compared to patients who had either therapy alone. There was no significant difference in OS or bPFS among patients who started chemotherapy first, WBRT first, or both concurrently. WBRT dose (30 Gy/10 fractions vs. 37.5 Gy/15 fractions) was not a significant predictor for OS or bPFS (Figure 3). Of the 90 patients who received WBRT, 21 had intracranial progression at 1 year, and 29 by 2 years. Fifteen of these patients had salvage brain radiation after their initial WBRT; nine had Gamma Knife radiosurgery, four had repeat WBRT, and two had both Gamma Knife radiosurgery followed by repeat WBRT for further progression.
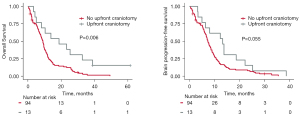
Thirteen of 107 patients (12.1%) received upfront craniotomy and brain tumor resection. Table 3 shows the characteristics for each patient who received upfront surgery as well as the indications for surgery. All presented with neurologic symptoms (altered mental status, motor dysfunction, aphasia, ataxia, dysarthria, cognitive/behavioral changes, seizures, or signs of increased intracranial pressure), and only one had histologically proven SCLC prior to surgery. Median OS for this surgery cohort was 18.7 months (interquartile range, 6.6–35.7 months). The surgical cohort had significantly longer OS but not bPFS compared to the non-surgical cohort (Figure 4). Eleven of the 13 patients had both chemotherapy and WBRT following surgery. All but one surgical patient received WBRT, and that patient died only 3.3 months after initial diagnosis.
Table 3
| Patient | Age (years) | Sex | Overall survival (months) | Reason for surgery | Number of brain metastases at diagnosis | Largest brain metastases size (cm) | KPS | TNM stage | Extracranial metastatic sites | Time from craniotomy to start of chemo or WBRT (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44.3 | M | 10.3 | Right frontal mass with acute left hemiparesis and AMS | 6 | 4.3 | 60 | T3N2M1c | Liver, adrenal, kidney | 18 |
| 2 | 59.6 | M | 38.8 | Cerebellar mass with ataxia and dysarthria | 3 | 3.0 | – | T2aN2M1c | Liver, bone | 15 |
| 3 | 62.7 | F | 18.7 | Cerebellar mass with headache, nausea, seizure | 10 | 3.4 | 90 | T1aNxM1c | None | 31 |
| 4 | 67.5 | M | 13.8 | Cerebellar mass with headache, ataxia, memory loss, nausea and vomiting | 2 | 4.6 | 80 | TxN2M1c | None | 22 |
| 5 | 65.9 | F | 25.3 | Cerebellar mass with ataxia | 1 | 3.3 | 30–40 | TxN2M1b | None | 20 |
| 6 | 53.3 | F | 32.7 | Pineal area mass with headache and diplopia | 1 | 2.6 | 90 | TxN2M1c | Adrenal | 16 |
| 7 | 81.2 | M | 5.0 | Left frontal mass with confusion and aphasia | 4 | 6.7 | 50–60 | TxN3M1c | Peritoneum, lymph node | 72 |
| 8 | 64.3 | M | 8.1 | Cerebellar mass with seizures, behavioral changes, headache, nausea and vomiting | 9 | 3.5 | 50–60 | T1bN3M1c | None | 16 |
| 9 | 53.8 | F | 61.5 | Right cerebral masses with left arm paresis | 6 | 4.4 | 70–80 | T4N2M1c | None | 27 |
| 10 | 62.5 | F | 23.2 | Right frontal mass with left arm paresis | 4 | 6.4 | 90 | T1aN2M1c | None | 23 |
| 11 | 86.7 | F | 3.3 | Cerebellar mass with ataxia and dysarthria | 1 | 5.1 | 50–60 | T1bN1M1c | Adrenal, bone | 42 |
| 12 | 64.7 | F | 4.2 | Left occipital mass with vertigo, headache, aphasia | 2 | 6.4 | 70–80 | TxN3M1c | None | 39 |
| 13 | 68.2 | F | 39.0 | Right parietal mass with ataxia, left visual field cut, dizziness | 12 | 5.5 | 70–80 | T1bN2M1c | None | 34 |
KPS, Karnofsky performance status; TNM, TNM classification of malignant tumors; WBRT, whole brain radiation therapy; M, male; AMS, altered mental status; F, female.
Subset analysis was performed on the cohort of 66 patients with recorded performance status using multivariable Cox regression to estimate the effect of upfront craniotomy on OS after controlling for the effect of performance status. Table 4 shows the results of this subset analysis. Upfront craniotomy remained significantly associated with improved OS (HR =0.37, P=0.006).
Table 4
| Factor | Hazard ratio (95% CI) | P value |
|---|---|---|
| Upfront craniotomy vs. no upfront craniotomy | 0.37 (0.18, 0.76) | 0.006 |
| KPS (≥70 vs. <70) | 0.3 (0.16, 0.57) | <0.001 |
CI, confidence interval; KPS, Karnofsky performance status.
Discussion
In this retrospective study of SCLC patients with synchronous brain metastases at initial diagnosis, we sought to identify factors associated with OS and bPFS. Of the 107 patients in this cohort, 13 had upfront craniotomy prior to chemotherapy or WBRT. On multivariate analysis, upfront craniotomy was a significant favorable predictor for OS but not bPFS. Receipt of both chemotherapy and WBRT was a predictor for improved OS and bPFS compared to receipt of either chemotherapy or WBRT alone. For patients who had both chemotherapy and WBRT, there was no significant difference in OS or bPFS among patients who started both concurrently (defined as starting within 14 days of each other), started chemotherapy before WBRT, or started WBRT before chemotherapy.
A recent study based on Surveillance, Epidemiology, and End Results (SEER) data included 33,169 SCLC patients, of whom 5,711 had synchronous brain metastases. This analysis found median OS of 5.0 months for SCLC patients with synchronous brain metastases. This is lower than the OS reported in our cohort (9.0 months), though this may be due to differences in treatment regimens and/or patient characteristics. This study also analyzed the impact of different treatment regimens. Similar to our findings, patients who received both radiation and chemotherapy had improved OS compared to receipt of either modality alone. However, data on surgical resection of brain metastases was not reported in this study (8).
Focal therapies such as SRS and surgical resection have historically not been used for SCLC brain metastases because of its radiosensitive nature and concern for diffuse spread leading to a short interval to distant intracranial failure. The role of radiation for the treatment for SCLC brain metastases is evolving (3,9-12). Two recent studies, the FIRE-SCLC multicenter cohort study and a systematic review and meta-analysis showed no survival detriment for upfront SRS compared to WBRT for selected SCLC patients (3,4). As such, the role of upfront SRS for the treatment of SCLC brain metastases is currently being evaluated in the NRG Oncology cooperative groups clinical trial, NRG-CC009. There remains a paucity of data on surgical resection for SCLC brain metastases aside from case reports (13-15). Prior randomized trials of surgical resection for solitary brain metastases have shown survival improvement compared to WBRT alone, but these trials excluded SCLC and other radiosensitive histologies (5-7,16). Current National Comprehensive Cancer Network (NCCN) guidelines do not list surgical resection as a standard treatment option for SCLC brain metastases (17). We do not view our study as suggestive for a change in the standard of care or guidelines, but rather as hypothesis-generating and warranting further exploration of potential patient characteristics that would help identify patients who may significantly benefit from upfront craniotomy for synchronous SCLC brain metastases.
Our retrospective analysis suggests that surgical resection of symptomatic SCLC brain metastases does not negatively impact survival, as the surgical cohort had a significantly longer OS compared to the overall cohort. There is likely selection bias; the surgical cohort was small (12% of patients) with a higher proportion of patients without extracranial metastases compared to the non-surgical cohort (61.5% vs. 37.2%), and these patients were healthy enough to undergo major surgery. However, the proportions of patients with single brain metastases were similar between cohorts (23.1% vs. 21.3%), and some of the longest-surviving surgical patients had more than three lesions. SCLC brain metastasis surgical specimens may open opportunities for genomic characterization of SCLC brain metastases as has been the case for brain metastases for NSCLC and other primary histologies (18,19). Such molecular studies may help elucidate why SCLC has such a high propensity for brain metastasis compared to other solid tumors.
Platinum doublet chemotherapy has long been the standard systemic treatment for ES-SCLC, and this may also be effective for intracranial disease. The optimal combination and sequence of chemotherapy and radiotherapy for SCLC brain metastases remains unclear. NCCN guidelines allow upfront chemotherapy with deferred WBRT if the brain metastases are asymptomatic (17). Our analysis showed improved OS and bPFS for patients who received both chemotherapy and WBRT as initial therapy compared to either modality alone. Selection bias may explain this, as patients with shorter survival or poorer performance status may not have lived long enough or have been eligible for chemotherapy. Whether these patients received WBRT before, during, or after the first cycle of chemotherapy did not significantly impact OS or bPFS.
WBRT dose (30 Gy/10 fractions vs. 37.5 Gy/15 fractions) was not a significant predictor of OS or bPFS in our study. A post hoc analysis of the N107C trial showed that WBRT 37.5 Gy/15 fractions did not improve cognitive outcomes, PFS, or OS compared to 30 Gy/10 fractions (20). This trial excluded radiosensitive histologies, and our analysis showed similar results for SCLC.
This study has multiple limitations. In particular, performance status data was not available for 38% of the entire patient cohort, and this is an important factor for brain metastasis prognosis (21-23). Our subset analysis on the patients with recorded performance status found that upfront craniotomy remained significantly associated with improved OS even when controlling for performance status. The proportion of patients with missing performance status certainly raises the possibility of selection bias. Moreover, we do not have patient quality of life data. There was also a lack of data on neurologic death vs. death from other causes, which would help elucidate the difference in OS seen between the surgical and non-surgical cohorts. Because this study included patients who were diagnosed with ES-SCLC between 1998 and 2018, the majority of patients were treated without immunotherapy, which is now a standard component of upfront systemic therapy for ES-SCLC after randomized trials showed OS benefit (17,24,25). The few patients in our cohort who did receive immunotherapy were treated with ipilimumab or nivolumab, whereas current standard systemic therapy regimens now incorporate atezolizumab or durvalumab following the publication of the IMpower133 and CASPIAN trials. However, IMpower133 included patients with treated asymptomatic brain metastases, but not untreated ones, limiting evaluation of the impact of atezolizumab on intracranial disease. While the CASPIAN trial did include patients with untreated brain metastases, they only made up about 10% of the cohort. Our study included patients with synchronous brain metastases at initial diagnosis of SCLC, representing a population that was not studied in IMpower133 and was a small minority in the CASPIAN trial. More prospective data is needed to evaluate the efficacy of immunotherapy for SCLC brain metastases. Other changes in treatment protocols between 1998 and 2018 could have impacted outcomes, but a linear regression model found no significant association between OS and year of diagnosis in our study.
Conclusions
In this retrospective analysis of SCLC patients with synchronous brain metastases at initial diagnosis, the combination of chemotherapy and WBRT was associated with improved OS and bPFS compared to either modality alone. Upfront brain metastasis resection was associated with increased OS but not bPFS. Further study is needed to better identify which patients with SCLC brain metastases may benefit from upfront surgical resection.
Acknowledgments
Some data from this manuscript was included in a poster presentation at the 2020 American Society for Radiation Oncology (ASTRO) Annual Meeting. The authors would like to thank Dr. Prashant Vempati for his critical review of the manuscript.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-641/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-641/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-641/coif). T.R.H. reports receiving consulting fees from Medtronics which is not relevant to the work in this paper. T.B. reports funding from National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP) as national principle investigator (PI) of a clinical trial 04/22-3/23, which is not relevant to this study. A.D. reports participation in data safety monitoring board or advisory board on Ipsen, Amgen, Jazz, BMS, Astra Zeneca. S.C. reports receiving funding from the National Institutes of Health 01/20-11/23 (grant No. K12CA076917 to S.C. for salary support and research funds); participation in advisory boards for Telix Pharmaceuticals, Seagen Inc. and GT Medical. A.E.S. reports funding from U01 CS236215 06/19-5/23 (PI: Sloan), R01 CA 217956 06/17-05/22 (PI: Brady-Kalnay), R21 CA256573 01/21-12/22 (PI: Sloan), P01 CA CA245705-01 09/20-08/25 (PI: Lathia), Merck Case 3316 12/17-11/21 (PI: Sloan), Coulter Translational Research Fund 09/17-08/22 (PI: Brady-Kalnay), Jobs Ohio 5/22-11/23 (PI: Brady-Kalnay); options for <1% of company shares of Surgical Theater; consulting fees from Medtronic (Visualase), Monteris Medical Inc. and Surgical Theater; patents on the usage of PS-Binding CAR-T Cells and the procoagulant function of cancer stem cells. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients treated for extensive-stage SCLC (ES-SCLC) at University Hospitals Cleveland Medical Center between 1998 and 2018 were identified using a University Hospitals Institutional Review Board (IRB) approved database (No. CHRV0021). There were minimal risks to the patients for study participation, thus obtaining consents was not required for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kromer C, Xu J, Ostrom QT, et al. Estimating the annual frequency of synchronous brain metastasis in the United States 2010-2013: a population-based study. J Neurooncol 2017;134:55-64. [Crossref] [PubMed]
- Seute T, Leffers P, ten Velde GP, et al. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI). Cancer 2008;112:1827-34. [Crossref] [PubMed]
- Rusthoven CG, Yamamoto M, Bernhardt D, et al. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA Oncol 2020;6:1028-37. [Crossref] [PubMed]
- Gaebe K, Li AY, Park A, et al. Stereotactic radiosurgery versus whole brain radiotherapy in patients with intracranial metastatic disease and small-cell lung cancer: a systematic review and meta-analysis. Lancet Oncol 2022;23:931-9. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 1993;33:583-90. [Crossref] [PubMed]
- Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996;78:1470-6. [Crossref] [PubMed]
- Zhou G, Zhang Z, Yu P, et al. Predictive value of clinical characteristics on risk and prognosis of synchronous brain metastases in small-cell lung cancer patients: A population-based study. Cancer Med 2023;12:1195-203. [Crossref] [PubMed]
- Gjyshi O, Lin SH, Pezzi TA, et al. Care Patterns for Stereotactic Radiosurgery in Small Cell Lung Cancer Brain Metastases. Clin Lung Cancer 2022;23:185-90. [Crossref] [PubMed]
- Jiang W, Haque W, Verma V, et al. Stereotactic radiosurgery for brain metastases from newly diagnosed small cell lung cancer: practice patterns and outcomes. Acta Oncol 2019;58:491-8. [Crossref] [PubMed]
- Robin TP, Jones BL, Amini A, et al. Radiosurgery alone is associated with favorable outcomes for brain metastases from small-cell lung cancer. Lung Cancer 2018;120:88-90. [Crossref] [PubMed]
- Rusthoven CG, Staley AW, Gao D, et al. Comparison of first-line radiosurgery for small-cell and non-small cell lung cancer brain metastases (CROSS-FIRE). J Natl Cancer Inst 2023;115:926-36. [Crossref] [PubMed]
- Imai R, Hayakawa K, Sakurai H, et al. Small cell lung cancer with a brain metastasis controlled for 5 years: a case report. Jpn J Clin Oncol 2001;31:116-8. [Crossref] [PubMed]
- Jesien-Lewandowicz E, Spych M, Fijuth J, et al. Solitary brain metastasis of an occult and stable small-cell lung cancer in a schizophrenic patient: a 3-year control. Lung Cancer 2010;69:245-8. [Crossref] [PubMed]
- Drpa G, Popovic F, Nikolic I, et al. Small cell lung cancer with solitary brain metastasis treated with complete resection. Precision Radiation Oncology 2018;2:61-3.
- Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 1994;29:711-7. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Small Cell Lung Cancer (Version 2.2024). Accessed December 10, 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- Wang H, Ou Q, Li D, et al. Genes associated with increased brain metastasis risk in non-small cell lung cancer: Comprehensive genomic profiling of 61 resected brain metastases versus primary non-small cell lung cancer (Guangdong Association Study of Thoracic Oncology 1036). Cancer 2019;125:3535-44. [Crossref] [PubMed]
- Brastianos PK, Twohy E, Anders CK, et al. Alliance A071701: Genomically guided treatment trial in brain metastases. J Clin Oncol 2020;38:TPS2573.
- Trifiletti DM, Ballman KV, Brown PD, et al. Optimizing Whole Brain Radiation Therapy Dose and Fractionation: Results From a Prospective Phase 3 Trial (NCCTG N107C [Alliance]/CEC.3). Int J Radiat Oncol Biol Phys 2020;106:255-60. Erratum in: Int J Radiat Oncol Biol Phys 2020;106:1111.
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Sperduto PW, Mesko S, Li J, et al. Survival in Patients With Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J Clin Oncol 2020;38:3773-84. [Crossref] [PubMed]
- Sperduto PW, De B, Li J, et al. Graded Prognostic Assessment (GPA) for Patients With Lung Cancer and Brain Metastases: Initial Report of the Small Cell Lung Cancer GPA and Update of the Non-Small Cell Lung Cancer GPA Including the Effect of Programmed Death Ligand 1 and Other Prognostic Factors. Int J Radiat Oncol Biol Phys 2022;114:60-74. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]


